Implementation of Individualized Low-Dose Computed Tomography-Guided Hook Wire Localization of Pulmonary Nodules: Feasibility and Safety in the Clinical Setting
Abstract
:1. Introduction
2. Material and Methods
2.1. General Information
2.2. Scanning Parameters
2.3. Computed Tomography-Guided Hook Wire Localization Procedure
2.4. Data Collection
2.5. Subjective Imaging Quality Evaluation
2.6. Statistical Analysis
3. Results
3.1. Demographics and Baseline Nodule Characteristics
3.2. Localization Procedure-Related Parameters
3.3. Evaluation of Nodule Identification Performance
3.4. Evaluation of Image Quality
3.5. Radiation Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, G.; Yu, X.; Chen, W.; Geng, G.; Li, N.; Liu, H.; Yin, P.; Sun, L.; Jiang, J. Computed tomography-guided preoperative semi-rigid hook-wire localization of small pulmonary nodules: 74 cases report. J. Cardiothorac. Surg. 2019, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Han, K.; Hur, J.; Lee, S.M.; Lee, J.W.; Hwang, S.H.; Seo, J.S.; Lee, K.H.; Kwon, W.; Kim, T.H.; et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: A systematic review and meta-analysis. Chest 2017, 151, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Yimin, N.; He, Z.; Chen, X. CT-guided hook-wire localization of malignant pulmonary nodules for video assisted thoracoscopic surgery. J. Cardiothorac. Surg. 2020, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gu, C. Expert consensus workshop report: Guidelines for preoperative assisted localization of small pulmonary nodules. J. Cancer Res. Ther. 2020, 16, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, T.; Chen, L.; Xing, P.; Wu, X.; Shao, C.; Huang, B.; Zang, W. Single-Stage Pulmonary Resection via a Combination of Single Hookwire Localization and Video-Assisted Thoracoscopic Surgery for Synchronous Multiple Pulmonary Nodules. Technol. Cancer Res. Treat. 2021, 20, 15330338211042511. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Ge, J.; Gong, Z.; Zhang, Y.; DiBardino, D.M.; Imperatori, A.; Tandon, Y.K.; Yanagiya, M.; Yao, F.; Qiu, Y. The incidence and risk factors of acute pain after preoperative needle localization of pulmonary nodules: A cross-sectional study. Transl. Lung Cancer Res. 2022, 11, 1667–1677. [Google Scholar] [CrossRef]
- Klinkenberg, T.J.; Dinjens, L.; Wolf, R.F.; van der Wekken, A.J.; van de Wauwer, C.; de Bock, G.H.; Timens, W.; Mariani, M.A.; Groen, H.J. Ct-guided percutaneous hookwire localization increases the efficacy and safety of vats for pulmonary nodules. J. Surg. Oncol. 2017, 115, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Hiss, S.; Mueller, D.; Hellmich, M.; Borggrefe, J.; Bunck, A.C.; Maintz, D.; Hackenbroch, M. Radiation Dose Reduction in Computed Tomography-Guided Lung Interventions using an Iterative Reconstruction Technique. Intervent. Radiol. 2015, 187, 906–914. [Google Scholar] [CrossRef]
