Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Overview
2.3. Animal Preparation
2.4. In Vivo Super-Resolution Ultrasound Imaging and Post-Processing
2.5. Ex Vivo µCT Imaging
2.6. Kidney Histology
2.7. Measurements of Metabolic Parameters
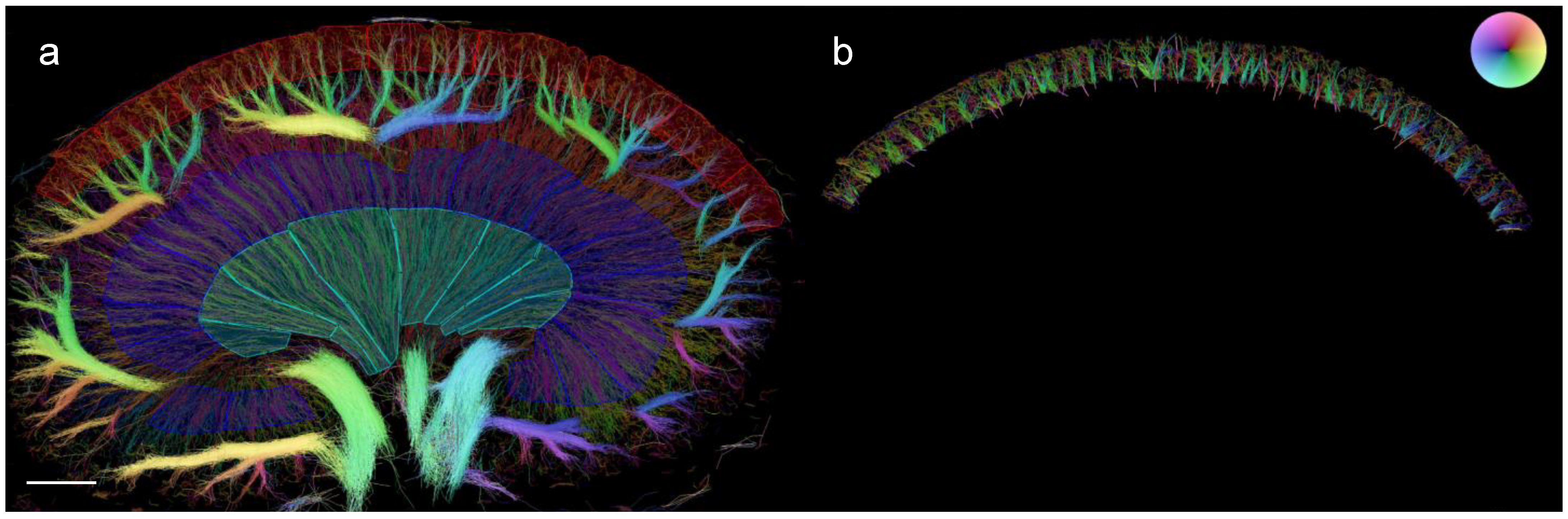
2.8. Quantification of Vascular Structural Alterations in the SRUS Images
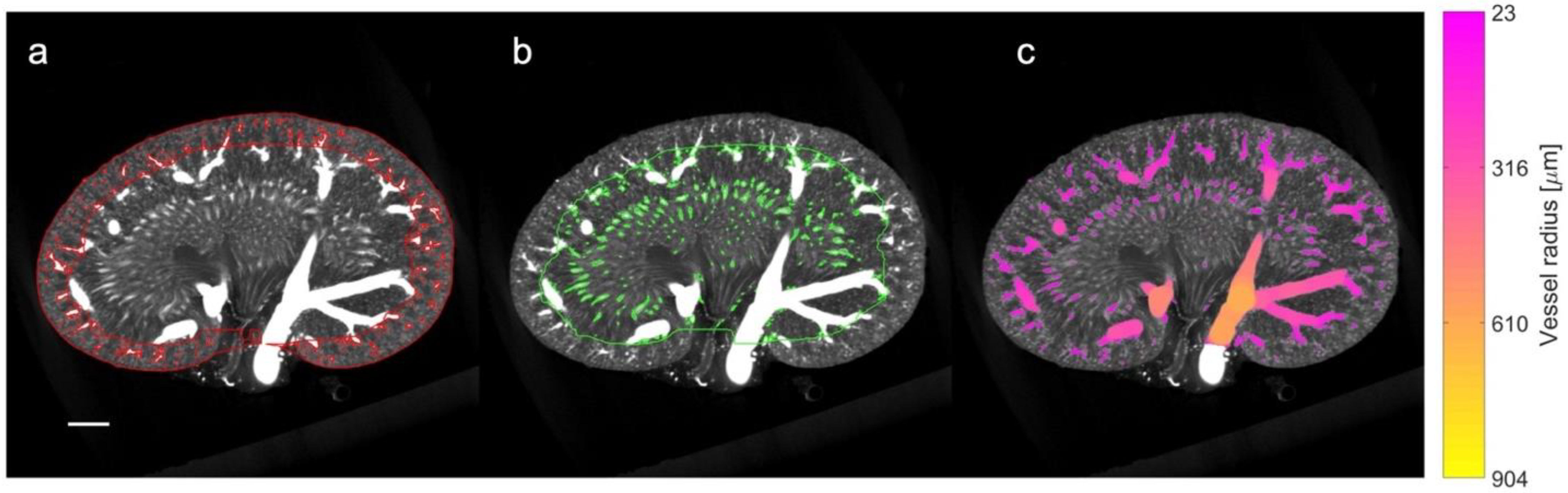
2.9. µCT Processing and Quantification of Vascular Density
