Derivation of a HEAR Pathway for Emergency Department Chest Pain Patients to Safely Avoid a Second Troponin Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Laboratory Measurements
2.3. Clinical Data Collection
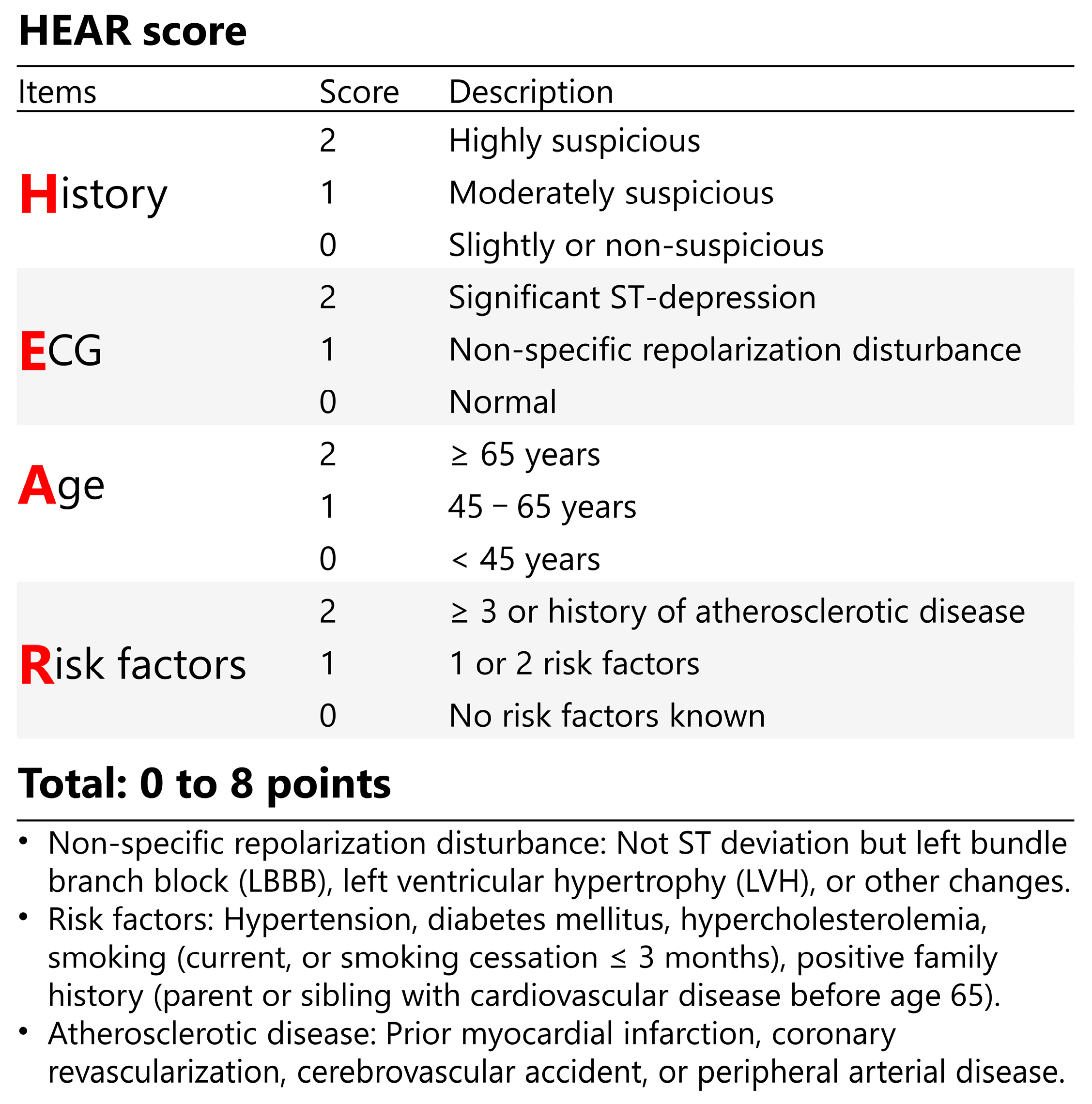
2.4. Risk Assessment Tools
2.5. Outcome Measures
2.6. HEAR Pathway Derivation
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Derivation of the HEAR Pathway
3.3. Safety and Efficacy of the HEAR Pathway for Rapid Rule-Out of NSTEMI
3.4. Safety and Efficacy of Different Sub-Strategies within the HEAR Pathway
3.5. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curfman, G. Acute Chest Pain in the Emergency Department. JAMA Intern. Med. 2018, 178, 220. [Google Scholar] [CrossRef]
- Hsia, R.Y.; Hale, Z.; Tabas, J.A. A National Study of the Prevalence of Life-Threatening Diagnoses in Patients with Chest Pain. JAMA Intern. Med. 2016, 176, 1029–1032. [Google Scholar] [CrossRef]
- Ekelund, U.; Akbarzadeh, M.; Khoshnood, A.; Björk, J.; Ohlsson, M. Likelihood of acute coronary syndrome in emergency department chest pain patients varies with time of presentation. BMC Res. Notes 2012, 5, 420. [Google Scholar] [CrossRef]
- Mehta, R.H.; Eagle, K.A. Missed Diagnoses of Acute Coronary Syndromes in the Emergency Room—Continuing Challenges. New Engl. J. Med. 2000, 342, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, E336–E367. [Google Scholar] [CrossRef]
- Wassie, M.; Lee, M.-S.; Sun, B.C.; Wu, Y.-L.; Baecker, A.S.; Redberg, R.F.; Ferencik, M.; Shen, E.; Musigdilok, V.; Sharp, A.L. Single vs Serial Measurements of Cardiac Troponin Level in the Evaluation of Patients in the Emergency Department with Suspected Acute Myocardial Infarction. JAMA Netw. Open 2021, 4, e2037930. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.A.; Riley, R.F.; Hiestand, B.C.; Russell, G.B.; Hoekstra, J.W.; Lefebvre, C.W.; Nicks, B.A.; Cline, D.M.; Askew, K.L.; Elliott, S.B.; et al. The HEART Pathway randomized trial: Identifying emergency department patients with acute chest pain for early discharge. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 195–203. [Google Scholar] [CrossRef]
- Than, M.P.; Pickering, J.W.; Dryden, J.M.; Lord, S.J.; Aitken, S.A.; Aldous, S.J.; Allan, K.E.; Ardagh, M.W.; Bonning, J.W.; Callender, R.; et al. ICare-ACS (Improving Care Processes for Patients with Suspected Acute Coronary Syndrome): A Study of Cross-System Implementation of a National Clinical Pathway. Circulation 2018, 137, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T.; Johannesson, E.; Lundager Forberg, J.; Mokhtari, A.; Ekelund, U. Diagnostic accuracy of the HEART Pathway and EDACS-ADP when combined with a 0-h/1-h hs-cTnT protocol for assessment of acute chest pain patients. Emerg. Med. J. EMJ 2021, 38, 808–813. [Google Scholar] [CrossRef]
