Abstract
Sentinel lymph node biopsy (SLNB) has been widely adopted in the management of early-stage gynaecological cancers such as endometrial, vulvar and cervical cancer. Comprehensive surgical staging is crucial for patients with early-stage ovarian cancer and currently, that includes bilateral pelvic and para-aortic lymph node assessment. SLNB allows the identification, excision and pathological assessment of the first draining lymph nodes, thus negating the need for a full lymphadenectomy. We systematically searched the MEDLINE, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) databases (from inception to 3 November 2022) in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Our search identified 153 articles from which 11 were eligible for inclusion. Patients with clinical stage I–II ovarian cancer undergoing sentinel lymph node biopsy were included. Statistical analysis was performed in RStudio using the meta package, where meta-analysis was performed for the detection. The risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies C (QUADAS-C) tool. Overall, 11 observational studies met the predetermined criteria and these included 194 women. The meta-analysis showed that the detection rate of sentinel lymph nodes in early-stage ovarian cancer was 94% (95% CI of 86% to 1.00%). Significant heterogeneity was noted among the studies with Q = 47.6, p < 0.0001, I2 = 79% and τ2 = 0.02. Sentinel lymph nodes in early-stage ovarian cancer have a high detection rate and can potentially have applicability in clinical practice. However, considering the small number of participants in the studies, the heterogeneity among them and the low quality of evidence, the results should be interpreted with caution. Larger trials are needed before a change in clinical practice is recommended.
1. Introduction
Ovarian cancer is the eighth most common cancer among women and the leading cause of death amongst gynaecological malignancies [1]. Around 17–24.3% of patients present with International Federation of Gynecology and Obstetrics (FIGO) stage 1 (disease confined to the ovary) and just 5% with FIGO stage 2 ovarian cancer (disease confined to pelvic organs but not involving lymph nodes) [2,3]. For patients with apparent early-stage disease on pre-operative imaging, complete surgical staging including full bilateral pelvic and para-aortic lymphadenectomy (except in stage 1 expansile mucinous adenocarcinomas) continues to be the primary management. Lymph node staging is not only important for prognostic information, but also for decisions regarding adjuvant therapeutic options in those who are upstaged [4].
The risk of microscopic pelvic or para-aortic lymph node metastases in apparent FIGO stages I–II disease is reported to be 14% (in the range 6.1–29.6%) [5,6]. Systematic lymphadenectomy is a complex procedure requiring advanced surgical skills. It is also associated with increased intra-operative morbidity (increased surgical time, blood loss and need for blood transfusion) and post-operative morbidity, including a 7-fold higher risk of lymphoedema and lymphocyst formation, vessel or nerve injury and reduced quality of life [7,8,9]. In advanced-stage ovarian cancer, systematic lymphadenectomy with clinically negative lymph nodes has not been shown to improve overall or progression-free survival [9]. Similarly, in early-stage ovarian cancer, systematic lymphadenectomy is only of diagnostic benefit and does not provide any survival benefit [10].
Sentinel lymph node biopsy (SLNB) is commonly used in gynaecological cancers such as breast and vulval cancer and has started to become an established approach in early-stage cervical and endometrial cancers [11,12,13,14,15,16,17]. It allows the first draining node from a cancer to be identified and undergo ultrastaging histological examination for metastatic cancer cells. Thus, SLNB aims to provide the same diagnostic information that a full lymphadenectomy would provide without the associated intra-operative risks and morbidity for a procedure that does not incur survival benefit. In parallel to developing SLNB procedures in gynaecological cancers, there has been a trend for minimally invasive approaches to managing early-stage ovarian cancer, in particular the use of laparoscopic or robotic staging in disease confined to the ovary [18,19].