2.10. Statistical Analyses
3. Results
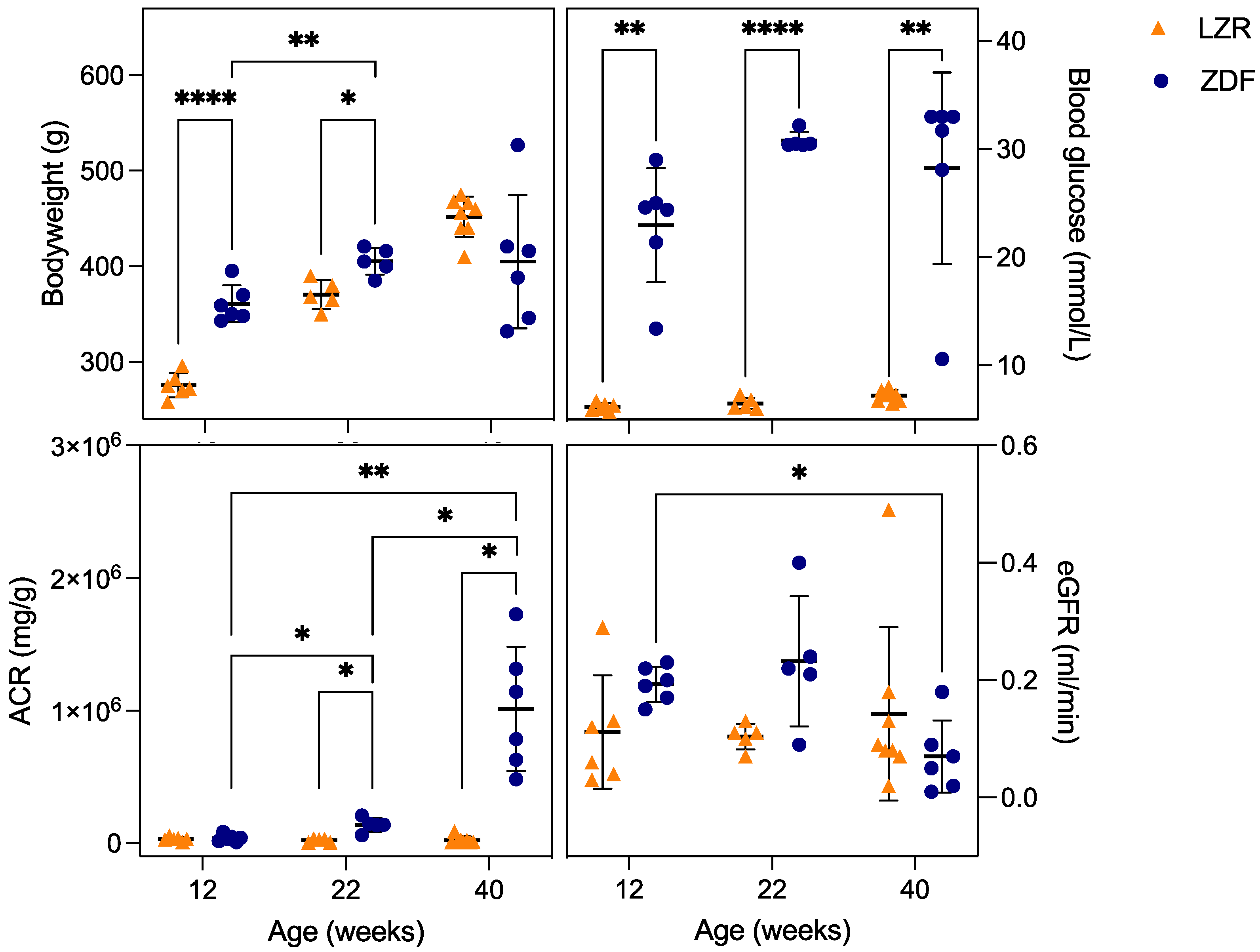
3.1. Metabolic Parameters
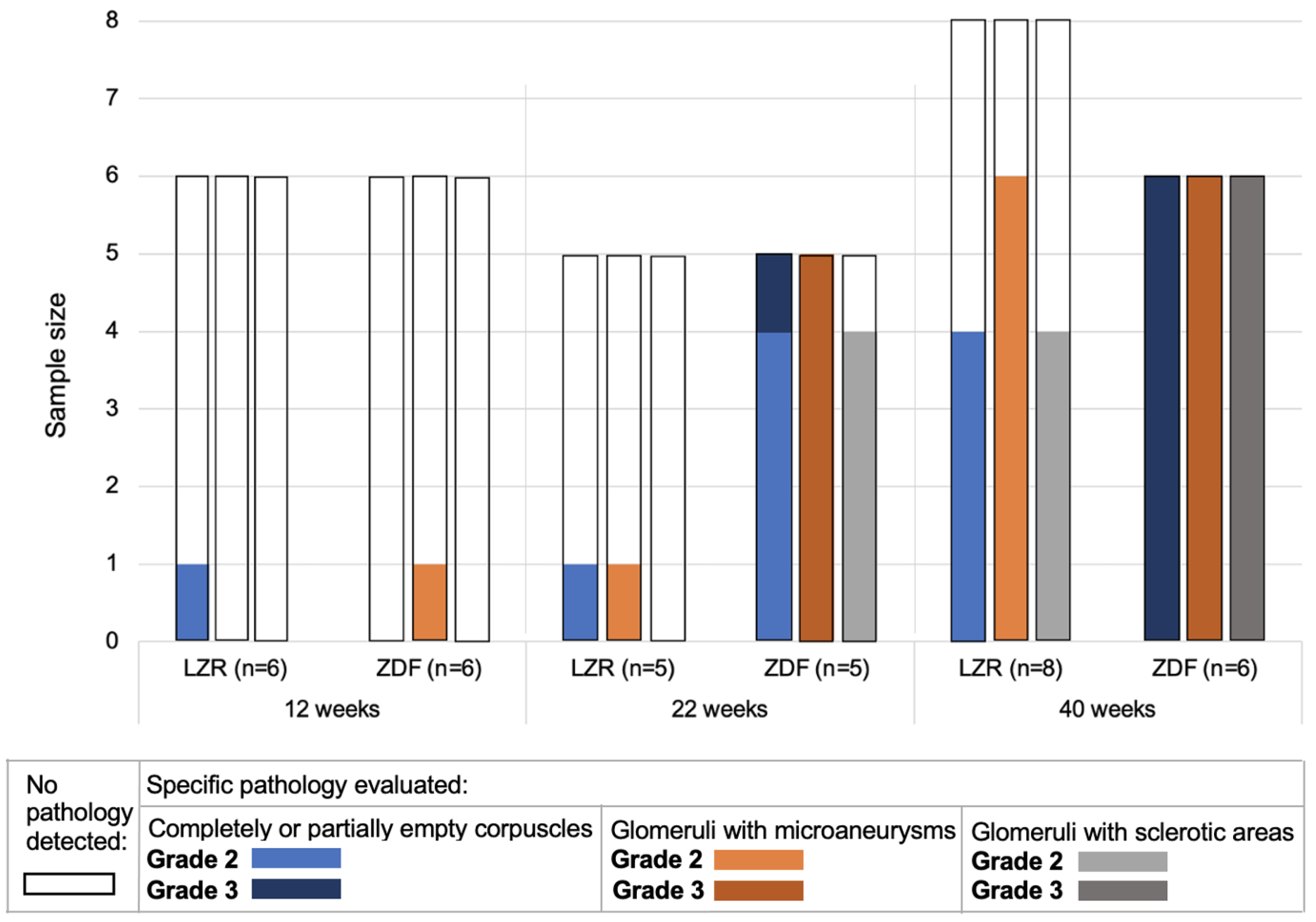
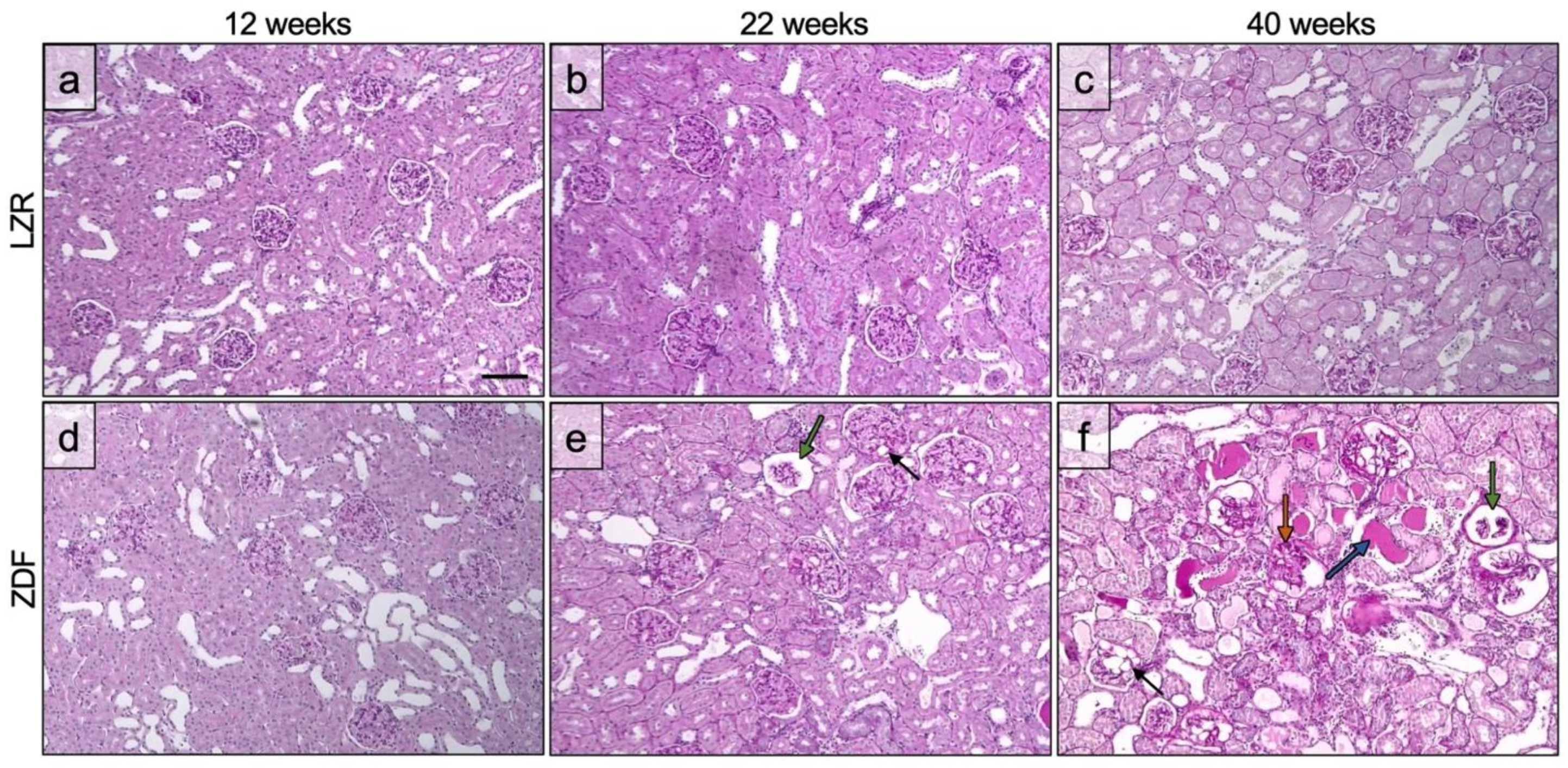
3.2. Glomerulopathy Grading