- Shimoni, Z.; Arbuzov, R.; Froom, P. Troponin Testing in Patients without Chest Pain or Electrocardiographic Ischemic Changes. Am. J. Med. 2017, 130, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Gunsolus, I.L.; Smith, S.W.; Sexter, A.; Thordsen, S.E.; Carlson, M.D.; Johnson, B.K.; Bruen, C.A.; Dodd, K.W.; Driver, B.E.; et al. Appropriateness of Cardiac Troponin Testing: Insights from the Use of TROPonin In Acute coronary syndromes (UTROPIA) Study. Am. J. Med. 2019, 132, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Viele, K.; Lewis, R.J. Bayesian Analysis: Using Prior Information to Interpret the Results of Clinical Trials. JAMA 2017, 318, 1605–1606. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.M. Bayes’ theorem, COVID-19, and screening tests. Am. J. Emerg. Med. 2020, 38, 2011–2013. [Google Scholar] [CrossRef]
- Bell, K.J.; Stanaway, F.F.; Irwig, L.M.; Horvath, A.R.; Teixeira-Pinto, A.; Loy, C. How to use imperfect tests for COVID-19 (SARS-CoV-2) to make clinical decisions. Med. J. Aust. 2021, 214, 69–73.e1. [Google Scholar] [CrossRef]
- Hobensack, M.; Phan, N. Derivation and Validation of a 4-Level Clinical Pretest Probability Score for Suspected Pulmonary Embolism to Safely Decrease Imaging Testing. J. Emerg. Med. 2021, 60, 827–828. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Raja, A.S. Arming the Bayesian physician to rule out pulmonary embolism: Using evidence-based diagnostics to combat overtesting. Acad. Emerg. Med. 2014, 21, 1036–1038. [Google Scholar] [CrossRef]
- Moumneh, T.; Sun, B.C.; Baecker, A.; Park, S.; Redberg, R.; Ferencik, M.; Lee, M.-S.; Douillet, D.; Roy, P.-M.; Sharp, A.L. Identifying Patients with Low Risk of Acute Coronary Syndrome Without Troponin Testing: Validation of the HEAR Score. Am. J. Med. 2020, 134, 499–506.e2. [Google Scholar] [CrossRef]
- Smith, L.M.; Ashburn, N.P.; Snavely, A.C.; Stopyra, J.P.; Lenoir, K.M.; Wells, B.J.; Hiestand, B.C.; Herrington, D.M.; Miller, C.D.; Mahler, S.A. Identification of very low-risk acute chest pain patients without troponin testing. Emerg. Med. J. 2020, 37, 690–695. [Google Scholar] [CrossRef]
- Stepinska, J.; Lettino, M.; Ahrens, I.; Bueno, H.; Garcia-Castrillo, L.; Khoury, A.; Lancellotti, P.; Mueller, C.; Muenzel, T.; Oleksiak, A.; et al. Diagnosis and risk stratification of chest pain patients in the emergency department: Focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur. Hear. J. Acute Cardiovasc. Care 2020, 9, 76–89. [Google Scholar] [CrossRef]
- Than, M.; Flaws, D.; Sanders, S.; Doust, J.; Glasziou, P.; Kline, J.; Aldous, S.; Troughton, R.; Reid, C.; A Parsonage, W.; et al. Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol. Emerg. Med. Australas. 2014, 26, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Cullen, L.; Parsonage, W.A.; Greenslade, J.; Twerenbold, R.; Moehring, B.; Wildi, K.; Mueller, S.; Zellweger, C.; Mosimann, T.; et al. Two-hour Algorithm for Triage Toward Rule-out and Rule-in of Acute Myocardial Infarction Using High-sensitivity Cardiac Troponin T. Am. J. Med. 2014, 128, 369–379.e4. