Clinical and Radiological Parameters to Discriminate Tuberculous Peritonitis and Peritoneal Carcinomatosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Work Up and Follow-Up
2.3. Definitions
2.4. CT Techniques and Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Clinical Differences
3.3. Radiological Differences
3.4. Peritoneal Involvement
3.5. Predictors of Peritoneal Carcinomatosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The history of tuberculosis: From the first historical records to the isolation of Koch’s bacillus. J. Prev. Med. Hyg. 2017, 58, E9–E12. [Google Scholar] [PubMed]
- Houben, R.M.G.J.; Esmail, H.; Cobelens, F.; Williams, C.M.L.; Coussens, A.K. Tuberculosis prevalence: Beyond the tip of the iceberg. Lancet Respir. Med. 2022, 10, 537–539. [Google Scholar] [CrossRef]
- Horvath, K.D.; Whelan, R.L. Intestinal tuberculosis: Return of an old disease. Am. J. Gastroenterol. 1998, 93, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Mak, J.W.; Maung, M.; Wong, S.F.; Kassim, A.I. Meta-analysis: The association between HIV infection and extrapulmonary tuberculosis. Lung 2013, 191, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Zanbagi, A.B.; Shariff, M.K. Gastrointestinal tuberculosis: A systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J. Gastroenterol. 2021, 27, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Sinan, T.; Sheikh, M.; Ramadan, S.; Sahwney, S.; Behbehani, A. CT features in abdominal tuberculosis: 20 years experience. BMC Med. Imaging. 2002, 2, 3. [Google Scholar] [CrossRef]
- Ahamed, Z.R.; Shah, J.; Agarwala, R.; Kumar-M, P.; Mandavdharem, H.S.; Gupta, P.; Singh, H.; Sharma, A.; Dutta, U.; Sharma, V. Controversies in classification of peritoneal tuberculosis and a proposal for clinico-radiological classification. Expert Rev. Anti Infect. Ther. 2019, 17, 547–555. [Google Scholar] [CrossRef]
- Jha, D.K.; Pathiyil, M.M.; Sharma, V. Evidence-based approach to diagnosis and management of abdominal tuberculosis. Indian J. Gastroenterol. 2023, 42, 17–31. [Google Scholar] [CrossRef]
- Sharma, V.; Soni, H.; Kumar-M, P.; Dawra, S.; Mishra, S.; Mandavdhare, H.S.; Singh, H.; Dutta, U. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: A systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2021, 19, 253–265. [Google Scholar] [CrossRef]
- Cortés-Guiral, D.; Hübner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef]
