Postoperative Seizure Prophylaxis in Meningioma Resection: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Risk of Bias Assessment
2.3. Statistical Analysis
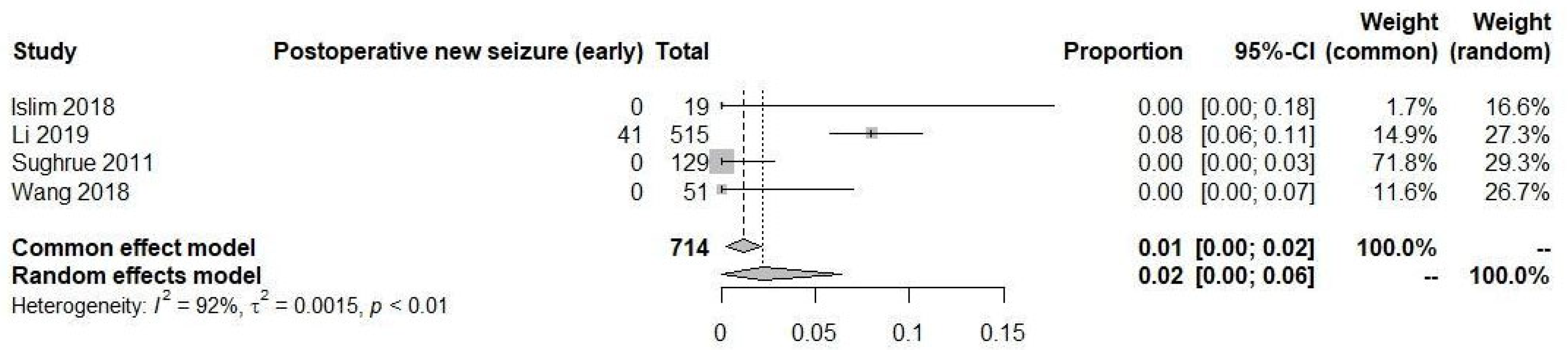
3. Results
3.1. Study Selection
3.2. Quality of the Included Studies
3.3. Baseline Characteristics of Included Studies
3.4. Postoperative Seizures in AEDs and No AEDs
3.5. Postoperative Seizures Prophylaxis in Early and Late Time
3.6. New Onset Postoperative Seizures
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, M.; Galldiks, N.; Iglseder, S.; Kickingereder, P.; von Deimling, A.; Bendszus, M.; Wick, W.; Sahm, F. Diagnostic challenges in meningioma. Neuro-Oncol. 2017, 19, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, A.; Kunimatsu, N.; Kamiya, K.; Katsura, M.; Mori, H.; Ohtomo, K. Variants of meningiomas: A review of imaging findings and clinical features. Jpn. J. Radiol. 2016, 34, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Simis, A.; de Aguiar, P.H.P.; Leite, C.C.; Santana, P.A.; Rosemberg, S.; Teixeira, M.J. Peritumoral brain edema in benign meningiomas: Correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg. Neurol. 2008, 70, 471–477. [Google Scholar] [CrossRef]
- Zouaoui, S.; Darlix, A.; Rigau, V.; Mathieu-Daudé, H.; Bauchet, F.; Bessaoud, F.; Fabbro-Peray, P.; Trétarre, B.; Figarella-Branger, D.; Taillandier, L.; et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie 2018, 64, 15–21. [Google Scholar] [CrossRef]
- Englot, D.J.; Magill, S.T.; Han, S.J.; Chang, E.F.; Berger, M.S.; McDermott, M.W. Seizures in supratentorial meningioma: A systematic review and meta-analysis. J. Neurosurg. 2016, 124, 1552–1561. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Tang, H.; Asemota, A.; Huang, L.; Boling, W.; Bannout, F. Meningioma Related Epilepsy- Pathophysiology, Pre/postoperative Seizures Predicators and Treatment. Front. Oncol. 2022, 12, 905976. [Google Scholar] [CrossRef]
- Mantegazza, M.; Curia, G.; Biagini, G.; Ragsdale, D.S.; Avoli, M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Singh, J.N.; Jain, G.; RamaRao, P.; Sharma, S.S. Inhibition of sodium current by carbamazepine in dorsal root ganglion neurons in vitro. Indian J. Physiol. Pharmacol. 2009, 53, 147–154. [Google Scholar]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef]
- Owens, M.J.; Nemeroff, C.B. Pharmacology of valproate. Psychopharmacol. Bull. 2003, 37 (Suppl. S2), 17–24. [Google Scholar] [PubMed]
- Mutanana, N.; Tsvere, M.; Chiweshe, M.K. General side effects and challenges associated with anti-epilepsy medication: A review of related literature. Afr. J. Prim. Health Care Fam. Med. 2020, 12, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, T.F.; Ridwan, S.; Grote, A.; Coras, R.; Simon, M. Early postoperative seizures (EPS) in patients undergoing brain tumour surgery. Sci. Rep. 2020, 10, 13674. [Google Scholar] [CrossRef] [PubMed]
- Islim, A.I.; McKeever, S.; Kusu-Orkar, T.-E.; Jenkinson, M.D. The role of prophylactic antiepileptic drugs for seizure prophylaxis in meningioma surgery: A systematic review. J. Clin. Neurosci. 2017, 43, 47–53. [Google Scholar] [CrossRef]