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Hesse, K.; Andrews, J.; Ken Lee, K.; Anand, A.; Shah, A.S.; Sandeman, D.; Ferry, A.V.; Jameson, J.; Piya, S.; et al. High-Sensitivity Cardiac Troponin I and Clinical Risk Scores in Patients with Suspected Acute Coronary Syndrome. Circulation 2018, 138, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.A.; Stubblefield, W.B. Clinician Gestalt Estimate of Pretest Probability for Acute Coronary Syndrome and Pulmonary Embolism in Patients with Chest Pain and Dyspnea. Ann. Emerg. Med. 2014, 63, 275–280. [Google Scholar] [CrossRef]
- A Kline, J.; Johnson, C.L.; PollackJr, C.V.; Diercks, D.B.; E Hollander, J.; Newgard, C.D.; Garvey, J.L. Pretest probability assessment derived from attribute matching. BMC Med. Inform. Decis. Mak. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Flaws, D.; Than, M.; Scheuermeyer, F.X.; Christenson, J.; Boychuk, B.; Greenslade, J.H.; Aldous, S.; Hammett, C.J.; A Parsonage, W.; Deely, J.M.; et al. External validation of the emergency department assessment of chest pain score accelerated diagnostic pathway (EDACS-ADP). Emerg. Med. J. 2016, 33, 618–625. [Google Scholar] [CrossRef]
- Mark, D.G.; Huang, J.; Chettipally, U.; Kene, M.V.; Anderson, M.L.; Hess, E.P.; Ballard, D.W.; Vinson, D.R.; Reed, M.E. Performance of Coronary Risk Scores Among Patients with Chest Pain in the Emergency Department. J. Am. Coll. Cardiol. 2018, 71, 606–616. [Google Scholar] [CrossRef]
- Than, M.P.; Pickering, J.W.; Aldous, S.J.; Cullen, L.; Frampton, C.M.; Peacock, W.F.; Jaffe, A.S.; Goodacre, S.W.; Richards, A.M.; Ardagh, M.W.; et al. Effectiveness of EDACS Versus ADAPT Accelerated Diagnostic Pathways for Chest Pain: A Pragmatic Randomized Controlled Trial Embedded Within Practice. Ann. Emerg. Med. 2016, 68, 93–102.e1. [Google Scholar] [CrossRef]
- van den Berg, P.; Body, R. The HEART score for early rule out of acute coronary syndromes in the emergency department: A systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 111–119. [Google Scholar] [CrossRef]
- Ke, J.; Chen, Y.; Wang, X.; Wu, Z.; Chen, F. Indirect comparison of TIMI, HEART and GRACE for predicting major cardiovascular events in patients admitted to the emergency department with acute chest pain: A systematic review and meta-analysis. BMJ Open 2021, 11, e048356. [Google Scholar] [CrossRef]