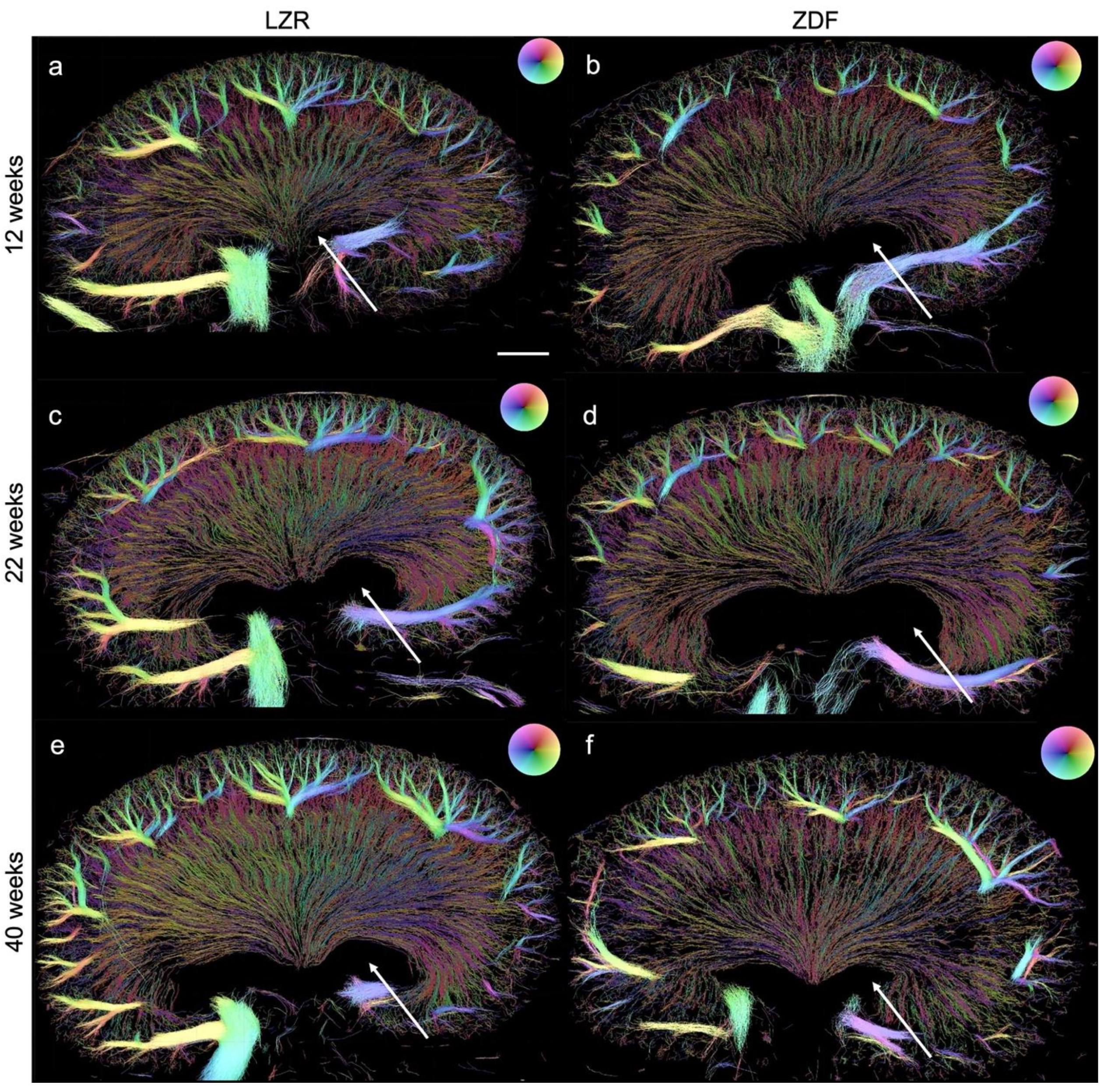
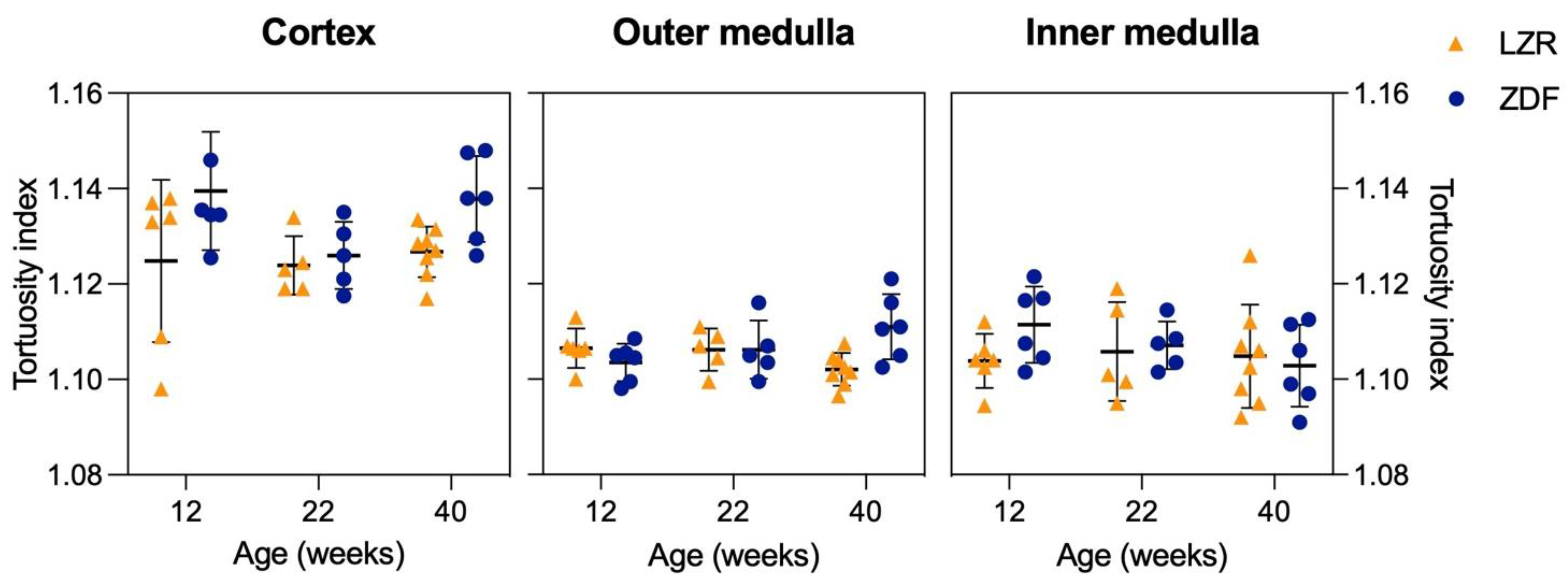
3.3. Quantified Measurements from SRUS
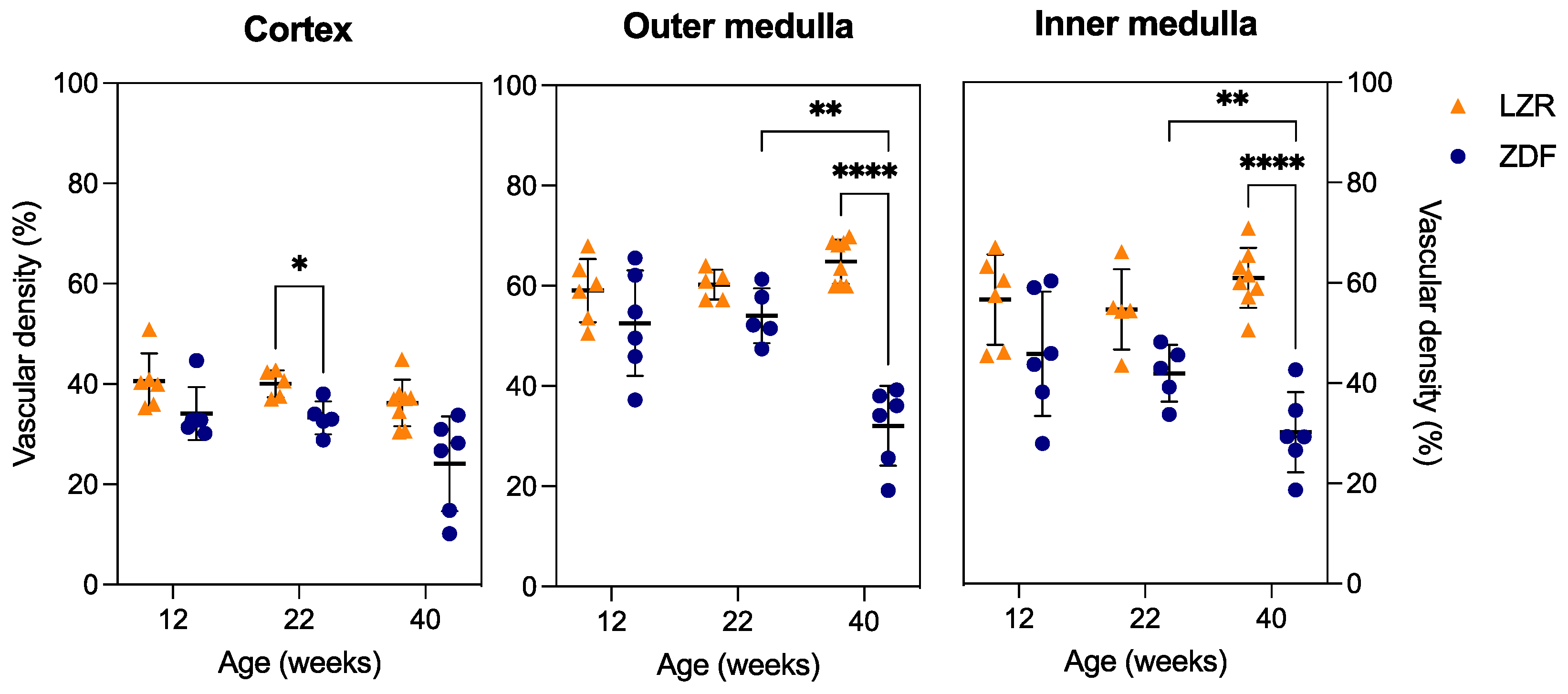
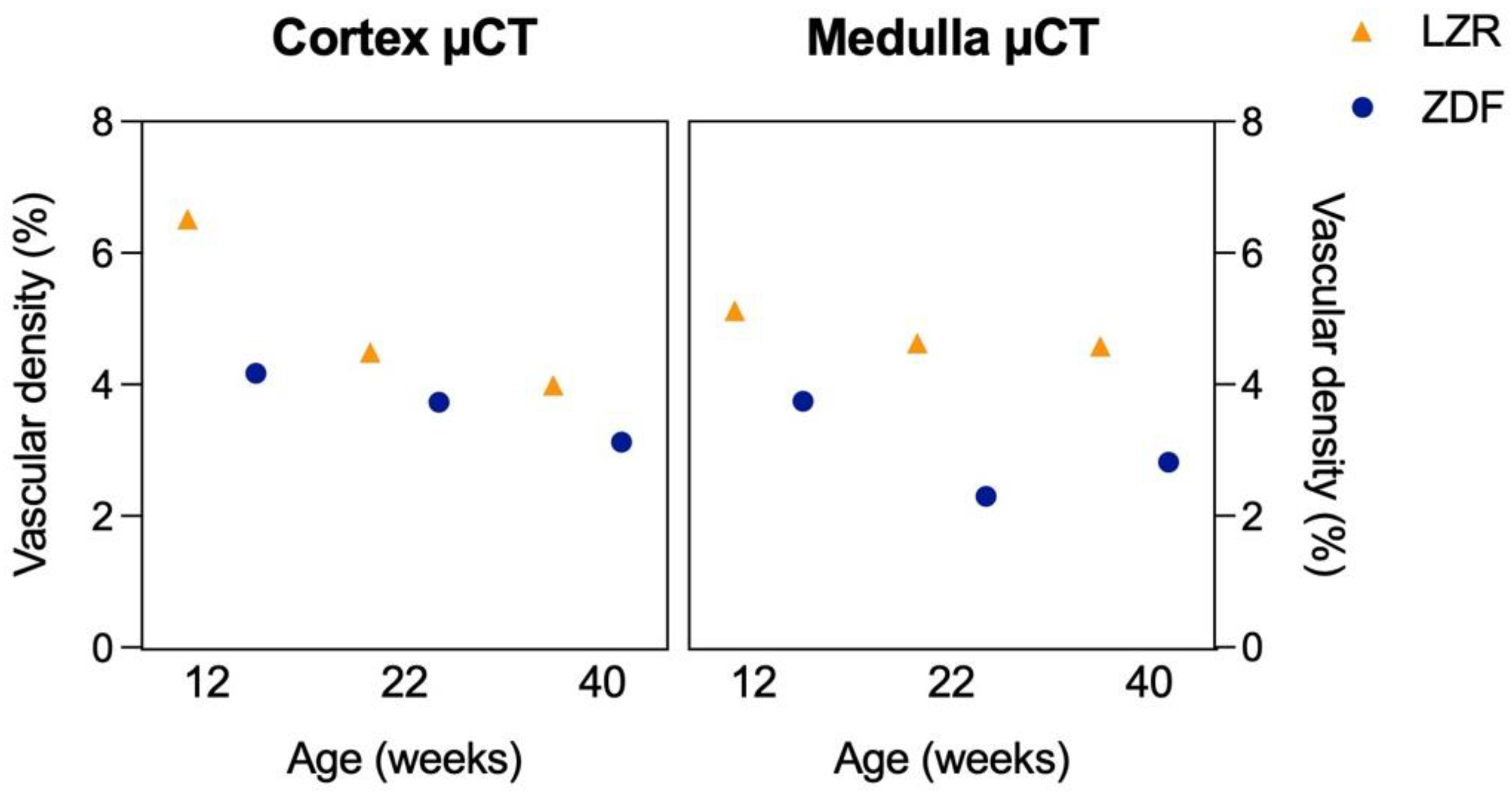
3.4. Qualitative and Quantitative Assessment in µCT
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation, Diabetes Atlas (10th ed.). Available online: www.diabetesatlas.org (accessed on 21 February 2022).
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 25 May 2022).