- Bakrin, N.; Bereder, J.M.; Decullier, E.; Classe, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Uzunkoy, A.; Harma, M.; Harma, M. Diagnosis of abdominal tuberculosis: Experience from 11 cases and review of the literature. World J. Gastroenterol. 2004, 10, 3647–3649. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Jha, D.K.; Sharma, V. Discriminating Tuberculous Peritonitis and Peritoneal Carcinomatosis. In Tuberculosis of the Gastrointestinal System; Sharma, V., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Ha, H.K.; Jung, J.I.; Choi, B.G.; Lee, M.G.; Kim, Y.H.; Kim, P.N.; Auh, Y.H.; Ha, J.I.J.H.K.; Walkey, M.; Friedman, A.; et al. CT differentiation of tuberculous peritonitis and peritoneal carcinomatosis. AJR Am. J. Roentgenol. 1996, 167, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.H.; Khan, M.S.; Sajan, A.; Williams, C.E.; Amodu, L.; Hakmi, H.; Hadi, Y.B.; Ismail, S.; Sohail, S.; Ahmad, M.N. Diagnostic accuracy of computed tomography in differentiating peritoneal tuberculosis from peritoneal carcinomatosis. Clin. Imaging 2022, 82, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.V.; Venu, V. Differentiation of peritoneal tuberculosis from peritoneal carcinomatosis by the Omental Rim sign. A new sign on contrast enhanced multidetector computed tomography. Eur. J. Radiol. 2019, 113, 124–134. [Google Scholar] [CrossRef]
- Chandekar, K.R.; Prashanth, A.; Vinjamuri, S.; Kumar, R. FAPI PET/CT Imaging-An Updated Review. Diagnostics 2023, 13, 2018. [Google Scholar] [CrossRef]
- Wang, S.-B.; Ji, Y.-H.; Wu, H.-B.; Wang, Q.-S.; Zhou, W.-L.; Lv, L.; Shou, T.; Hu, J. PET/CT for differentiating between tuberculous peritonitis and peritoneal carcinomatosis: The parietal peritoneum. Medicine 2017, 96, e5867. [Google Scholar] [CrossRef]
- Houshmand, S.; Salavati, A.; Segtnan, E.A.; Grupe, P.; Høilund-Carlsen, P.F.; Alavi, A. Dual-time-point Imaging and Delayed-time-point Fluorodeoxyglucose-PET/Computed Tomography Imaging in Various Clinical Settings. PET Clin. 2016, 11, 65–84. [Google Scholar] [CrossRef]
- Alçın, G.; Tatar, G.; Şahin, R.; Baloğlu, M.C.; Çermik, T.F. Peritoneal Tuberculosis Mimicking Peritoneal Carcinomatosis on 68 Ga-FAPI-04 and 18 F-FDG PET/CT. Clin. Nucl. Med. 2022, 47, e557–e558. [Google Scholar] [CrossRef]
- Sharma, V.; Jha, D.K.; Rohilla, M.; Das, C.K.; Singh, H.; Irrinki, S.; Arora, A.; Saha, S.C.; Gupta, P.; Mandavdhare, H.S.; et al. ‘Rollover’ abdominal paracentesis versus standard technique: Protocol of a crossover randomized comparative trial. Future Oncol. 2021, 17, 3425–3431. [Google Scholar] [CrossRef]
- Jha, D.K.; Rohilla, M.; Das, C.K.; Irrinki, S.; Singh, H.; Arora, A.; Saha, S.C.; Gupta, P.; Mandavdhare, H.S.; Dutta, U.; et al. Randomized crossover trial of ‘Roll-over’ technique of abdominal paracentesis versus standard technique in suspected malignant ascites. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qi, X.; Guo, X. Quantification of ascites based on abdomino-pelvic computed tomography scans for predicting the in-hospital mortality of liver cirrhosis. Exp. Ther. Med. 2017, 14, 5733–5742. [Google Scholar] [CrossRef][Green Version]