- Frisch, B.K.; Slebocki, K.; Mammadov, K.; Puesken, M.; Becker, I.; Maintz, D.; Chang, D.-H. Implementation of ultra-low-dose lung protocols in CT-guided lung biopsies: Feasibility and safety in the clinical setting. J. Int. Med. Res. 2017, 45, 2101–2109. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Cheng, H.; Li, G.-C. Computed tomography-guided core needle biopsy for lung nodules: Low-dose versus standard-dose protocols. Videosurg. Other Miniinvasive Tech. 2021, 16, 355–361. [Google Scholar] [CrossRef]
- Na Lee, H.; Lee, S.M.; Choe, J.; Chae, E.J.; Do, K.-H.; Seo, J.B. Diagnostic performance of CT-guided percutaneous transthoracic core needle biopsy using low tube voltage (100 kVp): Comparison with conventional tube voltage (120 kVp). Acta Radiol. 2018, 59, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Oda, S.; Nakaura, T.; Tsuji, A.; Urata, J.; Furusawa, M.; Utsunomiya, D.; Funama, Y.; Kidoh, M.; Yamashita, Y. Radiation Dose Reduction at Pediatric CT: Use of Low Tube Voltage and Iterative Reconstruction. RadioGraphics 2018, 38, 1421–1440. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Mardakhaev, E.; Shmukler, A.; Levsky, J.M.; Haramati, L.B. Meeting ACR Dose Guidelines for CT Lung Cancer Screening in an Overweight and Obese Population. Acad. Radiol. 2021, 28, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Harun, H.H.; Abdul Karim, M.K.; Abbas, Z.; Abdul Rahman, M.A.; Sabarudin, A.; Ng, K.H. Association of Radiation Doses and Cancer Risks from CT Pul-monary Angiography Examinations in Relation to Body Diameter. Diagnostics 2020, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, M.; Ji, Y.; Li, C.; Fang, X.; Zhang, S.; Wang, W.; Wang, L.; Liu, A. An Individualized Contrast-Enhanced Liver Computed Tomography Imaging Protocol Based on Body Mass Index in 126 Patients Seen for Liver Cirrhosis. Med. Sci. Monit. 2021, 27, e932109-1. [Google Scholar] [CrossRef] [PubMed]
- Svahn, T.M.; Sjöberg, T.; Ast, J.C. Dose estimation of ultra-low-dose chest CT to different sized adult patients. Eur. Radiol. 2019, 29, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Tækker, M.; Kristjánsdóttir, B.; Graumann, O.; Laursen, C.B.; Pietersen, P.I. Diagnostic accuracy of low-dose and ultra-low-dose ct in detection of chest pathology: A systematic review. Clin. Imag. 2021, 74, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.J.; Lewandowski, R.J.; Goodwin, L.; Yaghmai, V.; Nemcek, A.; Carr, J.C.; Collins, J.D. Reinforcing the Importance and Feasibility of Implementing a Low-dose Protocol for CT-guided Biopsies. Acad. Radiol. 2018, 25, 1146–1151. [Google Scholar] [CrossRef]