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global Estimates of the Prevalence of Diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- SAmerican Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Narres, M.; Claessen, H.; Droste, S.; Kvitkina, T.; Koch, M.; Kuss, O.; Icks, A. The Incidence of End-Stage Renal Disease in the Diabetic (Compared to the Non-Diabetic) Population: A Systematic Review. PLoS ONE 2016, 11, e0147329. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Alsaad, K.O.; Herzenberg, A.M. Distinguishing Diabetic Nephropathy from Other Causes of Glomerulosclerosis: An Update. J. Clin. Pathol. 2007, 60, 18–26. [Google Scholar] [CrossRef]
- Klessens, C.Q.F.; Woutman, T.D.; Veraar, K.A.M.; Zandbergen, M.; Valk, E.J.J.; Rotmans, J.I.; Wolterbeek, R.; Bruijn, J.A.; Bajema, I.M. An Autopsy Study Suggests That Diabetic Nephropathy Is Underdiagnosed. Kidney Int. 2016, 90, 149–156. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Hojs, R.; Trevisani, F.; Morales, E.; Fernández, G.; Bevc, S.; Cases Corona, C.M.; Cruzado, J.M.; Quero, M.; Navarro Díaz, M.; et al. The Role of Vascular Lesions in Diabetes Across a Spectrum of Clinical Kidney Disease. Kidney Int. Rep. 2021, 6, 2392–2403. [Google Scholar] [CrossRef]
- Lerman, L.O.; Textor, S.C. Renal Vascular Disease, 1st ed.; Springer: London, UK, 2014; ISBN 978-1447169680. [Google Scholar]
- Chade, A.R. Renovascular Disease, Microcirculation, and the Progression of Renal Injury: Role of Angiogenesis. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2011, 300, R783. [Google Scholar] [CrossRef]
- Pillebout, E.; Burtin, M.; Yuan, H.T.; Briand, P.; Woolf, A.S.; Friedlander, G.; Terzi, F. Proliferation and Remodeling of the Peritubular Microcirculation after Nephron Reduction: Association with the Progression of Renal Lesions. Am. J. Pathol. 2001, 159, 547–560. [Google Scholar] [CrossRef]
- Errico, C.; Pierre, J.; Pezet, S.; Desailly, Y.; Lenkei, Z.; Couture, O.; Tanter, M. Ultrafast Ultrasound Localization Microscopy for Deep Super-Resolution Vascular Imaging. Nature 2015, 527, 499–502. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.X.; et al. Super-Resolution Ultrasound Imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.B.; Taghavi, I.; Kjer, H.M.; Søgaard, S.B.; Gundlach, C.; Dahl, V.A.; Nielsen, M.B.; Dahl, A.B.; Jensen, J.A.; Sørensen, C.M. Evaluation of 2D Super-Resolution Ultrasound Imaging of the Rat Renal Vasculature Using Ex Vivo Micro-Computed Tomography. Sci. Rep. 2021, 11, 24335. [Google Scholar] [CrossRef] [PubMed]
- Demené, C.; Robin, J.; Dizeux, A.; Heiles, B.; Pernot, M.; Tanter, M.; Perren, F. Transcranial Ultrafast Ultrasound Localization Microscopy of Brain Vasculature in Patients. Nat. Biomed. Eng. 2021, 5, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, C.; Christensen-Jeffries, K.; Zhang, G.; Harput, S.; Dunsby, C.; Huang, P.; Tang, M.X. Super-Resolution Ultrasound Localization Microscopy of Microvascular Structure and Flow for Distinguishing Metastatic Lymph Nodes—An Initial Human Study. Ultraschall Med. 2022, 43, 592–598. [Google Scholar] [CrossRef]
- Opacic, T.; Dencks, S.; Theek, B.; Piepenbrock, M.; Ackermann, D.; Rix, A.; Lammers, T.; Stickeler, E.; Delorme, S.; Schmitz, G.; et al. Motion Model Ultrasound Localization Microscopy for Preclinical and Clinical Multiparametric Tumor Characterization. Nat. Commun. 2018, 9, 1527. [Google Scholar] [CrossRef]
- Bodard, S.; Denis, L.; Hingot, V.; Chavignon, A.; Hélénon, O.; Anglicheau, D.; Couture, O.; Correas, J.-M. Ultrasound Localization Microscopy of the Human Kidney Allograft on a Clinical Ultrasound Scanner. Kidney Int. 2023, 103, 930–935. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound Super-Resolution Imaging Provides a Noninvasive Assessment of Renal Microvasculature Changes during Mouse Acute Kidney Injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef]
- Andersen, S.B.; Taghavi, I.; Søgaard, S.B.; Hoyos, C.A.V.; Nielsen, M.B.; Jensen, J.A.; Sørensen, C.M. Super-Resolution Ultrasound Imaging Can Quantify Alterations in Microbubble Velocities in the Renal Vasculature of Rats. Diagnostics 2022, 12, 1111. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, J.; Yang, Y.; Zhang, H.; Lee, F.; He, Q.; Huang, C.; Huang, L.; Qian, L.; Luo, J. In Vivo Assessment of Hypertensive Nephrosclerosis Using Ultrasound Localization Microscopy. Med. Phys. 2022, 49, 2295–2308. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, L.; Yang, Y.; Qiu, L.; He, Q.; Liu, J.; Qian, L.; Luo, J. Evaluation of Early Diabetic Kidney Disease Using Ultrasound Localization Microscopy. J. Ultrasound Med. 2023, 9999, 1–16. [Google Scholar] [CrossRef]
- Yokoi, N.; Hoshino, M.; Hidaka, S.; Yoshida, E.; Beppu, M.; Hoshikawa, R.; Sudo, K.; Kawada, A.; Takagi, S.; Seino, S. A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. J. Diabetes Res. 2013, 2013, 103731. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Printz, R.L. Diabetes in Zucker Diabetic Fatty Rat. Methods Mol. Biol. 2012, 933, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Dvorakova, M.C. Future Perspective of Diabetic Animal Models. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 25–38. [Google Scholar] [CrossRef]
