Early Detection of Lung Nodules Using a Revolutionized Deep Learning Model
Abstract
:1. Introduction
1.1. Motivation
1.2. Scope
1.3. Objectives
- To enhance the early detection of lung cancer by accurately identifying potential indicators (lung nodules) at the earliest stages, improving treatment outcomes and increasing patient survival rates;
- To boost the accuracy and promptness of lung nodule detection via DL strategies, such as HFRCNN, that automate the detection process, provide an objective analysis of medical images, and reduce diagnostic errors.
1.4. Research Contribution
- This study successfully employs Hybridized Faster R-CNN (HFRCNN) to detect early-stage lung cancer in medical images, addressing a critical global health challenge;
- HFRCNN, a two-stage, region-based entity detector, demonstrates its efficacy in identifying crucial entities in medical imagery, showcasing its adaptability in health applications;
- The proposed model achieved a remarkable detection accuracy of over 97%, surpassing the performance of several previously established methods.
1.5. Research Questions (RQ)
2. Related Work
3. Methodology
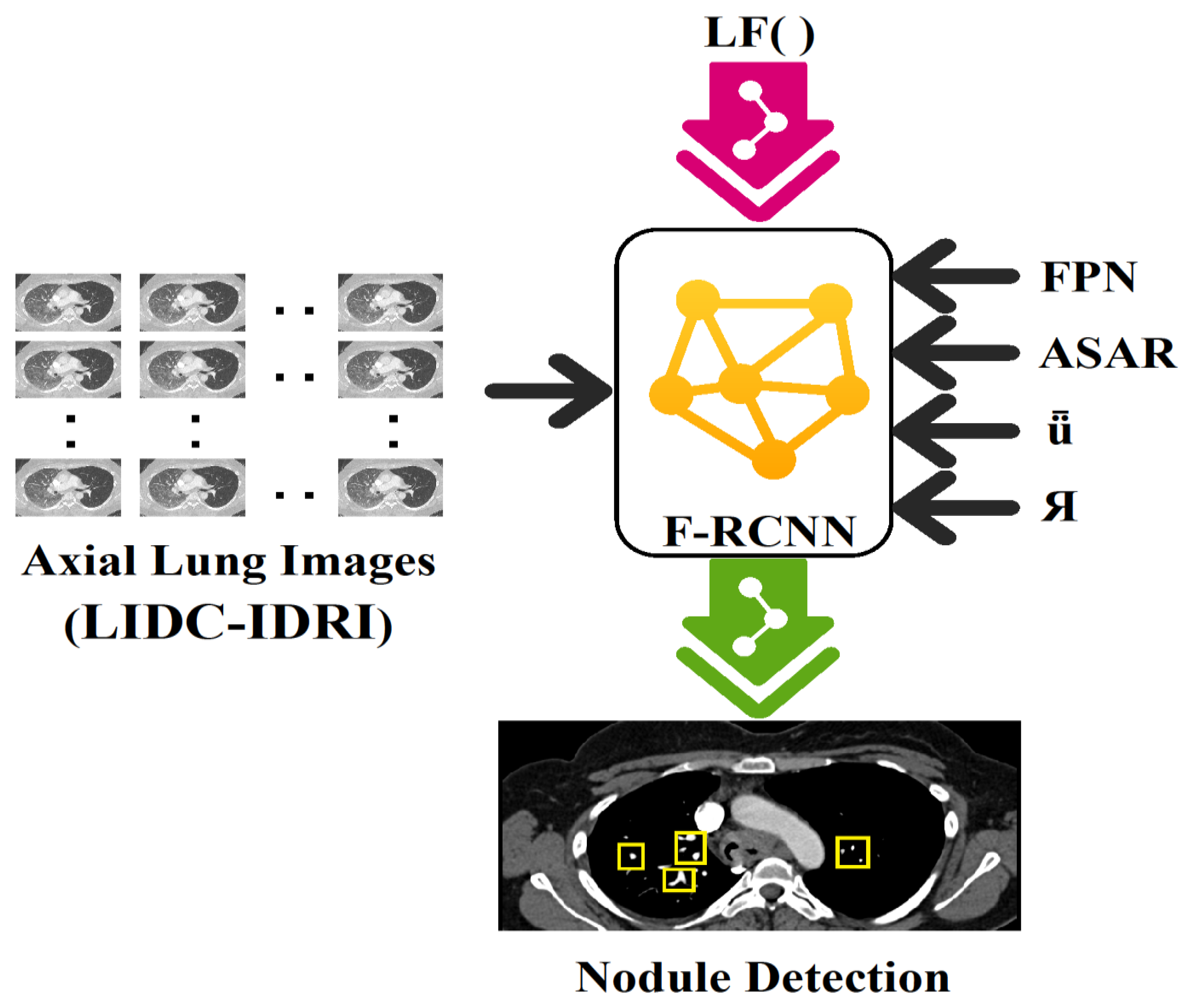
3.1. Dataset
3.2. HFRCNN Mechanism
3.2.1. Feature Pyramid Networks (FPN)
3.2.2. Adjusting Anchor Scales and Aspect Ratios (ASAR)
3.2.3. Intersection over Union (ǖ)
3.2.4. Bounding Box Regression (Я)
3.2.5. Loss Functions (LFs)
4. Implementation and Analysis
4.1. Empirical Requirements and Model Training
4.2. Performance Evaluation
- TP: True Positives (correctly detecting lung nodules);
- TN: True Negatives (correctly detecting non-nodules);
- FP: False Positives (incorrectly detecting as lung nodule cases instead of non-nodules);
- FN: False Negatives (incorrectly detecting as non-nodule cases instead of lung nodules).