- Adiga, S.; Athreya, S. Safety, efficacy, and feasibility of an ultra-low dose radiation protocol for ct-guided percutaneous needle biopsy of pulmonary lesions: Initial experience. Clin. Radiol. 2014, 69, 709–714. [Google Scholar] [CrossRef]
- Kallianos, K.G.; Elicker, B.M.; Henry, T.S.; Ordovas, K.G.; Nguyen, J.; Naeger, D.M. Instituting a Low-dose CT-guided Lung Biopsy Protocol. Acad. Radiol. 2016, 23, 1130–1136. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, S.; Li, J.; Yuan, W.; Song, Z.; Zhang, H. Preoperative CT-guided localization of pulmonary nodules with low-dose radiation. Quant. Imaging Med. Surg. 2023, 13, 4295–4304. [Google Scholar] [CrossRef]
- Ju, Y.H.; Lee, G.; Lee, J.W.; Hong, S.B.; Suh, Y.J.; Jeong, Y.J. Ultra-low-dose lung screening ct with model-based iterative reconstruction: An assessment of image quality and lesion conspicuity. Acta Radiol. 2018, 59, 553–559. [Google Scholar] [CrossRef]
- Steidel, J.; Maier, J.; Sawall, S.; Kachelrieß, M. Dose reduction potential in diagnostic single energy CT through patient-specific prefilters and a wider range of tube voltages. Med. Phys. 2022, 49, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Yang, P.; Luo, K.; He, X. Size-Specific Dose Estimates of Radiation Based on Body Weight and Body Mass Index for Chest and Abdomen-Pelvic CTs. BioMed. Res. Int. 2020, 2020, 6046501. [Google Scholar] [CrossRef] [PubMed]
- Saltybaeva, N.; Martini, K.; Frauenfelder, T.; Alkadhi, H. Organ dose and attributable cancer risk in lung cancer screening with low-dose computed tomography. PLoS ONE 2016, 11, e0155722. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; McMillan, K.; Bostani, M.; Cagnon, C.; McNitt-Gray, M. Patient Size—Specific Analysis of Dose Indexes from CT Lung Cancer Screening. Am. J. Roentgenol. 2017, 208, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, F.O.; Metaxas, V.I.; Dimitroukas, C.P.; Panayiotakis, G.S. Low BMI patient dose in digital radiography. Radiat. Prot. Dosimetry 2020, 189, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Macri, F.; Greffier, J.; Pereira, F.; Rosa, A.C.; Khasanova, E.; Claret, P.G.; Larbi, A.; Gualdi, G.; Beregi, J.P. Value of ultra-low-dose chest ct with iterative reconstruction for selected emergency room patients with acute dyspnea. Eur. J. Radiol. 2016, 85, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Jiang, B.; Zhang, S.; Zhang, L.; Zhang, L.; Feng, Y.; Li, J.; Zhang, Y.; Xie, X. Measurement Accuracy and Repeatability of RECIST-Defined Pulmonary Lesions and Lymph Nodes in Ultra-Low-Dose CT Based on Deep Learning Image Reconstruction. Cancers 2022, 14, 5016. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Wang, J.; Yao, J.; Xu, L.; Gao, L. Reevaluation of the efficacy of preoperative computed tomography-guided hook wire localization: A retrospective analysis. Int. J. Surg. 2018, 51, 24–30. [Google Scholar] [CrossRef]