- McKevitt, C.; Marenco-Hillembrand, L.; Bamimore, M.; Chandler, R.; Otamendi-Lopez, A.; Almeida, J.P.; Quiñones-Hinojosa, A.; Chaichana, K.L. Predictive factors for post operative seizures following meningioma resection in patients without preoperative seizures: A multicenter retrospective analysis. Acta Neurochir. 2023, 165, 1333–1343. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chozick, B.S.; Reinert, S.E.; Greenblatt, S.H. Incidence of seizures after surgery for supratentorial meningiomas: A modern analysis. J. Neurosurg. 1996, 84, 382–386. [Google Scholar] [CrossRef]
- Islim, A.I.; Ali, A.; Bagchi, A.; Ahmad, M.U.; Mills, S.J.; Chavredakis, E.; Brodbelt, A.R.; Jenkinson, M.D. Postoperative seizures in meningioma patients: Improving patient selection for antiepileptic drug therapy. J. Neurooncol. 2018, 140, 123–134. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Lin, Z.; Zhao, M.; Ren, X.; Zhang, X.; Jiang, Z. Risk factors and control of seizures in 778 Chinese patients undergoing initial resection of supratentorial meningiomas. Neurosurg. Rev. 2020, 43, 597–608. [Google Scholar] [CrossRef]
- Skardelly, M.; Rother, C.; Noell, S.; Behling, F.; Wuttke, T.V.; Schittenhelm, J.; Bisdas, S.; Meisner, C.; Rona, S.; Tabatabai, G.; et al. Risk Factors of Preoperative and Early Postoperative Seizures in Patients with Meningioma: A Retrospective Single-Center Cohort Study. World Neurosurg. 2017, 97, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Sughrue, M.E.; Rutkowski, M.J.; Chang, E.F.; Shangari, G.; Kane, A.J.; McDermott, M.W.; Berger, M.S.; Parsa, A.T.; Freund, B.; Probasco, J.C.; et al. Postoperative seizures following the resection of convexity meningiomas: Are prophylactic anticonvulsants indicated? J. Neurosurg. 2011, 114, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Chuang, C.-C.; Tu, P.-H.; Wei, K.-C.; Wu, C.-T.; Lee, C.-C.; Liu, Z.-H.; Chen, P.-Y. Seizures in surgically resected atypical and malignant meningiomas: Long-term outcome analysis. Epilepsy Res. 2018, 140, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.-G.; Morel, C.; Gmür, C.; Neidert, M.C.; Baumann, C.R.; Valavanis, A.; Rushing, E.J.; Krayenbühl, N.; Weller, M. Predicting outcome of epilepsy after meningioma resection. Neuro Oncol. 2016, 18, 1002–1010. [Google Scholar] [CrossRef]
- Xue, H.; Sveinsson, O.; Bartek, J.; Förander, P.; Skyrman, S.; Kihlström, L.; Shafiei, R.; Mathiesen, T.; Tomson, T. Long-term control and predictors of seizures in intracranial meningioma surgery: A population-based study. Acta Neurochir. 2018, 160, 589–596. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, G.; Yang, H.-F.; Wang, D.; Yu, J.-L.; Huang, H.-Y. Assessment of Risk Factors for Early Seizures following Surgery for Meningiomas Using Logistic Regression Analysis. J. Int. Med. Res. 2011, 39, 1728–1735. [Google Scholar] [CrossRef]
- Delgado-López, P.; Ortega-Cubero, S.; Bernal, J.G.; Cubo-Delgado, E. Seizure prophylaxis in meningiomas: A systematic review and meta-analysis. Neurologia 2023, 38, 291–302. [Google Scholar] [CrossRef]
- Abou-Khalil, B.W. Update on Antiepileptic Drugs 2019. Contin. Lifelong Learn. Neurol. 2019, 25, 508–536. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. Teratogenicity of antiepileptic drugs. Curr. Opin. Neurol. 2019, 32, 246–252. [Google Scholar] [CrossRef]