- Mahler, S.A.; Lenoir, K.M.; Wells, B.J.; Burke, G.L.; Duncan, P.W.; Case, L.D.; Herrington, D.M.; Diaz-Garelli, J.F.; Futrell, W.M.; Hiestand, B.C.; et al. Safely Identifying Emergency Department Patients with Acute Chest Pain for Early Discharge. Circulation 2018, 138, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.A.; Stopyra, J.P.; Apple, F.S.; Riley, R.F.; Russell, G.B.; Hiestand, B.C.; Hoekstra, J.W.; Lefebvre, C.W.; Nicks, B.A.; Cline, D.M.; et al. Use of the HEART Pathway with high sensitivity cardiac troponins: A secondary analysis. Clin. Biochem. 2017, 50, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Aung, S.S.M.; Roongsritong, C. A Closer Look at the HEART Score. Cardiol. Res. 2022, 13, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Dhruva, S.S.; Coon, E.R.; Wright, S.M.; Korenstein, D. 2018 Update on Medical Overuse. JAMA Intern. Med. 2019, 179, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Jülicher, P.; Greenslade, J.H.; A Parsonage, W.; Cullen, L. The organisational value of diagnostic strategies using high-sensitivity troponin for patients with possible acute coronary syndromes: A trial-based cost-effectiveness analysis. BMJ Open 2017, 7, e013653. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Pang, J.-J.; Cheng, K.; Xu, F.; Chen, Y.-G. Trends and challenges of emergency and acute care in Chinese mainland: 2005–2017. World J. Emerg. Med. 2021, 12, 5–11. [Google Scholar] [CrossRef]
- Dawson, L.; Nehme, E.; Nehme, Z.; Zomer, E.; Bloom, J.; Cox, S.; Anderson, D.; Stephenson, M.; Lefkovits, J.; Taylor, A.; et al. Healthcare cost burden of acute chest pain presentations. Emerg. Med. J. 2023, 40, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]




| Strategies | Clinical Criteria | 0 h hs-cTnT Cutoff | 0 h/1 h hs-cTnT Cutoff |
|---|---|---|---|
| 0 h/1 h hs-cTnT algorithm | Chest pain onset > 3 h (for 0 h rule-out) | 0 h hs-cTnT < 5 ng/L | 0 h hs-cTnT < 12 ng/L AND Δ1 h hs-cTnT increase < 3 ng/L |
| HEART pathway | HEART ≤ 3 | ||
| EDACS-ADP | EDACS < 16 points AND No signs of acute ischemia on ECG |
| Characteristics | Total | Not NSTEMI | NSTEMI | p Value |
|---|---|---|---|---|
| N = 7131 | N = 6549 (91.8%) | N = 582 (8.2%) | ||
| General information | ||||
| Age, years | 64 [55, 72] | 64 [55, 71] | 68 [60, 75] | <0.001 |
| Sex, male | 3912 (54.9) | 3459 (52.8) | 453 (77.8) | <0.001 |
| Symptoms | ||||
| Symptom onset time, hour | 2.0 [0.1, 12.0] | 2.0 [0.1, 12.0] | 3.0 [0.5, 24.0] | <0.001 |
| Diaphoresis | 941 (13.2) | 808 (12.3) | 133 (22.9) | <0.001 |
| Palpitation | 904 (12.7) | 867 (13.2) | 37 (6.4) | <0.001 |
| Dyspnea | 569 (8.0) | 510 (7.8) | 59 (10.1) | 0.054 |
| Signs | ||||
| Systolic BP, mmHg | 145.0 [129.0, 161.0] | 145.0 [129.0, 161.0] | 145.0 [129.0, 163.0] | 0.723 |
| Diastolic BP, mmHg | 79.0 [70.0, 89.0] | 79.0 [70.0, 88.0] | 78.0 [69.0, 90.0] | 0.803 |
| Heart rate, bpm | 82.0 [73.0, 93.0] | 82.0 [73.0, 93.0] | 81.0 [72.0, 92.8] | 0.232 |
| Ever smoked | 468 (6.6) | 390 (6.0) | 78 (13.4) | <0.001 |
| History of | ||||
| CAD | 1759 (24.7) | 1563 (23.9) | 196 (33.7) | <0.001 |
| AMI | 386 (5.4) | 328 (5.0) | 58 (10.0) | <0.001 |
| PCI | 998 (14.0) | 878 (13.4) | 120 (20.6) | <0.001 |
| CABG | 33 (0.5) | 26 (0.4) | 7 (1.2) | 0.015 |
| Hypertension | 3474 (48.7) | 3103 (47.4) | 371 (63.7) | <0.001 |