- Chadha, G.S.; Morris, M.E. Effect of Type 2 Diabetes Mellitus and Diabetic Nephropathy on IgG Pharmacokinetics and Subcutaneous Bioavailability in the Rat. AAPS J. 2015, 17, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Hempe, J.; Elvert, R.; Schmidts, H.L.; Kramer, W.; Herling, A.W. Appropriateness of the Zucker Diabetic Fatty Rat as a Model for Diabetic Microvascular Late Complications. Lab. Anim. 2012, 46, 32–39. [Google Scholar] [CrossRef]
- Schäfer, S.; Linz, W.; Bube, A.; Gerl, M.; Huber, J.; Kürzel, G.U.; Bleich, M.; Schmidts, H.L.; Busch, A.E.; Rutten, H. Vasopeptidase Inhibition Prevents Nephropathy in Zucker Diabetic Fatty Rats. Cardiovasc. Res. 2003, 60, 447–454. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Etgen, G.J.; Oldham, B.A. Profiling of Zucker Diabetic Fatty Rats in Their Progression to the Overt Diabetic State. Metab. Clin. Exp. 2000, 49, 684–688. [Google Scholar] [CrossRef]
- Taghavi, I.; Andersen, S.B.; Hoyos, C.A.V.; Nielsen, M.B.; Sorensen, C.M.; Jensen, J.A. In Vivo Motion Correction in Super Resolution Imaging of Rat Kidneys. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3082–3093. [Google Scholar] [CrossRef]
- Taghavi, I.; Andersen, S.B.; Hoyos, C.A.V.; Schou, M.; Gran, F.; Hansen, K.L.; Nielsen, M.B.; Sørensen, C.M.; Stuart, M.B.; Jensen, J.A. Ultrasound Super-Resolution Imaging with a Hierarchical Kalman Tracker. Ultrasonics 2022, 122, 106695. [Google Scholar] [CrossRef]
- Amann, K.; Haas, C.S. What You Should Know about the Work-up of a Renal Biopsy. Nephrol. Dial. Transplant. 2006, 21, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Moffat, D.B.; Fourman, J. The Vascular Pattern of the Rat Kidney. J. Anat. 1963, 97, 543. [Google Scholar] [PubMed]
- Zimmerhackl, B.; Robertson, C.R.; Jamison, R.L. The Microcirculation of the Renal Medulla. Off. J. Am. Heart Assoc. 1985, 57, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Johnson, R.; Feehally, J. Comprehensive Clinical Nephrology, 4th ed.; Mosby: Maryland Heights, MO, USA, 2010. [Google Scholar]
- Ehling, J.; Bábícková, J.; Gremse, F.; Klinkhammer, B.M.; Baetke, S.; Knuechel, R.; Kiessling, F.; Floege, J.; Lammers, T.; Boor, P. Quantitative Micro-Computed Tomography Imaging of Vascular Dysfunction in Progressive Kidney Diseases. J. Am. Soc. Nephrol. 2016, 27, 520–532. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Gostick, J.T.; Khan, Z.A.; Tranter, T.G.; Kok, M.D.R.; Agnaou, M.; Sadeghi, M.; Jervis, R. PoreSpy: A Python Toolkit for Quantitative Analysis of Porous Media Images Software. J. Open Source Softw. 2019, 4, 1296. [Google Scholar] [CrossRef]
- Baynes, J.; Murray, D.B. Cardiac and Renal Function Are Progressively Impaired with Aging in Zucker Diabetic Fatty Type II Diabetic Rats. Oxidative Med. Cell. Longev. 2009, 2, 328–334. [Google Scholar] [CrossRef]
- Silveiro, S.P.; Friedman, R.; Gross, J.L. Glomerular Hyperfiltration in NIDDM Patients without Overt Proteinuria. Diabetes Care 1993, 16, 115–119. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; Van Bommel, E.J.; Heerspink, H.J.L.; Van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef]
- Habib, S.L. Kidney Atrophy vs Hypertrophy in Diabetes: Which Cells Are Involved? Cell Cycle 2018, 17, 1683–1687. [Google Scholar] [CrossRef]
- Rave, K.; Nosek, L.; Posner, J.; Heise, T.; Roggen, K.; Hoogdalem, E.-J. van Renal Glucose Excretion as a Function of Blood Glucose Concentration in Subjects with Type 2 Diabetes—Results of a Hyperglycaemic Glucose Clamp Study. Nephrol. Dial. Transplant. 2006, 21, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Koya, D. Rodent Models of Diabetic Nephropathy: Their Utility and Limitations. Int. J. Nephrol. Renov. Dis. 2016, 9, 279. [Google Scholar] [CrossRef]
- Breyer, M.D.; Böttinger, E.; Brosius, F.C.; Coffman, T.M.; Harris, R.C.; Heilig, C.W.; Sharma, K. Mouse Models of Diabetic Nephropathy. J. Am. Soc. Nephrol. JASN 2005, 16, 27–45. [Google Scholar] [CrossRef]
- Maric-Bilkan, C.; Flynn, E.R.; Chade, A.R. Microvascular Disease Precedes the Decline in Renal Function in the Streptozotocin-Induced Diabetic Rat. Am. J. Physiol. -Ren. Physiol. 2012, 302, F308–F315. [Google Scholar] [CrossRef] [PubMed]
- Westergren, H.U.; Grönros, J.; Heinonen, S.E.; Miliotis, T.; Jennbacken, K.; Sabirsh, A.; Ericsson, A.; Jönsson-Rylander, A.C.; Svedlund, S.; Gan, L.M. Impaired Coronary and Renal Vascular Function in Spontaneously Type 2 Diabetic Leptin-Deficient Mice. PLoS ONE 2015, 10, e0130648. [Google Scholar] [CrossRef] [PubMed]
- Lowerison, M.R.; Sekaran, N.V.C.; Zhang, W.; Dong, Z.; Chen, X.; Llano, D.A.; Song, P. Aging-Related Cerebral Microvascular Changes Visualized Using Ultrasound Localization Microscopy in the Living Mouse. Sci. Rep. 2022, 12, 619. [Google Scholar] [CrossRef]