- Jouneau, S.; Ricard, J.D.; Seguin-Givelet, A.; Bigé, N.; Contou, D.; Desmettre, T.; Hugenschmitt, D.; Kepka, S.; Le Gloan, K.; Maitre, B.; et al. SPLF/SMFU/SRLF/SFAR/SFCTCV Guidelines for the management of patients with primary spontaneous pneumothorax. Ann. Intensive Care 2023, 13, 1–25. [Google Scholar] [CrossRef]


| Basic Information | Group A1 (n = 32) | Group B1 (n = 34) | p Value | Group A2 (n = 51) | Group B2 (n = 67) | p Value |
|---|---|---|---|---|---|---|
| Gender (F/M) | 17/8 | 22/6 | 0.384 | 25/14 | 37/21 | 0.975 |
| Age (Years) | 56.96 ± 11.05 | 52.93 ± 11.93 | 0.198 | 56.54 ± 8.99 | 57.89 ± 9.00 | 0.463 |
| BMI (kg/m2) | 20.58 ± 0.99 | 20.11 ± 1.33 | 0.154 | 24.50 ± 1.72 | 25.37 ± 2.55 | 0.064 |
| Lesion Type | ||||||
| pGGN | 20 (62.5%) | 19 (55.9%) | 34 (66.7%) | 34 (50.7%) | ||
| psGGN | 11 (34.4%) | 14 (41.2%) | 13 (25.5) | 29 (43.3%) | ||
| Solid | 1 (3.1%) | 1 (2.9%) | 4 (7.8%) | 4 (6.0%) | ||
| Lesion Size (mm) | ||||||
| Lesion ≤ 5 | 4 (12.5%) | 4 (11.8%) | 10 (19.6%) | 10/(14.9%) | ||
| 5 < Lesion ≤ 10 | 20 (62.5%) | 20 (58.8%) | 29 (56.9%) | 41 (61.2%) | ||
| 10 < Lesion ≤ 20 | 8 (25.0%) | 10 (29.4%) | 12 (23.5%) | 16 (23.9%) | ||
| Lesion Location | ||||||
| RUML | 8 (25.0%) | 9 (26.5%) | 13 (25.5%) | 24 (35.8%) | ||
| RLL | 7 (21.9%) | 9 (26.5%) | 11 (21.6%) | 13 (19.4%) | ||
| LUL | 9 (28.1%) | 3 (8.8%) | 16 (31.3) | 18 (26.9%) | ||
| LLL | 8 (25.0%) | 13 (38.2%) | 11 (21.6%) | 12 (17.9%) |
| Group | Nodule Density (HU) | Nodule Size (mm) | Patient Position Supine/Prone/Lateral (n) | Nodule Distance to Pleural Surface (mm) | Nodule Distance to Needle Tip (mm) | Needle Insertion Depth from Pleura (mm) | Pneumothorax (n) | Bleeding (n) | Number of Needle Insertions (n) | Localization Procedure Duration (min) | Surgery Duration (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A1 | −548.93 ± 202.82 | 8.82 ± 3.39 | 3/10/19 | 16.21 ± 12.72 | 15.01 ± 13.45 | 26.63 ± 13.85 | 9 (25) | 6 (25) | 1.76 ± 1.27 | 9.56 ± 4.37 | 1.38 ± 0.44 |
| Group B1 | −569.60 ± 139.36 | 8.59 ± 2.98 | 4/7/23 | 19.09 ± 13.54 | 15.13 ± 11.69 | 32.15 ± 16.65 | 6 (28) | 7 (28) | 1.62 ± 0.78 | 9.43 ± 2.57 | 1.21 ± 0.40 |
| p value | 0.629 | 0.773 | 0.377 | 0.969 | 0.108 | 0.360 | 1.000 | 0.635 | 0.894 | 0.154 | |