| Diabetes mellitus | 1245 (17.5) | 1061 (16.2) | 184 (31.6) | <0.001 |
| Risk scores | ||||
| HEAR | 4 [3, 5] | 4 [2, 5] | 5 [4, 7] | <0.001 |
| HEART | 4 [3, 6] | 4 [3, 5] | 7 [6, 8] | <0.001 |
| EDACS | 14 [10, 18] | 14 [10, 18] | 18 [14, 21] | <0.001 |
| HEART components | ||||
| Item: History | <0.001 | |||
| Score 0 | 2524 (35.4) | 2461 (37.6) | 63 (10.8) | |
| Score 1 | 1329 (18.6) | 1192 (18.2) | 137 (23.5) | |
| Score 2 | 3278 (46.0) | 2896 (44.2) | 382 (65.6) | |
| Item: ECG | <0.001 | |||
| Score 0 | 4546 (63.7) | 4352 (66.5) | 194 (33.3) | |
| Score 1 | 830 (11.6) | 719 (11.0) | 111 (19.1) | |
| Score 2 | 1755 (24.6) | 1478 (22.6) | 277 (47.6) | |
| Item: Age | <0.001 | |||
| Score 0 | 1008 (14.1) | 977 (14.9) | 31 (5.3) | |
| Score 1 | 2645 (37.1) | 2452 (37.4) | 193 (33.2) | |
| Score 2 | 3478 (48.8) | 3120 (47.6) | 358 (61.5) | |
| Item: Risk factors | <0.001 | |||
| Score 0 | 2744 (38.5) | 2638 (40.3) | 106 (18.2) | |
| Score 1 | 2954 (41.4) | 2675 (40.8) | 279 (47.9) | |
| Score 2 | 1433 (20.1) | 1236 (18.9) | 197 (33.8) | |
| Item: Initial troponin | <0.001 | |||
| Score 0 | 4431 (62.1) | 4421 (67.5) | 10 (1.7) | |
| Score 1 | 1892 (26.5) | 1778 (27.1) | 114 (19.6) | |
| Score 2 | 808 (11.3) | 350 (5.3) | 458 (78.7) | |
| 0-h hs-cTnT, ng/L | 11 [8, 18] | 11 [7, 16] | 119 [47, 354] | <0.001 |
| 0-h Rule-Out Strategies | HEAR Pathway | LoD Strategy 1 | HEART Pathway | EDACS-ADP |
|---|---|---|---|---|
| Patients ruled-out, n (%) | 1496 (21.0) | 196 (2.7) | 315 (4.4) | 350 (4.9) |
| Index visit NSTEMI, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sensitivity, % | 100.0 (99.4–100.0) | 100.0 (99.4–100.0) | 100.0 (99.4–100.0) | 100.0 (99.4–100.0) |
| NPV, % | 100.0 (99.8–100.0) | 100.0 (98.1–100.0) | 100.0 (98.8–100.0) | 100.0 (99.0–100.0) |
| 180-day MACE, n (%) | 6 (0.4) | 4 (2.0) | 1 (0.3) | 10 (2.9) |
| Sensitivity, % | 99.4 (98.6–99.8) | 99.6 (98.9–99.9) | 99.8 (99.2–100.0) | 99.9 (99.4–100.0) |
| NPV, % | 99.6 (99.1–99.9) | 98.0 (94.9–99.4) | 99.9 (99.5–100.0) | 99.7 (98.2–100.0) |
| 180-day MACE components, n (%) | ||||
| Cardiac death, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-fatal STEMI, n (%) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 1 (0.3) |
| Non-fatal NSTEMI, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ischemia-driven TLR, n (%) | 5 (0.3) | 4 (2.0) | 1 (0.3) | 10 (2.9) |
| 0-h Rule-Out Strategies | HEAR Pathway | Sub-Strategies | ||
|---|---|---|---|---|
| ① HEAR ≤ 2 AND 0 h hs-cTnT < 5 ng/L | ② HEAR ≤ 2 AND 5 ≤ 0 h hs-cTnT < 14 ng/L | ③ HEAR > 2 AND 0 h hs-cTnT < 5 ng/L 1 | ||
| Patients ruled-out, n (%) | 1496 (21.0) | 212 (3.0) | 1188 (16.7) | 96 (1.3) |
| Index visit NSTEMI, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sensitivity, % | 100.0 (99.4–100.0) | 100.0 (99.4, 100.0) | 100.0 (99.4, 100.0) | 100.0 (99.4, 100.0) |