Several observational studies have examined the hypothesis that SLNB could potentially provide the diagnostic and prognostic accuracy that systematic lymphadenectomy does, without a significant increase in the morbidity or surgical time. This systematic review and meta-analysis aim to evaluate the current evidence available for the role of SLNB in early-stage ovarian cancer.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
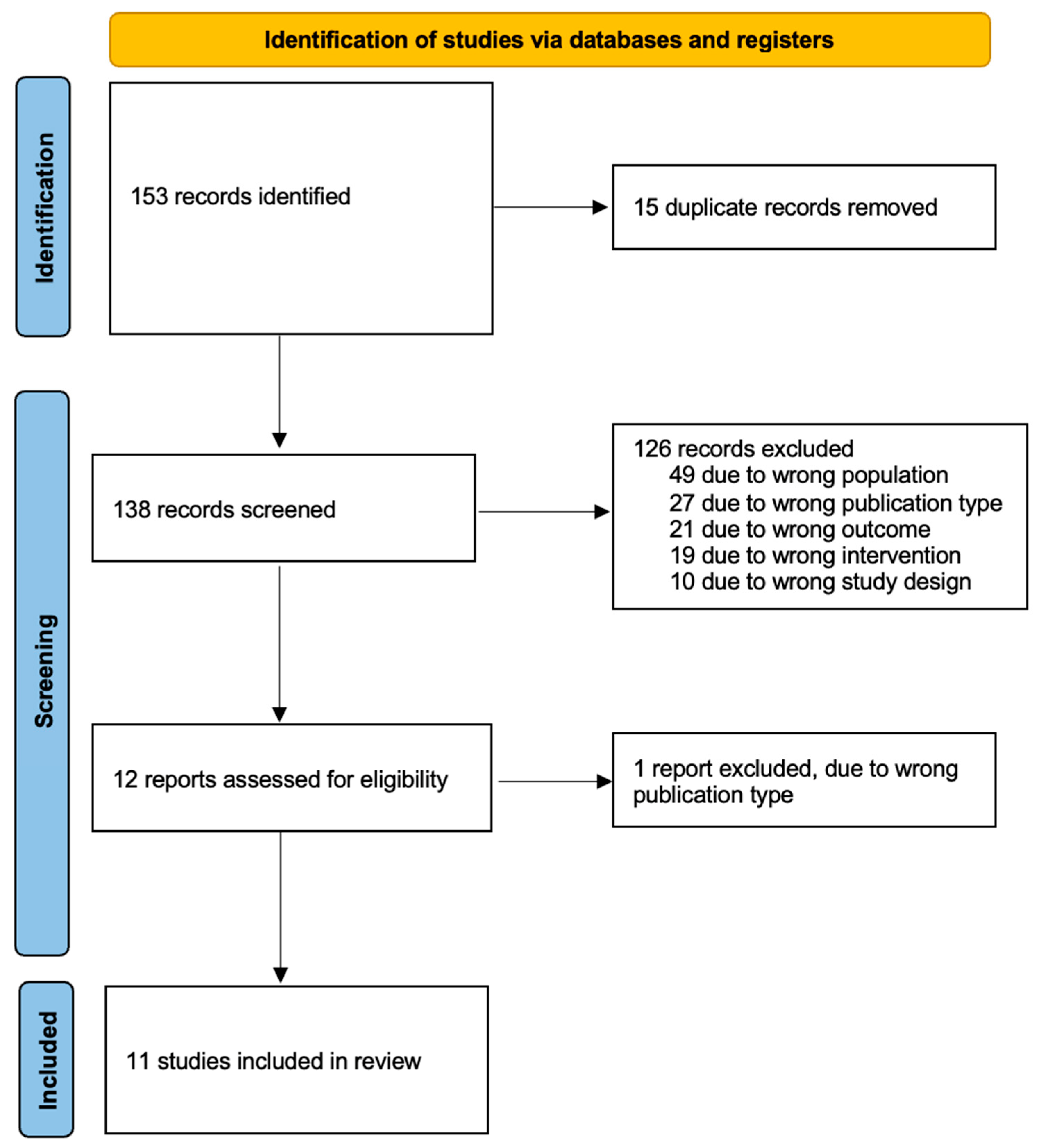
This systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Figure 1) [20]. A systematic literature search was performed for relevant studies from inception to 3 November 2022 across the following databases: Embase (OVID), MEDLINE (OVID), Google Scholar, ClinicalTrials.gov and the Cochrane Central Register of Controlled Trials (CENTRAL). Review articles were also hand searched for relevant studies. The following search terms were used in OVID: (“ovarian neoplasms” (MeSH terms) OR “ovarian neoplasms” (MeSH terms)) AND (“radioactive tracers” (MeSH terms) OR “coloring agents” (MeSH terms) OR “indocyanine green” (MeSH terms) OR “technetium” (MeSH terms) OR “spectroscopy, near infrared” (MeSH terms) OR “methylene blue” (MeSH terms) OR “fluorescent antibody technique” (MeSH terms)) AND (“sentinel lymph node biopsy” (MeSH terms) OR “lymph nodes” (MeSH terms) OR “lymph node excision” (MeSH terms)). The same search strategy was adapted to Google Scholar.
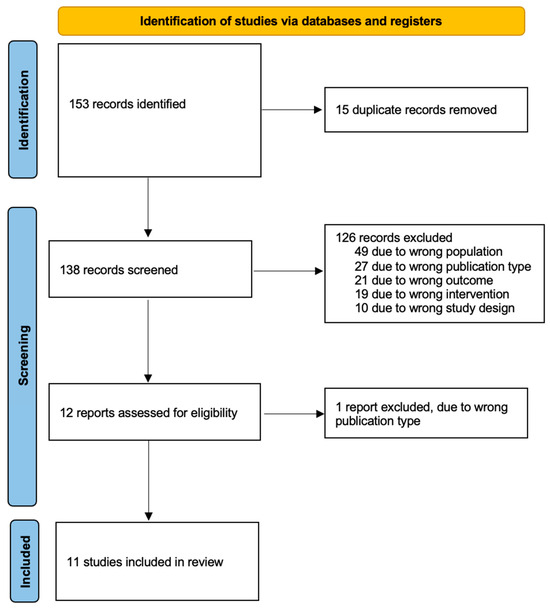
Figure 1.
PRISMA flow diagram.
2.2. Inclusion and Exclusion Criteria
Randomised controlled trials (RCTs), cohorts and case–control studies looking at SLNB in early-stage epithelial ovarian cancer were included. Studies included patients with early-stage epithelial ovarian cancer who had undergone SLNB detection (index test). For diagnostic accuracy, the studies had to have proceeded with full pelvic and para-aortic lymphadenectomy (reference standard). The study design could have been prospective or retrospective. As we expected to find studies with small numbers of participants, all the relevant studies irrespective of the numbers of participants were included.
2.3. Data Extraction
Titles and abstracts retrieved by electronic searching were exported to the systematic review management tool, Rayyan (Rayyan.ai), and duplicates were removed using the “find duplicates” software tool and by manual checking [21]. Two review authors (G.Z. and G.Y.) independently assessed the eligibility of the papers. Studies that clearly did not meet the inclusion criteria were excluded, 49 of them due to the wrong population, 27 due to the wrong publication type, 21 due to the wrong outcome, 19 due to the wrong drug and 10 due to their study design. Copies of the full text of potentially relevant references were obtained. After the full-text screening, we excluded 1 study due to the wrong publication type. Any disagreements between authors were resolved by discussion or by the involvement of a third review author (D.C.).
The following data were collected for each study: first investigator’s name, publication year, country, sample size, age, body mass index, menopausal status, type of surgery, surgical approach, FIGO stage, histological type, SLNB technique, location of injection, type of tracer, number of LNs, location of LN, detection rate and intra-operative complications.
2.4. Outcome
The primary outcome of interest was the detection rate in order to investigate the feasibility of SLNB. The secondary reported outcomes were the intra-operative complications and the diagnostic accuracy.