| Group A2 | −518.66 ± 222.88 | 8.29 ± 3.78 | 9/15/27 | 21.05 ± 14.95 | 19.97 ± 18.16 | 30.24 ± 12.49 | 15 (39) | 13 (39) | 2.15 ± 1.44 | 10.49 ± 4.08 | 1.55 ± 0.63 |
| Group B2 | −460.22 ± 223.05 | 8.31 ± 2.83 | 10/21/36 | 22.83 ± 16.61 | 16.69 ± 12.10 | 29.15 ± 14.48 | 13 (58) | 19 (58) | 2.05 ± 1.39 | 10.47 ± 4.58 | 1.43 ± 0.42 |
| p value | 0.161 | 0.963 | 0.550 | 0.243 | 0.668 | 0.111 | 1.000 | 0.735 | 0.988 | 0.298 |
| Group | Nodule Density | Nodule Size |
|---|---|---|
| Group B1 | −546.64 ± 136.67 | 8.59 ± 2.98 |
| Group C1 | −544.91 ± 134.27 | 8.75 ± 3.10 |
| p value | 0.458 | 0.135 |
| Group B2 | −460.22 ± 223.05 | 8.31 ± 2.83 |
| Group C2 | −443.68 ± 225.56 | 8.45 ± 2.86 |
| p value | 0.108 | 0.350 |
| Group | Score | |
|---|---|---|
| BMI ≤ 21 kg/m2 | BMI > 21 kg/m2 | |
| Group A | 4.92 ± 0.277 | 4.92 ± 0.270 |
| Group B | 3.86 ± 0.356 | 3.95 ± 0.223 |
| p value | 0.000 | 0.000 |
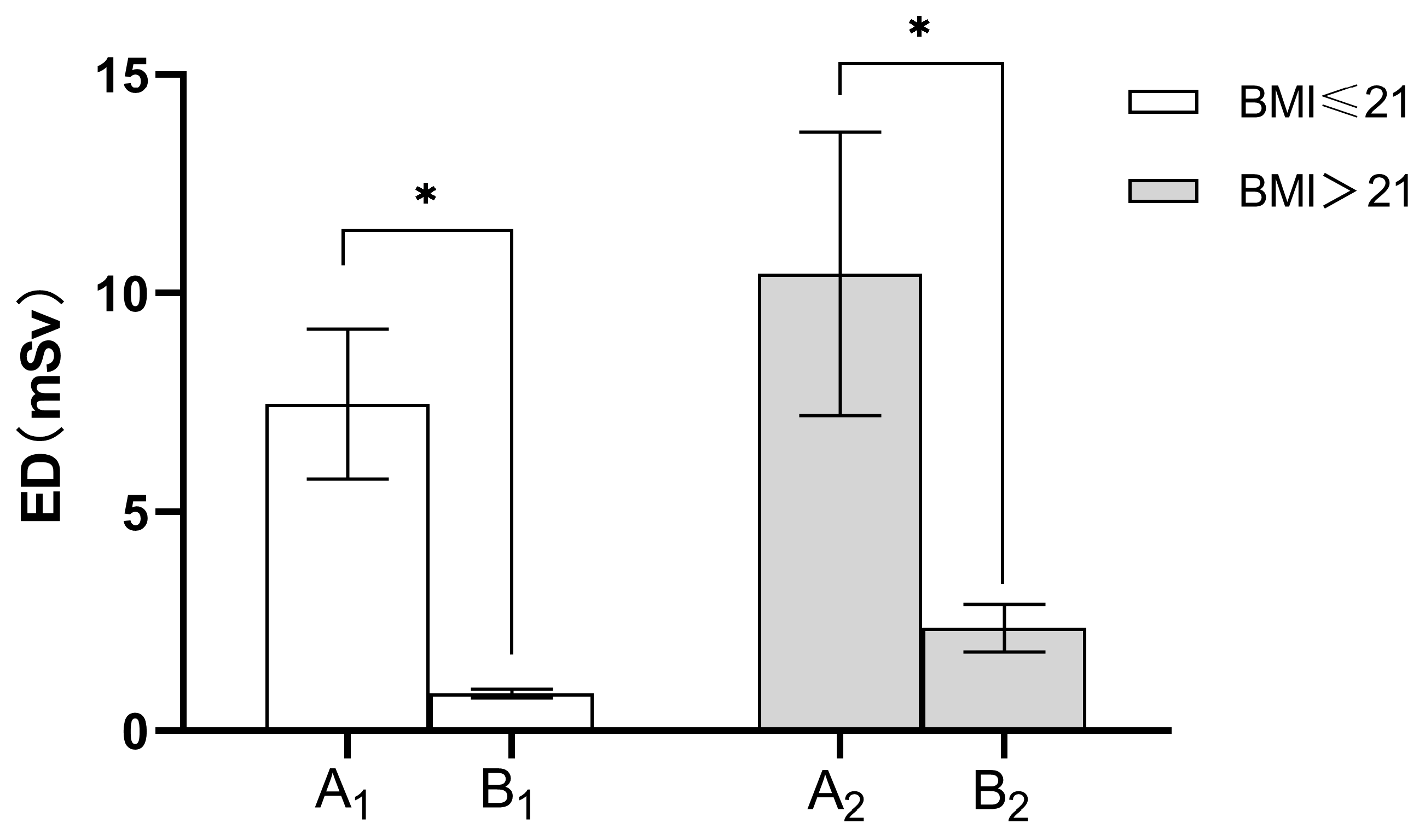
| Group | CTDI (mGy) | DLP (mGy.cm) | ED (mSv) |
|---|---|---|---|
| Group A1 | 5.24 ± 0.95 | 533.58 ± 122.06 | 7.47 ± 1.71 |
| Group B1 | 0.56 ± 0.00 | 56.86 ± 4.73 | 0.79 ± 0.07 |
| p value | <0.001 | <0.001 | <0.001 |
| Group A2 | 6.69 ± 1.47 | 746.01 ± 230.91 | 10.44 ± 3.23 |
| Group B2 | 1.48 ± 0.00 | 167.02 ± 38.76 | 2.33 ± 0.54 |
| p value | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Wang, S.-G.; Zhang, J.-Y.; Togn, X.-Y.; Li, B.-B.; Fang, X.; Pu, R.-W.; Zhou, Y.-J.; Liu, Y.-J. Implementation of Individualized Low-Dose Computed Tomography-Guided Hook Wire Localization of Pulmonary Nodules: Feasibility and Safety in the Clinical Setting. Diagnostics 2023, 13, 3235. https://doi.org/10.3390/diagnostics13203235
Wei W, Wang S-G, Zhang J-Y, Togn X-Y, Li B-B, Fang X, Pu R-W, Zhou Y-J, Liu Y-J. Implementation of Individualized Low-Dose Computed Tomography-Guided Hook Wire Localization of Pulmonary Nodules: Feasibility and Safety in the Clinical Setting. Diagnostics. 2023; 13(20):3235. https://doi.org/10.3390/diagnostics13203235
Chicago/Turabian StyleWei, Wei, Shi-Geng Wang, Jing-Yi Zhang, Xiao-Yu Togn, Bei-Bei Li, Xin Fang, Ren-Wang Pu, Yu-Jing Zhou, and Yi-Jun Liu. 2023. "Implementation of Individualized Low-Dose Computed Tomography-Guided Hook Wire Localization of Pulmonary Nodules: Feasibility and Safety in the Clinical Setting" Diagnostics 13, no. 20: 3235. https://doi.org/10.3390/diagnostics13203235
APA StyleWei, W., Wang, S.-G., Zhang, J.-Y., Togn, X.-Y., Li, B.-B., Fang, X., Pu, R.-W., Zhou, Y.-J., & Liu, Y.-J. (2023). Implementation of Individualized Low-Dose Computed Tomography-Guided Hook Wire Localization of Pulmonary Nodules: Feasibility and Safety in the Clinical Setting. Diagnostics, 13(20), 3235. https://doi.org/10.3390/diagnostics13203235