- Nougaret, S.; Sadowski, E.; Lakhman, Y.; Rousset, P.; Lahaye, M.; Worley, M.; Sgarbura, O.; Shinagare, A.B. The BUMPy road of peritoneal metastases in ovarian cancer. Diagn. Interv. Imaging 2022, 103, 448–459. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Sathyakumar, K.; Bindra, M.S.; Eapen, A. Is MDCT an accurate tool to differentiate between benign and malignant etiology in diffuse peritoneal disease? Abdom. Radiol. 2022, 47, 3921–3929. [Google Scholar] [CrossRef]
- Johnson, C.C.; Baldessarre, J.; Levison, M.E. Peritonitis: Update on pathophysiology, clinical manifestations, and management. Clin. Infect. Dis. 1997, 24, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Raptopoulos, V.; Gourtsoyiannis, N. Peritoneal carcinomatosis. Eur. Radiol. 2001, 11, 2195–2206. [Google Scholar] [CrossRef]
- Charoensak, A.; Nantavithya, P.; Apisarnthanarak, P. Abdominal CT findings to distinguish between tuberculous peritonitis and peritoneal carcinomatosis. J. Med. Assoc. Thai. 2012, 95, 1449–1456. [Google Scholar]
- Rodríguez, E.; Pombo, F. Peritoneal tuberculosis versus peritoneal carcinomatosis: Distinction based on CT findings. J. Comput. Assist. Tomogr. 1996, 20, 269–272. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, J.W.; Baek, J.H.; Kim, S.H.; Kim, B.G.; Lee, K.L.; Jeong, J.B.; Jung, Y.J.; Kim, J.S.; Jung, H.C.; et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J. Gastroenterol. 2012, 18, 2837–2843. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Tang, Y.; Zhang, X.; Cao, M.; Xiao, Z.; Ren, M.; Chen, T. Diagnostic performance of CT for differentiating peritoneal tuberculosis from peritoneal carcinomatosis: A systematic review and meta-analysis. Clin. Radiol. 2020, 75, 396.e7–396.e14. [Google Scholar] [CrossRef]
- Sharma, M.P.; Bhatia, V. Abdominal tuberculosis. Indian J. Med. Res. 2004, 120, 305–315. [Google Scholar] [PubMed]
- Pang, Y.; Li, Y.; Xu, D.; Sun, X.; Hou, D. Differentiating peritoneal tuberculosis and peritoneal carcinomatosis based on a machine learning model with CT: A multicentre study. Abdom. Radiol. 2023, 48, 1545–1553. [Google Scholar] [CrossRef]
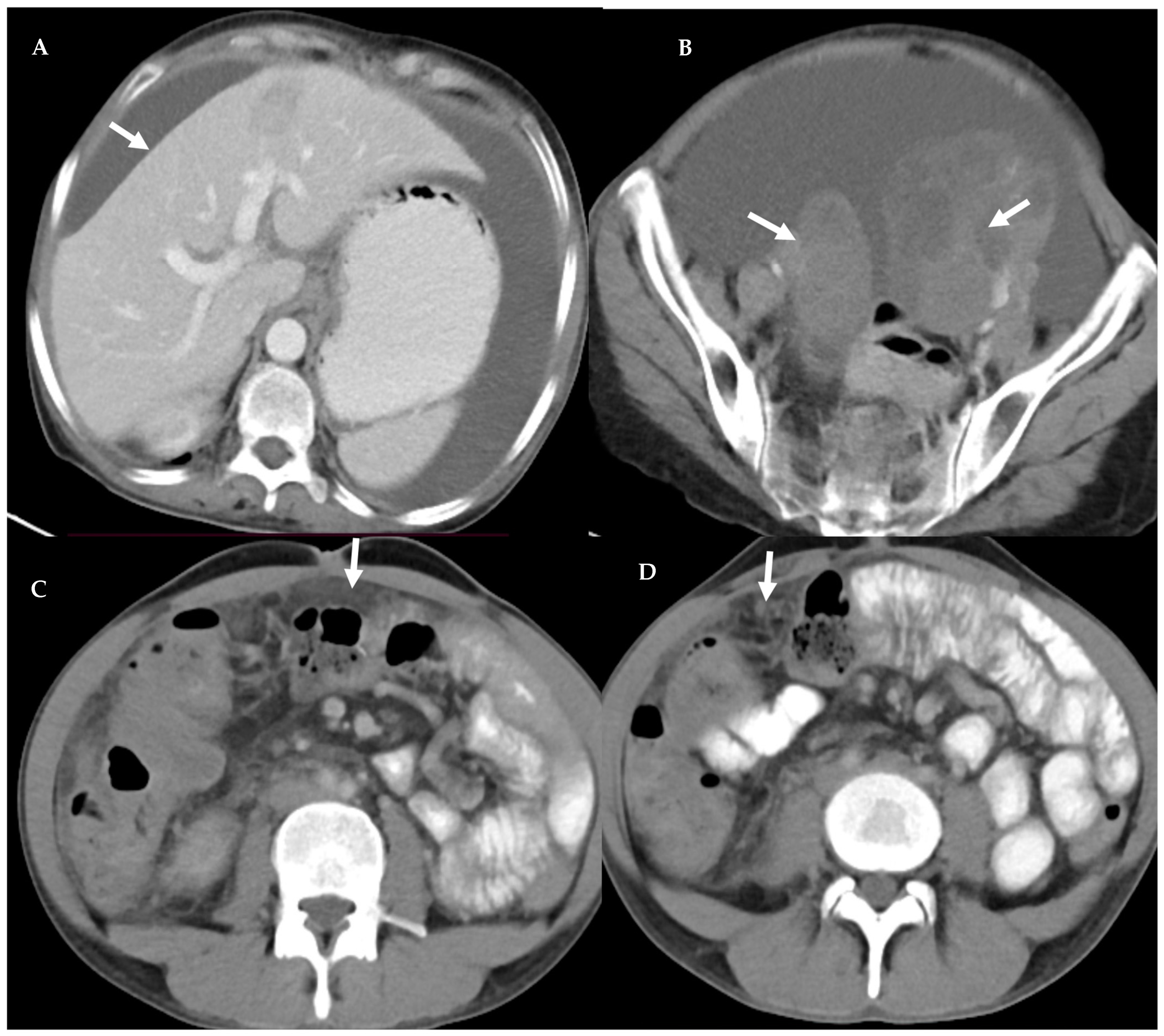



| S. No | Character | Tuberculous Peritonitis (n-44) | Peritoneal Carcinomatosis (n-45) | p Value |
|---|---|---|---|---|
| 1. | Age (yrs) (Median, IQR) | 31.5 (23.5–40) | 52 (46–61) | <0.001 |
| 2. | Male (%) | 19 (43.2%) | 16 (35.5%) | 0.461 |
| 3. | Distension | 33 (75%) | 40 (88.8%) | 0.088 |