- Vidaurre, J.; Gedela, S.; Yarosz, S. Antiepileptic Drugs and Liver Disease. Pediatr. Neurol. 2017, 77, 23–36. [Google Scholar] [CrossRef]
- Tanti, M.J.; Marson, A.G.; Jenkinson, M.D. Epilepsy and adverse quality of life in surgically resected meningioma. Acta Neurol. Scand. 2017, 136, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.A.; Raza, M.L.; Shafiq, Y.; Asghar, M.A. Analysis of treatment adherence and cost among patients with epilepsy: A four-year retrospective cohort study in Pakistan. BMC Health Serv. Res. 2021, 21, 72. [Google Scholar] [CrossRef]
- Faught, E.; Duh, M.S.; Weiner, J.R.; Guerin, A.; Cunnington, M.C. Nonadherence to antiepileptic drugs and increased mortality: Findings from the RANSOM Study. Neurology 2008, 71, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, Y.-R.; Zhou, M.-Y.; Wang, M.-W.; Ye, L.; Xu, Z.-C.; Feng, Z.-H.; Ma, X.-T. Prophylactic AEDs Treatment for Patients With Supratentorial Meningioma Does Not Reduce the Rate of Perioperative Seizures: A Retrospective Single-Center Cohort Study. Front. Oncol. 2020, 10, 568369. [Google Scholar] [CrossRef]
- Zachenhofer, I.; Donat, M.; Oberndorfer, S.; Roessler, K. Perioperative levetiracetam for prevention of seizures in supratentorial brain tumor surgery. J. Neurooncol. 2010, 101, 101–106. [Google Scholar] [CrossRef]
- Siomin, V.; Angelov, L.; Li, L.; Vogelbaum, M.A. Results of a Survey of Neurosurgical Practice Patterns Regarding the Prophylactic use of Anti-Epilepsy Drugs in Patients with Brain Tumors. J. Neurooncol. 2005, 74, 211–215. [Google Scholar] [CrossRef]



| Study | n | Mean Age | Sex (M/F) | Drugs | Preoperative Seizures | Patients without Preoperative Seizures | Size of Tumor | Tumor Location | WHO Grade | SG/Resection |
|---|---|---|---|---|---|---|---|---|---|---|
| Chozick, 1996 [18] | 158 | 60 | 50/108 | NA | 63 | 95 | NA | Skull base: 141 * (Frontal 38 (26.9%), Parietal 34 (24.1%), Middle cranial fossa 12 (8.5%), Occipital 6 (4.2%), Sphenoid wing 33 (23.4%), Petrous 8 (5.6%), Dorsum/tuberculum sella 7 (4.9%), Cavernous sinus 3 (2.1%)) Non-skull base: 60 * (Parasagittal 56 (93.3%), Intraventricular 4 (6.6%)) | NA | SG NA GTR 119; STR 39 |
| Islim, 2018 [19] | 283 | 57 | 69/214 | P 48%, L 26% | 68 | 215 | ≤10 cm3 57 > 10 cm3 221 | Skull base: 76 (Sphenoid 34 (44.7%), Olfactory groove 18 (23.6%), Suprasellar 10 (13.1%), Posterior fossa 2 (2.6%), Others 12 (15.7%)) Non-skull base: 207 (Convexity 98 (47.3%), Parafalcine 39 (18.8%), Tentorial 24 (11.5%), Convexity/parafalcine 17 (8.2%), Parasagittal 12 (5.7%), Posterior fossa 5 (2.4%), Others 12 (5.7%)) | WHO GRADE I 233, II 47, III 3 | NA |
| Li, 2019 [20] | 778 | 50 | 241/537 | NA | 87 | 586 | Range 1.2–11.5 cm | Skull base: 291 Non-skull base: 487 | WHO GRADE I 699, II 74, III 5 | SG I-III 735 SG IV 43 GTR 735 STR 43 |
| Skardelly, 2017 [21] | 634 | 58 | 176/458 | L: 76, other: 30, missing: 3/L: (median/10–90/range)—1000 mg/1000–2000 mg/500–3000 mg | 97 | 537 | Tumor volume (Median) 17.4 cm3 skull base meningioma 10.8 cm3 cranial roof meningioma 26.4 cm3 | Skull base: 350 Non-skull base: 284 | WHO GRADE I 423, II 208, III 3 | SG I 142 II 83 III 181 IV 115 Missing 16. Resection: NA |