2.5. Assessment of Risk of Bias in Included Studies
The quality of the studies was assessed with the “Quality Assessment of Diagnostic Accuracy Studies” (QUADAS-C) tool [22]. This tool assesses four domains: patient selection, index test, reference standard, and flow and timing. The risk of bias was judged as “low”, “high” or “unclear” in each domain.
2.6. Statistical Analysis
The Hartung, Knapp, Sidik and Jonkman (HKSJ) approach was used to calculate the detection rates (proportion) and their 95% confidence intervals (CI) instead of the DerSimonian and Laird (DL) method for random effects meta-analysis using the metafor package in RStudio [23]. The HKSJ method seems to outperform the DL method, resulting more adequate error rates [24]. The statistical heterogeneity was assessed using the I2 statistics and Cochrane Q tests. Subgroup analyses were not performed in view of the limited number of cases in each study and the underreporting of demographic parameters. The possibility of publication bias was assessed visually by funnel plot and formally by the Egger’s regression test for funnel plot asymmetry using RStudio [25].
3. Results
3.1. Study Selection
Our initial search yielded 153 relevant studies. After duplicates were removed, a total of 138 studies remained. A total of 12 of them were deemed eligible for full-text screening from which 11 studies met our inclusion criteria and were included in our review [26,27,28,29,30,31,32,33,34,35,36] (Figure 1). All of them were prospective studies; no randomised control trial was identified. Of the included studies, three of them were conducted in Spain [27,28,30], three in Italy [29,32,33], two in the Netherlands [26,35], one in Finland [31], one in Iran [34] and one in Japan [36].
3.2. Quality Assessment
The quality of the studies was assessed with the QUADAS-C tool, although due to the aforementioned methodological heterogeneity, this should be interpreted with caution (Table 1). It was unclear in all studies if consecutive or random sampling of participants was enrolled. Regarding the index and the reference test, none of the studies were designed so that the index test results were interpreted without knowledge of the result of the reference standard, or the reference standard results interpreted without knowledge