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sreekumar, A.; Nair, K.R.; Sudheer, S.; Nayar, H.G.; Nair, J.J. Malignant Lung Nodule Detection using Deep Learning. In Proceedings of the 2020 International Conference on Communication and Signal Processing (ICCSP), Chennai, India, 28–30 July 2020. [Google Scholar] [CrossRef]
- Balyan, A.K.; Ahuja, S.; Lilhore, U.K.; Sharma, S.K.; Manoharan, P.; Algarni, A.D.; Elmannai, H.; Raahemifar, K. A Hybrid Intrusion Detection Model Using EGA-PSO and Improved Random Forest Method. Sensors 2022, 22, 5986. [Google Scholar] [CrossRef]
- Barbouchi, K.; El Hamdi, D.; Elouedi, I.; Ben Aïcha, T.; Echi, A.K.; Slim, I. A transformer-based deep neural network for detection and classification of lung cancer via PET/CT images. Int. J. Imaging Syst. Technol. 2023, 33, 1383–1395. [Google Scholar] [CrossRef]
- Bharati, S.; Mondal, M.R.H.; Podder, P. A Review on Explainable Artificial Intelligence for Healthcare: Why, How, and When? IEEE Trans. Artif. Intell. 2023, 1–15. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Today. Iarc.fr. Available online: https://gco.iarc.fr/today/home (accessed on 3 August 2023).
- Bharati, S.; Podder, P.; Mondal, M.R.H. Hybrid deep learning for detecting lung diseases from X-ray images. Inform. Med. Unlocked 2020, 20, 100391. [Google Scholar] [CrossRef]
- Dhiman, P.; Kukreja, V.; Manoharan, P.; Kaur, A.; Kamruzzaman, M.M.; Ben Dhaou, I.; Iwendi, C. A Novel Deep Learning Model for Detection of Severity Level of the Disease in Citrus Fruits. Electronics 2022, 11, 495. [Google Scholar] [CrossRef]
- Dai, D.; Sun, Y.; Dong, C.; Yan, Q.; Li, Z.; Xu, S. Effectively fusing clinical knowledge and AI knowledge for reliable lung nodule diagnosis. Expert Syst. Appl. 2023, 230, 120634. [Google Scholar] [CrossRef]
- Holbrook, M.D.; Clark, D.P.; Patel, R.; Qi, Y.; Bassil, A.M.; Mowery, Y.M.; Badea, C.T. Detection of Lung Nodules in Micro-CT Imaging Using Deep Learning. Tomography 2021, 7, 32. [Google Scholar] [CrossRef]
- LUNA16—Grand Challenge. Grand-Challenge.org. Available online: https://luna16.grand-challenge.org/Download/ (accessed on 3 August 2023).