| 4. | Pain in abdomen | 24 (54.5%) | 34 (75.5%) | 0.038 |
| 5. | History of intestinal obstruction | 8 (18.2%) | 3 (6.6%) | 0.099 |
| 6. | Fever | 32 (72.7%) | 5 (11.1%) | <0.001 |
| 7. | Lump in abdomen | 8 (18.2%) | 10 (22.3%) | 0.635 |
| 8. | Loss of weight | 41 (93.2%) | 29 (64.4%) | 0.001 |
| 9. | Past history of TB | 4 (9.1%) | 0 | 0.038 |
| 10. | History of malignancy | 0 | 17 (37.7%) | <0.001 |
| S. No | Character | Tuberculous Peritonitis (n-44) | Peritoneal Carcinomatosis (n-45) | p Value | |
|---|---|---|---|---|---|
| 1. | Ascites | 42 (95.4%) | 42 (93.3%) | 0.664 | |
| Quantity | Mild to moderate | 20(47.6%) | 18(42.8%) | 0.661 | |
| Severe | 22(52.3%) | 24(57.14%) | 0.661 | ||
| 2. | Loculated ascites | 27 (64.3%) | 9 (21.4%) | <0.001 | |
| 3. | Low ascitic attenuation | 23 (54.7%) | 27 (64.2%) | 0.373 | |
| 4. | Lymphadenopathy | 13 (29.5%) | 22 (48.8%) | 0.121 | |
| Mean size in cm Lymph node necrosis | 1.37 | 1.32 | - | ||
| 6 (46.1%) | 8 (36.3%) | 0.592 | |||
| Conglomeration | 4 (30.7%) | 0 | 0.038 | ||
| Location | Periportal | 3 (23.1%) | 10 (45.4%) | 0.220 | |
| Mesenteric | 9 (69.2%) | 4 (18.2%) | 0.001 | ||
| Retroperitoneal | 0 | 3 (13.5%) | 0.173 | ||
| Para aortic | 1 (7.6%) | 3 (13.5%) | 0.623 | ||
| Peri gastric | 0 | 2 (9.1%) | 0.273 | ||
| Peri pancreatic | 0 | 1 (4.5%) | 0.445 | ||
| 5. | Bowel involvement | 17 (38.6%) | 11 (24.4%) | 0.149 | |
| Bowel characters | Clumping | 12 (70.5%) | 6 (54.5%) | 0.102 | |
| Membrane | 10 (58.8%) | 0 | 0.001 | ||
| Dilation | 10 (58.8%) | 0 | 0.001 | ||
| 6. | Liver involvement | 13 (29.5%) | 26 (57.8%) | 0.007 | |
| Reduced attenuation | 5 (11.4%) | 17 (37.7%) | 0.004 | ||
| Scalloping | 11 (25%) | 17 (37.8%) | 0.194 | ||
| Focal | 11 (25%) | 23 (51.1%) | 0.011 | ||
| 7. | Splenomegaly | 6 (13.6%) | 0 | 0.010 | |
| Spleen | SOL | 4 (9.1%) | 1 (2.2%) | 0.159 | |
| Scalloping | 2 (4.5%) | 4 (8.8%) | 0.414 | ||
| 8. | Pleural effusion | 23 (52.3%) | 21 (46.7%) | 0.597 | |
| 9. | Adnexal involvement | 14 (56%) | 11 (37.9%) | 0.184 | |
| S. No | Character | Tuberculous Peritonitis (TBP) (n-44) | Peritoneal Carcinomatosis (PC) (n-45) | p Value | |
|---|---|---|---|---|---|
| 1. | Mesentery | Changes | 35 (79.5%) | 29 (64.4%) | 0.113 |
| Stranding | 32 (91.4%) | 27 (93.1%) | 0.095 | ||
| Nodularity | 10 (28.6%) | 7 (24.2%) | 0.390 | ||
| 2. | Peritoneal involvement | 32 (72.7%) | 33 (73.3%) | 0.949 | |
| Peritoneal enhancement | 32 (100%) | 33 (100%) | 0.949 | ||
| Peritoneal thickening type | Symmetric | 23 (71.9%) | 20 (60.6%) | ||
| Asymmetric | 9 (28.1%) | 13 (39.4%) | 0.337 | ||
| 3. | Omental involvement | 32 (72.7%) | 28 (62.2%) | 0.4 | |