of the results of the index tests. Different surgical approaches, laparotomy, laparoscopy or robotic surgery, were adopted among and within the studies. It is unclear how this may have affected the detection of SLN (the index test) or the ability to perform full lymphadenectomy (the reference standard). These lead to unclear risk of bias for all studies about the conduct and interpretation of the index test and reference standard. There were no concerns regarding the time interval between the index and the reference standard. Most studies used full lymphadenectomy as the reference standard, except for Kleppe et al.’s, which could have introduced bias in the flow and timing domain [35]. There were no concerns regarding the applicability.

Table 1.
QUADAS-C Bias tool.
Significant heterogeneity was noted in the demographic and methodological characteristics of the included studies (Table 2 and Table 3). Specifically, three studies used indocyanine green (ICG) and radioisotope [27,28,30], three studies used blue dye and radioisotope [26,31,35], three studies used ICG alone [29,32,33], one used radioisotope alone [34] and one used charcoal solution [36]. Also, the surgical approach to identifying sentinel lymph nodes (SLNs) was different with four studies performing laparotomy only [31,34,35,36], two studies performing laparoscopy only [32,33], two studies using a combination of laparoscopy and laparotomy [28,30] and one study using the robotic system as well [29]. For two studies, the surgical route was not available [26,27]. Patient characteristics are summarised in Table 2. There were significant differences among studies in terms of median age, body mass index (BMI), immediate or delayed surgical staging, FIGO staging and histological type.

Table 2.
Demographic characteristics of the included studies.

Table 3.
Technical study characteristics and outcomes.
3.3. Sentinel Detection
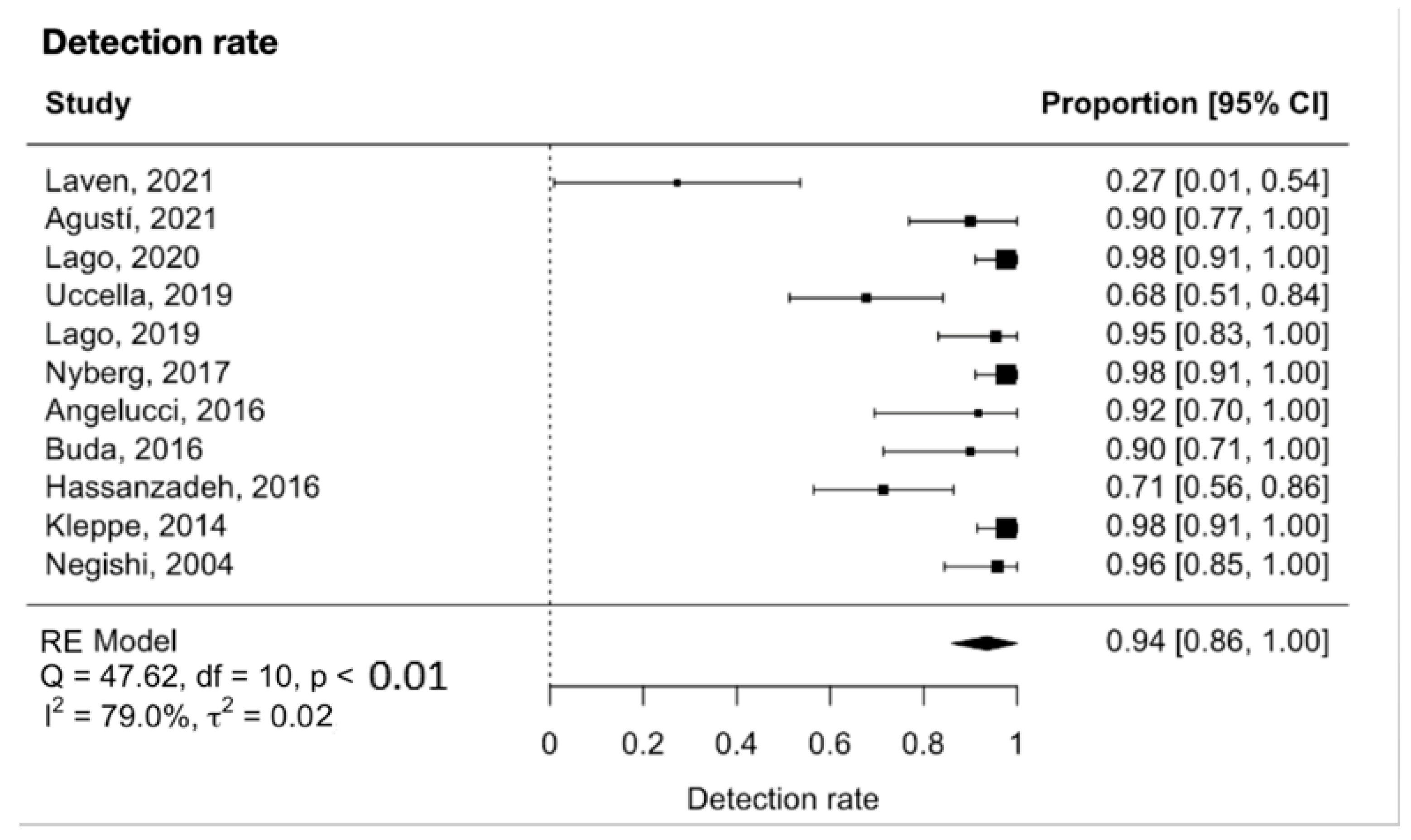
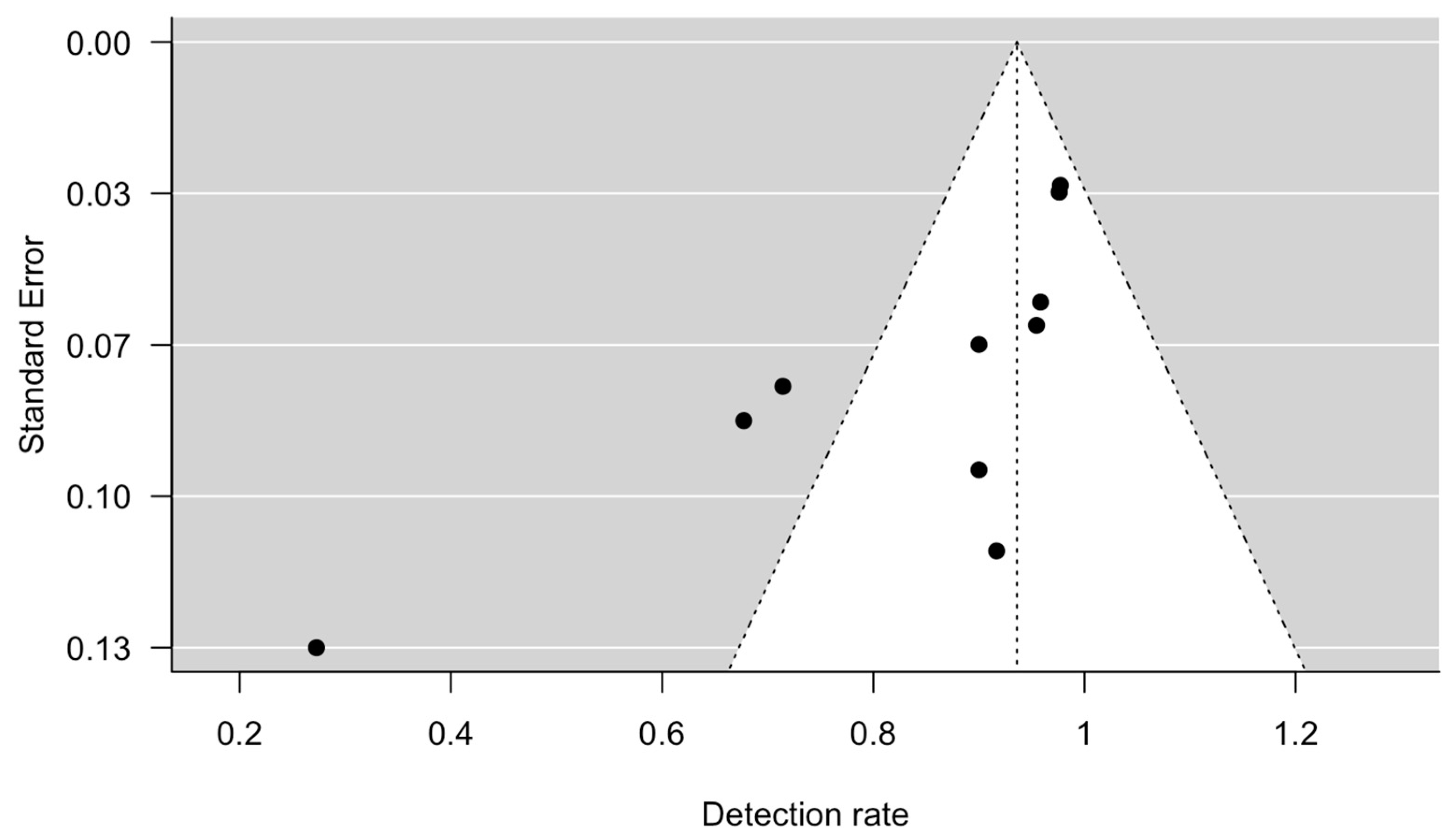
The meta-analysis showed that the detection rate of SLN in early-stage ovarian cancer was 0.94 (95% CI 0.86 to 1.00, Figure 2). Significant heterogeneity was noted with Q = 47.6, p < 0.0001, I2 = 79% and τ2 = 0.02. A funnel plot was used to visually assess for potential publication bias (Figure 3).