- Elnakib, A.; Amer, H.M.; Abou-Chadi, F.E.Z. Early Lung Cancer Detection using Deep Learning Optimization. Int. J. Online Biomed. Eng. 2020, 16, 82. [Google Scholar] [CrossRef]
- Musthafa, A.S.; Sankar, K.; Benil, T.; Rao, Y.N. A hybrid machine learning technique for early prediction of lung nodules from medical images using a learning-based neural network classifier. Concurr. Comput. Pr. Exp. 2022, 35, e7488. [Google Scholar] [CrossRef]
- Sheriff, S.T.M.; Kumar, J.V.; Vigneshwaran, S.; Jones, A.; Anand, J. Lung Cancer Detection using VGG NET 16 Architecture. J. Phys. Conf. Ser. 2021, 2040, 012001. [Google Scholar] [CrossRef]
- Cui, S.; Ming, S.; Lin, Y.; Chen, F.; Shen, Q.; Li, H.; Chen, G.; Gong, X.; Wang, H. Development and clinical application of deep learning model for lung nodules screening on CT images. Sci. Rep. 2020, 10, 13657. [Google Scholar] [CrossRef]
- Thaseen, M.; UmaMaheswaran, S.; Naik, D.A.; Aware, M.S.; Pundhir, P.; Pant, B. A Review of Using CNN Approach for Lung Cancer Detection Through Machine Learning. In Proceedings of the 2022 2nd International Conference on Advance Computing and Innovative Technologies in Engineering (ICACITE), Greater Noida, India, 28–29 April 2022. [Google Scholar] [CrossRef]
- Prasad, P.H.S.; Daswanth, N.M.V.S.; Kumar, C.V.S.P.; Yeeramally, N.; Mohan, V.M.; Satish, T. Detection of Lung Cancer using VGG-16. In Proceedings of the 2023 7th International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 23–25 February 2023. [Google Scholar] [CrossRef]
- OpenMRS. Available online: https://openmrs.org/ (accessed on 3 August 2023).
- Li, F.; Huang, H.; Wu, Y.; Cai, C.; Huang, Y.; Ding, X. Lung Nodule Detection with a 3D ConvNet via IoU Self-normalization and Maxout Unit. In Proceedings of the ICASSP 2019–2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Brighton, UK, 12–17 May 2019. [Google Scholar] [CrossRef]
- Li, X.; Shen, L.; Xie, X.; Huang, S.; Xie, Z.; Hong, X.; Yu, J. Multi-resolution convolutional networks for chest X-ray radiograph based lung nodule detection. Artif. Intell. Med. 2019, 103, 101744. [Google Scholar] [CrossRef] [PubMed]
- Saab, S., Jr.; Fu, Y.; Ray, A.; Hauser, M. A dynamically stabilized recurrent neural network. Neural Process. Lett. 2022, 54, 1195–1209. [Google Scholar] [CrossRef]
- Lian, Z.; Zeng, Q.; Wang, W.; Gadekallu, T.R.; Su, C. Blockchain-Based Two-Stage Federated Learning with Non-IID Data in IoMT System. IEEE Trans. Comput. Soc. Syst. 2022, 10, 1701–1710. [Google Scholar] [CrossRef]
- Negi, C. Deep Learning-Based Automated Detection of Lung Cancer from CT Scans: A Comparative Study. Math. Stat. Eng. Appl. 2021, 70, 312–323. [Google Scholar] [CrossRef]
- Vohra, B.; Mittal, S. Deep Learning Paradigms for Existing and Imminent Lung Diseases Detection: A Review. J. Exp. Biol. Agric. Sci. 2023, 11, 226–235. [Google Scholar] [CrossRef]
- GNU Health. Freedom and Equity in Healthcare. Available online: https://www.gnuhealth.org/ (accessed on 3 August 2023).