| NPV, % | 100.0 (99.8–100.0) | 100.0 (98.3, 100.0) | 100.0 (99.7, 100.0) | 100.0 (96.2, 100.0) |
| 180-day MACE, n (%) | 6 (0.4) | 0 (0.0) | 2 (0.2) | 4 (4.2) |
| Sensitivity, % | 99.4 (98.6–99.8) | 100.0 (99.6, 100.0) | 99.8 (99.2, 100.0) | 99.6 (98.9–99.9) |
| NPV, % | 99.6 (99.1–99.9) | 100.0 (98.3, 100.0) | 99.8 (99.4, 100.0) | 95.8 (89.7–98.9) |
| 180-day MACE events, n (%) | ||||
| Cardiac death, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-fatal STEMI, n (%) | 1 (0.1) | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Non-fatal NSTEMI, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ischemia-driven TLR, n (%) | 5 (0.3) | 0 (0.0) | 1 (0.1) | 4 (4.2) |
| Patient Characteristics, n | Patients Ruled-Out, n (%) | |||||
|---|---|---|---|---|---|---|
| HEAR Pathway | LoD Strategy 1 | HEART Pathway | EDACS-ADP | |||
| Total | n = 7131 | 1496 (21.0) | 196 (2.7) *** | 315 (4.4) *** | 350 (4.9) *** | |
| CPO | ≤3 h | n = 4077 | 741 (18.2) | / | 176 (4.3) *** | 205 (5.0) *** |
| >3 h | n = 3054 | 755 (24.7) | 196 (6.4) *** | 139 (4.6) *** | 145 (4.7) *** | |
| Sex | Females | n = 3219 | 773 (24.0) | 134 (4.2) *** | 203 (6.3) *** | 245 (7.6) *** |
| Males | n = 3912 | 723 (18.5) | 62 (1.6) *** | 112 (2.9) *** | 105 (2.7) *** | |
| Age | ≤65 years | n = 3938 | 1321 (33.5) | 166 (4.2) *** | 294 (7.5) *** | 314 (8.0) *** |
| >65 years | n = 3193 | 175 (5.5) | 30 (0.9) *** | 21 (0.7) *** | 36 (1.1) *** | |
| eGFR 2 | <60 mL·min−1·1.73 m2 | n = 528 | 9 (1.7) | 1 (0.2) * | 0 (0.0) * | 1 (0.2) * |
| >60 mL·min−1·1.73 m2 | n = 4247 | 785 (18.5) | 97 (2.3) *** | 155 (3.6) *** | 173 (4.1) *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yu, Y.; Chen, D.; Cai, C.; Zhou, Y.; Liao, F.; Humarbek, A.; Li, X.; Song, Z.; Sun, Z.; et al. Derivation of a HEAR Pathway for Emergency Department Chest Pain Patients to Safely Avoid a Second Troponin Test. Diagnostics 2023, 13, 3217. https://doi.org/10.3390/diagnostics13203217
Chen C, Yu Y, Chen D, Cai C, Zhou Y, Liao F, Humarbek A, Li X, Song Z, Sun Z, et al. Derivation of a HEAR Pathway for Emergency Department Chest Pain Patients to Safely Avoid a Second Troponin Test. Diagnostics. 2023; 13(20):3217. https://doi.org/10.3390/diagnostics13203217
Chicago/Turabian StyleChen, Chen, Yao Yu, Dongxu Chen, Canguang Cai, Yannan Zhou, Fengqing Liao, Alima Humarbek, Xuan Li, Zhenju Song, Zhan Sun, and et al. 2023. "Derivation of a HEAR Pathway for Emergency Department Chest Pain Patients to Safely Avoid a Second Troponin Test" Diagnostics 13, no. 20: 3217. https://doi.org/10.3390/diagnostics13203217
APA StyleChen, C., Yu, Y., Chen, D., Cai, C., Zhou, Y., Liao, F., Humarbek, A., Li, X., Song, Z., Sun, Z., Tong, C., Yao, C., & Gu, G. (2023). Derivation of a HEAR Pathway for Emergency Department Chest Pain Patients to Safely Avoid a Second Troponin Test. Diagnostics, 13(20), 3217. https://doi.org/10.3390/diagnostics13203217