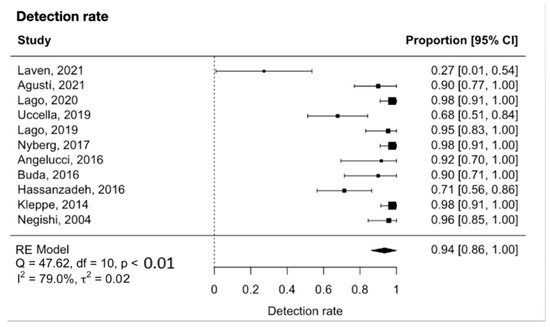
Figure 2.
Forest plot for the detection rate [26,27,28,29,30,31,32,33,34,35,36].
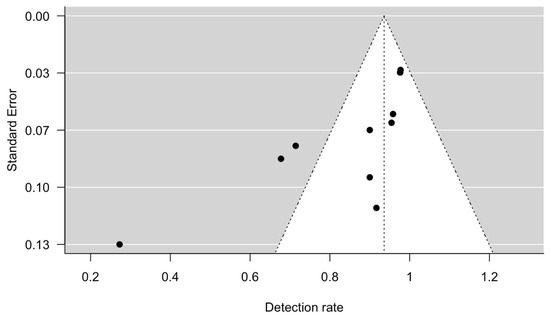
Figure 3.
Funnel plot, using detection rate and standard error.
4. Discussion
Full pelvic and para-aortic lymphadenectomy is currently recommended for complete staging of early ovarian cancer as radiological methods of detecting lymph nodes are inadequate [4]. Sensitivity of radiological detection of lymph node metastasises ranges from 73.2% in positron emission tomography (PET), to 42.6% for computed tomography (CT) and 54.7% in magnetic resonance imaging (MRI) [37]. However, complete lymphadenectomy is associated with increased intra- and post-operative complications [7,8]. SLNB, if proven to be oncologically non-inferior, could have a significant impact in patient morbidity and quality of life.
In our meta-analysis, the pooled detection rate of SLNB in early-stage ovarian cancer was 94% (95% CI 86% to 100%, I2 = 79%, p < 0.0001). This is in agreement with a recent meta-analysis on lymph node detection in early-stage ovarian cancer, which included nine studies and reported a detection rate of 93.3% per patient (95% CI 77.8% to 100%; I2 = 74.3%, p < 0.0001) [38]. This is also comparable to the detection rates in other more well-established malignancies such as endometrial cancer, with a detection rate of 87%; breast cancer, with a detection rate of 97.9% with ICG; vulvar cancer, with a detection rate of 98% with blue dye and 99mTc radioisotope; and cervical cancer, with a detection rate of 98% with ICG [12,13,16,39]. It is likely that the SLNB detection rate in ovarian cancer will improve further with increased experience and refinement of technique.
4.1. Tracers
All the studies used one of or a combination of blue dyes, ICG or 99mTc radioisotope, except for Negishi et al.’s who used carbon nanoparticles (CH40) [36]. These tracers, apart from CH40, are commonly used in other gynaecological malignancies and are those generally recommended in national and international guidelines [40].
Blue dye, of which methylene blue is the most commonly used, has an affinity to nucleic acids and is the cheapest tracer as it does not require any additional equipment. Its visibility is not as robust as other tracers because it is not radioactive or penetrating and can therefore be easily obscured by overlying tissue [41]. Blue dye can be detected in SLNs within 15–20 min after its injection and tends to dissipate after 50 min. It has a higher rate of allergic reaction of 2% vs. 0.05% for ICG [42].
ICG is a water-soluble tricarboxycyanine which binds to albumin and fluoresces under near-infrared (NIR) light. It is highly effective, quickly absorbed and has a low toxicity. It can penetrate into tissues by 5–8 mm, allowing it to have better visibility [43]. It does, however, require extra equipment in the form of a near-infrared light source at 800 nm [41]. ICG is generally considered a more superior dye in comparison to blue dye and is the European Society of Gynaecological Oncology (ESGO)’s tracer of choice for endometrial SLNB [40]. However, it should be avoided in those with an iodine allergy or significant hepatic impairment.
99mTC binds to mannose receptors in reticuloendothelial cells found in lymph nodes. A handheld radio detector is required to locate sentinel lymph nodes intra-operatively. It has a short, six-hour half-life and a photopeak of gamma ray emission of just 140.5 keV, minimising its toxicity to patients [44]. It is also the preferred tracer for SLNB in vulvar cancer in combination with blue dye or ICG [45].
CH40 has been more commonly used in breast and gastric carcinomas. CH40 is injected as a suspension with a suspending agent and saline. Due to their size, the nanoparticles cannot enter capillaries, but instead entre the lymphatics and undergo phagocytosis by macrophages [46]. They stain the lymphatics black and have a half-life of 3 days, and no toxic side effects have been reported in the literature.
New tracers are being developed including “magnetic dyes” such as iron oxide nanoparticles, biodegradable tracers and tumour-targeted tracers. The latter are the most interesting development, as they aim to identify pathological lymph nodes without surgical removal and histopathological assessment. One such example is panitumubab-IRDye900CW. Analysis of its five most highly fluorescent lymph nodes has demonstrated 100% sensitivity, 100% negative predictive value and 85.8% specificity in identifying tumour metastases [47].
4.2. Tracer Injection Sites
There are three lymphatic drainage pathways described in ovarian cancer [48]. The first and most prominent is via the infundibulopelvic (IP) ligament which drains to the common iliacs bilaterally and then to the aorta on the left and the caval vein on the right. A total of 50% of patients solely drain through this route and a further 30%, in combination with the following route. The second pathway is through the ovarian ligament to the internal iliac artery plexi and the obturator fossa. A total of 20% of patients will solely drain through the ovarian ligament. Lastly, the round ligament, which drains to the inguinal nodes contributes to the rare cases of isolated inguinal metastases. Consequently, the majority of studies chose to inject their tracers into both the IP ligament and ovarian ligament stumps [26,27,28,29,30,33,34,35]. Three studies chose to inject, in some or all of their patients, into the ovarian cortex [31,32,34,36]. This technique has been criticised due to its poor detection rate, as low as 40%; the difficulty in identifying normal cortex to injected; and the risk of tumour puncture and seeding [34].