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 1137–1149. [Google Scholar] [CrossRef]
- Naqi, S.M.; Sharif, M.; Yasmin, M. Multistage segmentation model and SVM-ensemble for precise lung nodule detection. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1083–1095. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Wu, W. Pyramid Focusing Network for mutation prediction and classification in CT images. arXiv 2020, arXiv:2004.03302. [Google Scholar]
- Saab, S., Jr.; Saab, K.; Phoha, S.; Zhu, M.; Ray, A. A multivariate adaptive gradient algorithm with reduced tuning efforts. Neural Netw. 2022, 152, 499–509. [Google Scholar] [CrossRef]
- Murugesan, M.; Kaliannan, K.; Balraj, S.; Singaram, K.; Kaliannan, T.; Albert, J.R. A Hybrid deep learning model for effective segmentation and classification of lung nodules from CT images. J. Intell. Fuzzy Syst. 2022, 42, 2667–2679. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; Hu, R.; Yang, B.; Liu, S.; Yin, L.; Zheng, W. Improved Feature Point Pair Purification Algorithm Based on SIFT During Endoscope Image Stitching. Front. Neurorobotics 2022, 16, 840594. [Google Scholar] [CrossRef] [PubMed]
- Candemir, S.; Jaeger, S.; Palaniappan, K.; Musco, J.P.; Singh, R.K.; Xue, Z.; Karargyris, A.; Antani, S.; Thoma, G.; McDonald, C.J. Lung Segmentation in Chest Radiographs Using Anatomical Atlases with Nonrigid Registration. IEEE Trans. Med. Imaging 2013, 33, 577–590. [Google Scholar] [CrossRef] [PubMed]
- LHNCBC. Available online: https://lhncbc.nlm.nih.gov/LHC-downloads/downloads.html#tuberculosis-image-data-sets (accessed on 3 August 2023).
- JSRT Database. Japanese Society of Radiological Technology. Available online: http://db.jsrt.or.jp/eng.php (accessed on 18 October 2023).
- Judith, A.M.; Priya, S.B.; Mahendran, R.K.; Gadekallu, T.R.; Ambati, L.S. Two-phase classification: ANN and A-SVM classifiers on motor imagery BCI. Asian J. Control 2022, 25, 3318–3329. [Google Scholar]
- Lu, S.; Liu, S.; Hou, P.; Yang, B.; Liu, M.; Yin, L.; Zheng, W. Soft Tissue Feature Tracking Based on Deep Matching Network. Comput. Model. Eng. Sci. 2023, 136, 363–379. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Yang, B.; Yin, Z.; Liu, S.; Yin, L.; Zheng, W. Three-Dimensional Modeling of Heart Soft Tissue Motion. Appl. Sci. 2023, 13, 2493. [Google Scholar] [CrossRef]
- Data from LIDC-IDRI. Available online: https://doi.org/10.7937/K9/TCIA.2015.LO9QL9SX (accessed on 3 August 2023).