| Sughrue, 2011 [22] | 180 | 55 | 50/130 | P and L | 0 | 180 | NA | NA | WHO GRADE I 129, II 30, III 21 | SG I 102 II 59 III 10 IV 9. Resection: NA |
| Wang, 2018 [23] | 102 | 57 | 45/57 | Va, L or P | 15 | 87 | Tumor Diameter (cm) Mean. 5.2 ± 2.0 | Skull base: 29 Non-skull base: 73 (convexity 33 (45.2%), parasagittal 32 (43.8%), posterior fossa 8 (10.9%)) | WHO GRADE II 86, III 16 | SG NA GTR 69 STR 33 |
| Wirsching, 2016 [24] | 779 | 57 | 247/532 | P and CB | 244 | 535 | Maximal diameter (mm) Median 40.0 | Skull base: 260 Non-skull base: 401 * (convexity 167 (41.6%), parasagittal 131 (32.6%), posterior fossa 81 (20.1%), other location 22 (5.4%)) | WHO GRADE I 638, II 119, III 22 | SG I 143 II 221 III 41 IV 55 V11 GTR was achieved in 531 patients. A subset of 129 patients underwent 2 or more meningiomas |
| Xue, 2018 [25] | 113 | 53 | 19/94 | CB | NA | 92 | <3.5 cm (65) and ≥3.5 cm (50)//median (range): 3.51 ± 1.58 cm | Skull base: 82 (spenhoid wing 25 (30.4%), posterior fossa 19 (23.1%), middle cranial fossa 9 (10.9%), olfactory groove 8 (9.7%), tuberculum sellae 8 (9.7%), tentorial 7 (8.5%), foramen magnun 5 (6.09%), foramen jugular 1 (1.2%)) Non-skull base: 31 (convexity 17 (54.8%), parasaggital 8 (25.8%), falcine 4 (12.9%), intra-lateral ventricle 2 (6.4%)) | WHO GRADE I 110, II 3 | SG I–III 78, IV–V 35 GTR 78 STR 35 |
| Zhang, 2011 [26] | 222 | 50 | 72/150 | P 5 mg/kg per day | NA | NA | Maximum tumour diameter—(1) 3–5 cm; (2) <3 cm; and (3) >5 cm | Skull base and non-skull base: * Spine of sphenoid bone, parietooccipital, posterior fossa, occipitotemporal area, occiput, cerebellopontine angle, cerebellar hemisphere, frontotemporal, middle cranial fossa, petroclival, paracele, anterior cranial fossa, forehead, saddle region, and temporoparietal region, temporal, parietal lobe, frontal and parietal lobes | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, S.; Bertani, R.; Palavani, L.B.; de Barros Oliveira, L.; Borges, P.; Koester, S.W.; Paiva, W.S. Postoperative Seizure Prophylaxis in Meningioma Resection: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3415. https://doi.org/10.3390/diagnostics13223415
Batista S, Bertani R, Palavani LB, de Barros Oliveira L, Borges P, Koester SW, Paiva WS. Postoperative Seizure Prophylaxis in Meningioma Resection: A Systematic Review and Meta-Analysis. Diagnostics. 2023; 13(22):3415. https://doi.org/10.3390/diagnostics13223415
Chicago/Turabian StyleBatista, Sávio, Raphael Bertani, Lucca B. Palavani, Leonardo de Barros Oliveira, Pedro Borges, Stefan W. Koester, and Wellingson Silva Paiva. 2023. "Postoperative Seizure Prophylaxis in Meningioma Resection: A Systematic Review and Meta-Analysis" Diagnostics 13, no. 22: 3415. https://doi.org/10.3390/diagnostics13223415
APA StyleBatista, S., Bertani, R., Palavani, L. B., de Barros Oliveira, L., Borges, P., Koester, S. W., & Paiva, W. S. (2023). Postoperative Seizure Prophylaxis in Meningioma Resection: A Systematic Review and Meta-Analysis. Diagnostics, 13(22), 3415. https://doi.org/10.3390/diagnostics13223415