All of the studies, except two, performed immediate SLNB as opposed to delayed SLNB as part of a two-stage surgery following confirmation of malignancy on the tumour specimen. Uccella et al. found that the SLN detection rate was higher in patients undergoing immediate staging (88.9%) in comparison to those undergoing delayed staging (41.7%) [29]. These results were replicated by Laven et al., who in their small study of 11 patients, concluded that their low detection rate of just 27% was attributable to the fact that their tracers were injected after oophorectomy either at primary or secondary surgery [26]. This could be explained by the fact that lymphatic pathways are occluded and diverted following loss of perfusion of lymph fluid from the original organ. Lago et al., in both of their studies, had a 100% detection rate following injection of tracers after adnexectomy and frozen sectioning, confirming malignancy at primary surgery [28,30]. Thus, this suggests that tracers can be injected either before or after adnexectomy, but are most accurate in the primary surgical setting.
4.3. Ultrastaging
It is recommended that SLN undergo ultrastaging as it improves the detection of metastasis by of lymph node by 37–43% in endometrial cancer and 10–15% in cervical cancer [49]. This is due to the fact that ultrastaging identifies micrometastases and isolated tumour cells that would otherwise have gone undetected by traditional techniques. There are numerous protocols published in the literature, but generally, it involves the SLN being divided into serial sections of 50–250 μM [50]. Each slice is assessed using H&E and immunohistochemistry.
4.4. Strengths and Limitations
Our meta-analysis was conducted according to the PRISMA guidelines. Statistical models (HKSJ) that aim to reduce the methodological heterogeneity among the included studies were used. However, the results should be interpreted with caution due to the observational design of the included studies which affects the strength of our meta-analysis. The low quality of evidence, the small number of included studies, the sample sizes and the heterogeneity among the studies influenced the results of our meta-analysis.
5. Conclusions
In conclusion, SLNB holds a promising prospect for the future surgical management of early-stage ovarian cancer. Although current evidence is heterogeneous, it appears that the detection rate of ovarian SLN is comparable to that in other more established gynaecological SLNBs. However, there is not enough good quality evidence to recommend that this procedure should yet become standard practice. We look forward to receiving the results of ongoing and future trials which will add to the evidence on the sentinel detection rate as well as their diagnostic accuracy.
6. Future Directions
Currently, full pelvic and para-aortic lymphadenectomy is recommended for complete staging of early-stage ovarian cancer [4]. SLNB has emerged as a standard approach for staging in endometrial, cervical and vulvar cancers. Given its success in these gynaecological malignancies, there is a growing interest in investigating the feasibility and oncological accuracy of SLNB in early-stage ovarian cancer.
This systematic review and meta-analysis have evaluated the SLNB detection rate, which is one aspect that would define the success of LN mapping in early-stage ovarian cancer. Although the data are limited, the detection rate appears to be comparable to those in other gynaecological cancers in which SLNB is more established. As the studies included in this analysis have small population sizes, it was difficult to assess the sensitivity and false-negative rate of detecting lymph node metastasis, which would be crucial information prior to implementing SLNB as standard practice in early-stage ovarian cancer. Additionally, more information with regards to tracer type, injection site and mapping techniques will be welcomed.
Apropos the limited quality of evidence, larger prospective studies are needed to assess these parameters. Ongoing trials will provide vital information before the routine implementation of SLNB in early-stage ovarian cancer (NCT05184140, NCT03563781, NCT05937620, NCT05375526, NCT04051502, NCT04714931 and NCT05927818) (Table 4) [51,52,53,54,55,56,57].

Table 4.
Ongoing trials in sentinel node mapping in early-stage ovarian cancer.
Author Contributions
D.C. conceptualized the project, G.Z. and G.Y. reviewed the literature, extracted the data and wrote the article, G.Z. performed the statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics; Surveillance Research Program; National Cancer Institute. SEER Incidence Data, November 2022 Submission (1975–2020). SEER 22 Registries. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 19 April 2023).
- NHS England. Case-Mix Adjusted Percentage of Cancers Diagnosed at Stages 1 and 2 in England. 2020. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/case-mix-adjusted-percentage-of-cancers-diagnosed-at-stages-1-and-2-in-england/2020 (accessed on 15 December 2022).