| Study | Research Objective | Methodology | Outcomes Measured |
|---|---|---|---|
| [14] | Create an LDCT-based DL method for identifying lung nodules and analyzing their occurrence in China. | Deep learning algorithm: TS-DL | ROC (Receiver Operating Curves)–AUC (Area Under the Curve), Free-response ROC Score, Average Duration |
| [15] | Early identification of lung nodule anomalies using DL | U-net Design | Detection of Lung Tumor Regions, Lung Nodule Segmentation (U-Net Architecture), Lung Cancer Classification (Detecting normalcy and abnormalities) |
| [16] | Fast and accurate lung tumor detection (via a CNN) | CNN | Precision, Recall, ROC, AUC |
| [17] | Lung lesion detection and prognosis using a mixed neural network framework | ISSO-B and CASO techniques | AUC, Sensitivity, Accuracy, Specificity |
| [18] | Deep learning-based lung nodule detection method | Fusion Algorithms (FAs) and patch-based multi-resolution neural networks | Lung Nodule Detection, False Positives per Image (FPs/Image), FAUC (False Positive Area Under the Curve), R-CPM (Relative Cumulative Performance Measure) |
| [1] | Detection of malignant pulmonary nodules using deep learning from CT scans | Preprocessing pipeline to mask lung regions; feature extraction using 3D CNN based on a C3D network | Sensitivity: 86% |
| Feature | Value |
|---|---|
| Dataset Type | Medical Imaging |
| Dataset Size | 888 CT Scans |
| Source | Lung Image Database Consortium (LIDC-IDRI) |
| Annotation Type | Expert Radiologists’ Markings for Lung Nodules |
| Nodule Types | Benign and Malignant |
| Nodule Annotations | Yes |
| Nodule Sizes (in millimeters, mm) | Minimum: 3 mm Maximum: 30 mm |
| Nodule Shapes | Round, Oval, Irregular, Spiculated, Lobulated, Spherical |
| Purpose | Lung Cancer Detection and Research |
| Released By | RSNA and NCI |
| Year of Release | 2016 |
| Approaches | Specificity (%) | Sensitivity (%) | F1-Score (%) |
|---|---|---|---|
| TS-DL | 88.16 | 90.14 | 89.14 |
| CNN | 89.23 | 87.34 | 88.32 |
| ISSO-B + CASO | 90.08 | 91.11 | 91.03 |
| FA | 92.24 | 90.24 | 91.34 |
| HFRCNN | 94.32 | 94.23 | 94.54 |
| Patient Survival Rates | Early detection rates Reduction in misdiagnoses |
| Healthcare Costs | Reduction in treatment costs due to early detection Savings from minimizing unnecessary procedures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, D.; Srivastava, S.K.; Khan, S.B.; Singh, H.R.; Maakar, S.K.; Agarwal, A.K.; Malibari, A.A.; Albalawi, E. Early Detection of Lung Nodules Using a Revolutionized Deep Learning Model. Diagnostics 2023, 13, 3485. https://doi.org/10.3390/diagnostics13223485
Srivastava D, Srivastava SK, Khan SB, Singh HR, Maakar SK, Agarwal AK, Malibari AA, Albalawi E. Early Detection of Lung Nodules Using a Revolutionized Deep Learning Model. Diagnostics. 2023; 13(22):3485. https://doi.org/10.3390/diagnostics13223485
Chicago/Turabian StyleSrivastava, Durgesh, Santosh Kumar Srivastava, Surbhi Bhatia Khan, Hare Ram Singh, Sunil K. Maakar, Ambuj Kumar Agarwal, Areej A. Malibari, and Eid Albalawi. 2023. "Early Detection of Lung Nodules Using a Revolutionized Deep Learning Model" Diagnostics 13, no. 22: 3485. https://doi.org/10.3390/diagnostics13223485
APA StyleSrivastava, D., Srivastava, S. K., Khan, S. B., Singh, H. R., Maakar, S. K., Agarwal, A. K., Malibari, A. A., & Albalawi, E. (2023). Early Detection of Lung Nodules Using a Revolutionized Deep Learning Model. Diagnostics, 13(22), 3485. https://doi.org/10.3390/diagnostics13223485