| Omental pattern | Nodular | 0 | 4 (14.3%) | 0.0269 | |
| Caking | 10 (31.3%) | 8 (28.6%) | 0.399 | ||
| Smudged | 22 (68.7%) | 16 (57.1%) | 0.352 | ||
| S. No | Radiological Findings | TBP n-44 | PC n-45 | TP | FP | FN | TN | Sn (95% CI) | Sp (95% CI) | PPV (%) | NPV (%) | Diagnostic Accuracy (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favoring TBP | 1. | Loculated ascites | 27 | 9 | 27 | 9 | 17 | 36 | 61 (45.50–75.64) | 80 (65.40–90.42) | 75 | 68 | 71 |
| 2. | LN conglomeration | 4 | 0 | 4 | 0 | 40 | 45 | 9 (2.53–21.67) | 100 (92.13–100.0) | 100 | 53 | 55 | |
| 3. | Bowel membrane | 10 | 0 | 10 | 0 | 34 | 45 | 23 (11.47–37.84) | 100 (92.13–100.0) | 100 | 57 | 62 | |
| 4. | Bowel dilatation | 10 | 0 | 10 | 0 | 34 | 45 | 23 (11.47–37.84) | 100 (92.13–100.0) | 100 | 57 | 62 | |
| 5. | Splenomegaly | 6 | 0 | 6 | 0 | 38 | 45 | 14 (5.17–27.35) | 100 (92.13–100.0) | 100 | 54 | 57 | |
| 6. | Mesenteric LN | 9 | 4 | 9 | 4 | 35 | 41 | 20 (9.80–35.30) | 91 (78.78–97.52) | 69 | 54 | 56 | |
| Favoring PC | 7. | Focal liver lesions | 11 | 23 | 23 | 11 | 22 | 33 | 51 (35.77–66.30) | 75 (59.66–86.81) | 68 | 60 | 63 |
| 8. | Reduced liver attenuation | 5 | 17 | 17 | 05 | 28 | 39 | 38 (23.77–53.46) | 89 (75.44–96.21) | 77 | 58 | 63 | |
| 9. | Omental nodularity | 0 | 6 | 6 | 0 | 39 | 44 | 13 (5.05–26.79) | 100 (91.96–100.0) | 100 | 53 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, D.K.; Gupta, P.; Neelam, P.B.; Kumar, R.; Krishnaraju, V.S.; Rohilla, M.; Prasad, A.S.; Dutta, U.; Sharma, V. Clinical and Radiological Parameters to Discriminate Tuberculous Peritonitis and Peritoneal Carcinomatosis. Diagnostics 2023, 13, 3206. https://doi.org/10.3390/diagnostics13203206
Jha DK, Gupta P, Neelam PB, Kumar R, Krishnaraju VS, Rohilla M, Prasad AS, Dutta U, Sharma V. Clinical and Radiological Parameters to Discriminate Tuberculous Peritonitis and Peritoneal Carcinomatosis. Diagnostics. 2023; 13(20):3206. https://doi.org/10.3390/diagnostics13203206
Chicago/Turabian StyleJha, Daya K., Pankaj Gupta, Pardhu B. Neelam, Rajender Kumar, Venkata S. Krishnaraju, Manish Rohilla, Ajay S. Prasad, Usha Dutta, and Vishal Sharma. 2023. "Clinical and Radiological Parameters to Discriminate Tuberculous Peritonitis and Peritoneal Carcinomatosis" Diagnostics 13, no. 20: 3206. https://doi.org/10.3390/diagnostics13203206
APA StyleJha, D. K., Gupta, P., Neelam, P. B., Kumar, R., Krishnaraju, V. S., Rohilla, M., Prasad, A. S., Dutta, U., & Sharma, V. (2023). Clinical and Radiological Parameters to Discriminate Tuberculous Peritonitis and Peritoneal Carcinomatosis. Diagnostics, 13(20), 3206. https://doi.org/10.3390/diagnostics13203206