- Shelton, S.E.; Lee, Y.Z.; Lee, M.; Cherin, E.; Foster, F.S.; Aylward, S.R.; Dayton, P.A. Quantification of Microvascular Tortuosity during Tumor Evolution Utilizing Acoustic Angiography. Ultrasound Med. Biol. 2015, 41, 1896. [Google Scholar] [CrossRef]
- Vora, J.P.; Zimsen, S.M.; Houghton, D.C.; Anderson, S.; Vora, J.P.; Zimsen, S.M.; Anderson, S.; Houghton, D.C. Evolution of Metabolic and Renal Changes in the ZDF/Drt-Fa Rat Model of Type II Diabetes. J. Am. Soc. Nephrol. 1996, 7, 113–117. [Google Scholar] [CrossRef]
- Marsh, S.A.; Powell, P.C.; Agarwal, A.; Dell’Italia, L.J.; Chatham, J.C. Cardiovascular Dysfunction in Zucker Obese and Zucker Diabetic Fatty Rats: Role of Hydronephrosis. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H292–H298. [Google Scholar] [CrossRef]
- Chade, A.R. Renal Vascular Structure and Rarefaction. Compr. Physiol. 2013, 3, 817–831. [Google Scholar] [CrossRef]
- Querfeld, U.; Mak, R.H.; Pries, A.R. Microvascular Disease in Chronic Kidney Disease: The Base of the Iceberg in Cardiovascular Comorbidity. Clin. Sci. 2020, 134, 1333–1356. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease: A Statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Paul Sidhu, A.S.; Cantisani, V.; Dietrich, C.F.; Helge Gilja, O.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, 2–44. [Google Scholar] [CrossRef]
- von Stillfried, S.; Apitzsch, J.C.; Ehling, J.; Penzkofer, T.; Mahnken, A.H.; Knüchel, R.; Floege, J.; Boor, P. Contrast-Enhanced CT Imaging in Patients with Chronic Kidney Disease. Angiogenesis 2016, 19, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Dencks, S.; Piepenbrock, M.; Opacic, T.; Krauspe, B.; Stickeler, E.; Kiessling, F.; Schmitz, G. Clinical Pilot Application of Super-Resolution US Imaging in Breast Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 517–526. [Google Scholar] [CrossRef]
- Chavignon, A.; Heiles, B.; Hingot, V.; Orset, C.; Vivien, D.; Couture, O. 3D Transcranial Ultrasound Localization Microscopy in the Rat Brain with a Multiplexed Matrix Probe. IEEE Trans. Bio-Med. Eng. 2022, 69, 2132–2142. [Google Scholar] [CrossRef]


| 12 Weeks | 22 Weeks | 40 Weeks | ||||
|---|---|---|---|---|---|---|
| Basic characteristics | LZR (n = 6) | ZDF (n = 6) | LZR (n = 5) | ZDF (n = 5) | LZR (n = 8) | ZDF (n = 6) |
| Body weight (g) | 276 ± 12.74 | 361 ± 19.26 * | 371 ± 15.22 | 405 ± 14.15 * | 424 ± 21.09 | 405 ± 69.80 |
| Kidney weight (g) | 1.28 ± 0.09 | 1.67 ± 0.09 * | 1.35 ± 0.08 | 2.20 ± 0.46 * | 1.57 ± 0.20 | 2.41 ± 0.29 * |
| KW/BW (%) | 0.46 ± 0.04 | 0.46 ± 0.02 | 0.37 ± 0.02 | 0.54 ± 0.10 * | 0.35 ± 0.04 | 0.60 ± 0.07 * |
| Mean arterial pressure (mmHg) | 104 ± 13.26 | 118 ± 15.52 | 110 ± 9.67 | 119 ± 14.69 | 110 ± 9.68 | 100 ± 16.65 |
| Biochemical biomarkers | ||||||
| Blood glucose (mmol/L) | 6.17 ± 0.37 | 22.97 ± 5.28 * | 6.48± 0.55 | 30.8 ± 0.78 * | 7.2 ± 0.53 | 28.23 ± 8.84 * |
| P-creatinine (mg/dL) | 2.08 ± 0.59 | 1.33 ± 0.17 * | 2.03 ± 0.26 | 1.54 ± 0.51 | 1.88 ± 0.30 | 2.76 ± 2.09 |
| Urinary creatinine (mg/dL) | 72.92 ± 33.09 | 14.50 ± 6.21 * | 75.60 ± 36.84 | 9.89 ± 2.69 * | 28.66 ± 15.67 | 5.09 ± 2.53 * |
| Urinary albumin (mg/mL) | 0.66 ± 0.67 | 2.41 ± 1.48 * | 1.59 ± 1.21 | 1.29 ± 0.40 | 0.62 ± 0.66 | 4.78 ± 3.27 * |
| Creatinine excretion rate (µg/min) | 4.90 ± 1.51 | 2.55 ± 0.40 | 2.14 ± 0.61 | 3.12 ± 0.78 | 2.76 ± 3.36 | 4.56 ± 6.73 |
| Albumin excretion rate (µg/min) | 5.21 ± 2.28 | 10.29 ± 8.67 | 4.89 ± 3.44 | 41.72 ± 14.48 * | 4.85 ± 5.11 | 148.93 ± 101.67 * |
| Albumin/creatinine ratio (mg/g) | 32,114 ± 15,416 | 38,341 ± 27,739 | 21,729 ± 14,143 | 138,633 ± 52,788 * | 23,497 ± 27,843 | 1,014,106 ± 468,583 * |
| GFR/Creatinine clearance (mL/min) | 0.11 ± 0.10 | 0.19 ± 0.03 | 0.10 ± 0.02 | 0.23 ± 0.11 | 0.14 ± 0.15 | 0.07 ± 0.06 |
| Diuresis (µL/min) | 3.52 ± 3.71 | 20.47 ± 8.92 * | 3.28 ± 1.71 | 32.75 ± 9.65 * | 8.84 ± 7.25 | 36.63 ± 26.72 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Søgaard, S.B.; Andersen, S.B.; Taghavi, I.; Schou, M.; Christoffersen, C.; Jacobsen, J.C.B.; Kjer, H.M.; Gundlach, C.; McDermott, A.; Jensen, J.A.; et al. Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease. Diagnostics 2023, 13, 3197. https://doi.org/10.3390/diagnostics13203197
Søgaard SB, Andersen SB, Taghavi I, Schou M, Christoffersen C, Jacobsen JCB, Kjer HM, Gundlach C, McDermott A, Jensen JA, et al. Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease. Diagnostics. 2023; 13(20):3197. https://doi.org/10.3390/diagnostics13203197
Chicago/Turabian StyleSøgaard, Stinne Byrholdt, Sofie Bech Andersen, Iman Taghavi, Mikkel Schou, Christina Christoffersen, Jens Christian Brings Jacobsen, Hans Martin Kjer, Carsten Gundlach, Amy McDermott, Jørgen Arendt Jensen, and et al. 2023. "Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease" Diagnostics 13, no. 20: 3197. https://doi.org/10.3390/diagnostics13203197