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Powless, C.A.; Aletti, G.D.; Bakkum-Gamez, J.N.; Cliby, W.A. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: Implications for surgical staging. Gynecol. Oncol. 2011, 122, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, M.; Wang, T.; Van Gorp, T.; Slangen, B.; Kruse, A.; Kruitwagen, R. Lymph node metastasis in stages I and II ovarian cancer: A review. Gynecol. Oncol. 2011, 123, 610–614. [Google Scholar] [CrossRef]
- Bizzarri, N.; du Bois, A.; Fruscio, R.; De Felice, F.; De Iaco, P.; Casarin, J.; Vizza, E.; Chiantera, V.; Corrado, G.; Cianci, S.; et al. Is there any therapeutic role of pelvic and para-aortic lymphadenectomy in apparent early stage epithelial ovarian cancer? Gynecol. Oncol. 2021, 160, 56–63. [Google Scholar] [CrossRef]
- Maggioni, A.; Panici, P.B.; Dell’Anna, T.; Landoni, F.; Lissoni, A.; Pellegrino, A.; Rossi, R.S.; Chiari, S.; Campagnutta, E.; Greggi, S.; et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br. J. Cancer 2006, 95, 699–704. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef]
- Deng, T.; Huang, Q.; Wan, T.; Luo, X.; Feng, Y.; Huang, H.; Liu, J. The impact of lymph node dissection on survival in patients with clinical early-stage ovarian cancer. J. Gynecol. Oncol. 2021, 32, e40. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Mok, C.W.; Tan, S.; Zheng, Q.; Shi, L. Network meta-analysis of novel and conventional sentinel lymph node biopsy techniques in breast cancer. BJS Open 2019, 3, 445–452. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Patel, A.; Martin-Hirsch, P.P.; Bryant, A.; Ratnavelu, N.D.; Naik, R.; Ralte, A. Sentinel node assessment for diagnosis of groin lymph node involvement in vulval cancer. Cochrane Database Syst. Rev. 2014, 2016, CD010409. [Google Scholar] [CrossRef] [PubMed]
- Covens, A.; Vella, E.T.; Kennedy, E.B.; Reade, C.J.; Jimenez, W.; Le, T. Sentinel lymph node biopsy in vulvar cancer: Systematic review, meta-analysis and guideline recommendations. Gynecol. Oncol. 2015, 137, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.A.; Webster, K.E.; Bryant, A.; Morrison, J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst. Rev. 2017, 10, CD007585. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.; Wietek, N.; Goodall, R.J.; Hughes, W.; Schmidt-Hansen, M.; Morrison, J. Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst. Rev. 2021, 2021, CD013021. [Google Scholar] [CrossRef]
- Bats, A.-S.; Mathevet, P.; Buenerd, A.; Orliaguet, I.; Mery, E.; Zerdoud, S.; Le Frère-Belda, M.-A.; Froissart, M.; Querleu, D.; Martinez, A.; et al. The Sentinel Node Technique Detects Unexpected Drainage Pathways and Allows Nodal Ultrastaging in Early Cervical Cancer: Insights from the Multicenter Prospective SENTICOL Study. Ann. Surg. Oncol. 2012, 20, 413–422. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.W.; Paek, J.; Lee, S.H.; Yim, G.W.; Kim, J.W.; Kim, Y.T.; Nam, E.J. Comparisons of Surgical Outcomes, Complications, and Costs Between Laparotomy and Laparoscopy in Early-Stage Ovarian Cancer. Int. J. Gynecol. Cancer 2011, 21, 251–256. [Google Scholar] [CrossRef]
- Koo, Y.-J.; Kim, J.-E.; Kim, Y.-H.; Hahn, H.-S.; Lee, I.-H.; Kim, T.-J.; Lee, K.-H.; Shim, J.-U.; Lim, K.-T. Comparison of laparoscopy and laparotomy for the management of early-stage ovarian cancer: Surgical and oncological outcomes. J. Gynecol. Oncol. 2014, 25, 111–117. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Yang, B.; Mallett, S.; Takwoingi, Y.; Davenport, C.F.; Hyde, C.J.; Whiting, P.F.; Deeks, J.J.; Leeflang, M.M.; QUADAS-C Group. QUADAS-C: A Tool for Assessing Risk of Bias in Comparative Diagnostic Accuracy Studies. Ann. Intern. Med. 2021, 174, 1592–1599. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Laven, P.; Kruitwagen, R.; Zusterzeel, P.; Slangen, B.; Van Gorp, T.; van der Pol, J.; Lambrechts, S. Sentinel lymph node identification in early stage ovarian cancer: Is it still possible after prior tumor resection? J. Ovarian Res. 2021, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Agustí, N.; Paredes, P.; Vidal-Sicart, S.; Glickman, A.; Fusté, P.; Carreras, N.; Pahisa, J.; Del Pino, M.; Fristch, A.; Torne, A.; et al. 834 Study of the lymphatic map and detection of the sentinel lymph node in ovaric masses with suspected malignancy. Int. J. Gynecol. Cancer 2021, 31, A278–A279. [Google Scholar] [CrossRef]
- Lago, V.; Bello, P.; Montero, B.; Matute, L.; Padilla-Iserte, P.; Lopez, S.; Marina, T.; Agudelo, M.; Domingo, S. Sentinel lymph node technique in early-stage ovarian cancer (SENTOV): A phase II clinical trial. Int. J. Gynecol. Cancer 2020, 30, 1390–1396. [Google Scholar] [CrossRef]
- Uccella, S.; Nero, C.; Vizza, E.; Vargiu, V.; Corrado, G.; Bizzarri, N.; Ghezzi, F.; Cosentino, F.; Turco, L.C.; Fagotti, A.; et al. Sentinel-node biopsy in early-stage ovarian cancer: Preliminary results of a prospective multicentre study (SELLY). Am. J. Obstet. Gynecol. 2019, 221, 324.e1–324.e10. [Google Scholar] [CrossRef]
- Lago, V.; Bello, P.; Montero, B.; Matute, L.; Padilla-Iserte, P.; Lopez, S.; Agudelo, M.; Domingo, S. Clinical application of the sentinel lymph node technique in early ovarian cancer: A pilot study. Int. J. Gynecol. Cancer 2018, 29, 377–381. [Google Scholar] [CrossRef]
- Nyberg, R.H.; Korkola, P.; Maenpaa, J.U. Sentinel Node and Ovarian Tumors: A Series of 20 Patients. Int. J. Gynecol. Cancer 2017, 27, 684–689. [Google Scholar] [CrossRef]
- Angelucci, M.; Corrado, G.; Mancini, E.; Baiocco, E.; Chiofalo, B.; Zampa, A.; Bufalo, A.; Vizza, E. Laparoscopic indocyanine green sentinel lymph node mapping in early ovarian cancer. A pilot study and review of the literature. Ital. J. Gynaecol. Obstet. 2016, 28, 23–28. [Google Scholar]
- Buda, A.; Passoni, P.; Corrado, G.; Bussi, B.; Cutillo, G.; Magni, S.; Vizza, E. Near-infrared Fluorescence-guided Sentinel Node Mapping of the Ovary with Indocyanine Green in a Minimally Invasive Setting: A Feasible Study. J. Minim. Invasive Gynecol. 2017, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, M.; Farahabadi, E.H.; Yousefi, Z.; Kadkhodayan, S.; Zarifmahmoudi, L.; Sadeghi, R. Lymphatic mapping and sentinel node biopsy in ovarian tumors: A study using intra-operative Tc-99m-Phytate and lymphoscintigraphy imaging. J. Ovarian Res. 2016, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, M.; Brans, B.; Van Gorp, T.; Slangen, B.F.; Kruse, A.J.; Pooters, I.N.; Lotz, M.G.; Van de Vijver, K.K.; Kruitwagen, R.F. The Detection of Sentinel Nodes in Ovarian Cancer: A Feasibility Study. J. Nucl. Med. 2014, 55, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Takeda, M.; Fujimoto, T.; Todo, Y.; Ebina, Y.; Watari, H.; Yamamoto, R.; Minakami, H.; Sakuragi, N. Lymphatic mapping and sentinel node identification as related to the primary sites of lymph node metastasis in early stage ovarian cancer. Gynecol. Oncol. 2004, 94, 161–166. [Google Scholar] [CrossRef]
- Yuan, Y.; Gu, Z.-X.; Tao, X.-F.; Liu, S.-Y. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: A meta-analysis. Eur. J. Radiol. 2012, 81, 1002–1006. [Google Scholar] [CrossRef]
- Agusti, N.; Viveros-Carreño, D.; Grillo-Ardila, C.; Izquierdo, N.; Paredes, P.; Vidal-Sicart, S.; Torne, A.; Díaz-Feijoo, B. Sentinel lymph node detection in early-stage ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 1493–1501. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Xu, T.; Yuan, L.; Yang, X. Sentinel lymph node mapping in early-stage cervical cancer: Meta-analysis. Medicine 2021, 100, e27035. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Deken, M.M.; van Doorn, H.C.; Verver, D.; Boogerd, L.S.; de Valk, K.S.; Rietbergen, D.D.; van Poelgeest, M.I.; de Kroon, C.D.; Beltman, J.J.; van Leeuwen, F.W.; et al. Near-infrared fluorescence imaging compared to standard sentinel lymph node detection with blue dye in patients with vulvar cancer—A randomized controlled trial. Gynecol. Oncol. 2020, 159, 672–680. [Google Scholar] [CrossRef]
- Freytag, D.; Pape, J.; Dhanawat, J.; Günther, V.; Maass, N.; Gitas, G.; Laganà, A.S.; Allahqoli, L.; Meinhold-Heerlein, I.; Moawad, G.N.; et al. Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer. J. Clin. Med. 2020, 9, 4107. [Google Scholar] [CrossRef]
- Zammarrelli, W.A.; Afonso, A.M.; Broach, V.; Sonoda, Y.; Zivanovic, O.; Mueller, J.J.; Leitao, M.M.; Chan, A.; Abu-Rustum, N.R. Sentinel lymph node biopsy in patients with endometrial cancer and an indocyanine green or iodinated contrast reaction—A proposed management algorithm. Gynecol. Oncol. 2021, 162, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Green, C. Technetium-99m production issues in the United Kingdom. J. Med. Phys. 2012, 37, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Ming, J.; Liu, J.; Zhong, L.; Liang, Q.; Fan, L.; Jiang, J. Evaluation of the tracing effect of carbon nanoparticle and carbon nanoparticle-epirubicin suspension in axillary lymph node dissection for breast cancer treatment. World J. Surg. Oncol. 2016, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Berg, N.S.v.D.; Nishio, N.; Juniper, G.; Pei, J.; Zhou, Q.; Lu, G.; Lee, Y.-J.; Ramos, K.; Iagaru, A.H.; et al. Metastatic and sentinel lymph node mapping using intravenously delivered Panitumumab-IRDye800CW. Theranostics 2021, 11, 7188–7198. [Google Scholar] [CrossRef]
- Kleppe, M.; Kraima, A.C.; Kruitwagen, R.F.; Van Gorp, T.; Smit, N.N.; van Munsteren, J.C.; DeRuiter, M.C. Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 1405–1414. [Google Scholar] [CrossRef]
- Collins, A.; Phillips, A. Sentinel lymph node mapping in the modern management of gynaecological malignancy. Obstet. Gynaecol. 2023, 25, 210–219. [Google Scholar] [CrossRef]
- Euscher, E.D.; Malpica, A.; Atkinson, E.N.; Levenback, C.F.; Frumovitz, M.; Deavers, M.T. Ultrastaging Improves Detection of Metastases in Sentinel Lymph Nodes of Uterine Cervix Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2008, 32, 1336–1343. [Google Scholar] [CrossRef]
- NCT05184140—Mapping Sentinel Lymph Node in Initial Stages of Ovarian Cancer (MELISA). Available online: https://clinicaltrials.gov/ct2/show/NCT05184140 (accessed on 30 July 2023).
- NCT03563781—SEntine Lymph Node in Early Ovarian Cancer (SELLY). Available online: https://clinicaltrials.gov/ct2/show/NCT03563781 (accessed on 30 July 2023).
- NCT05937620—Sentinel Node Detection with Technetium-99m Albumin Nanocolloid and ICG in Patients with Epithelial Ovarian Cancer (Melisa-II). Available online: https://clinicaltrials.gov/ct2/show/NCT05937620 (accessed on 30 July 2023).
- NCT05375526—Magtrial: Magtrace® as Tracer for Sentinel Lymph Node Detection in Early Stage Epithelial Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05375526 (accessed on 30 July 2023).
- NCT04051502—ICG-Enabled Mapping of Ovarian Sentinel Lymph Nodes: A Feasibility Study. Available online: https://clinicaltrials.gov/ct2/show/NCT04051502 (accessed on 30 July 2023).
- NCT04714931—Sentinel Lymph Node Assessment in Ovarian Cancer (TRSGO-SLN-OO5). Available online: https://clinicaltrials.gov/ct2/show/NCT04714931 (accessed on 30 July 2023).
- NCT05927818—Sentinel Lymph Node Biopsy in Early-Stage Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05927818 (accessed on 30 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).