Comparative Evaluation of a Standard M10 Assay with Xpert Xpress for the Rapid Molecular Diagnosis of SARS-CoV-2, Influenza A/B Virus, and Respiratory Syncytial Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Study Design
2.2. Index Test
2.3. Comparator Test
2.4. Standard Tests
2.4.1. RT-qPCR for SARS-CoV-2
2.4.2. Immunofluorescence Assay
2.4.3. Virus Culture
2.5. Study Endpoint and Data Analysis
3. Results
3.1. Final Sample Size and Diagnostic Performance of STANDARD M10
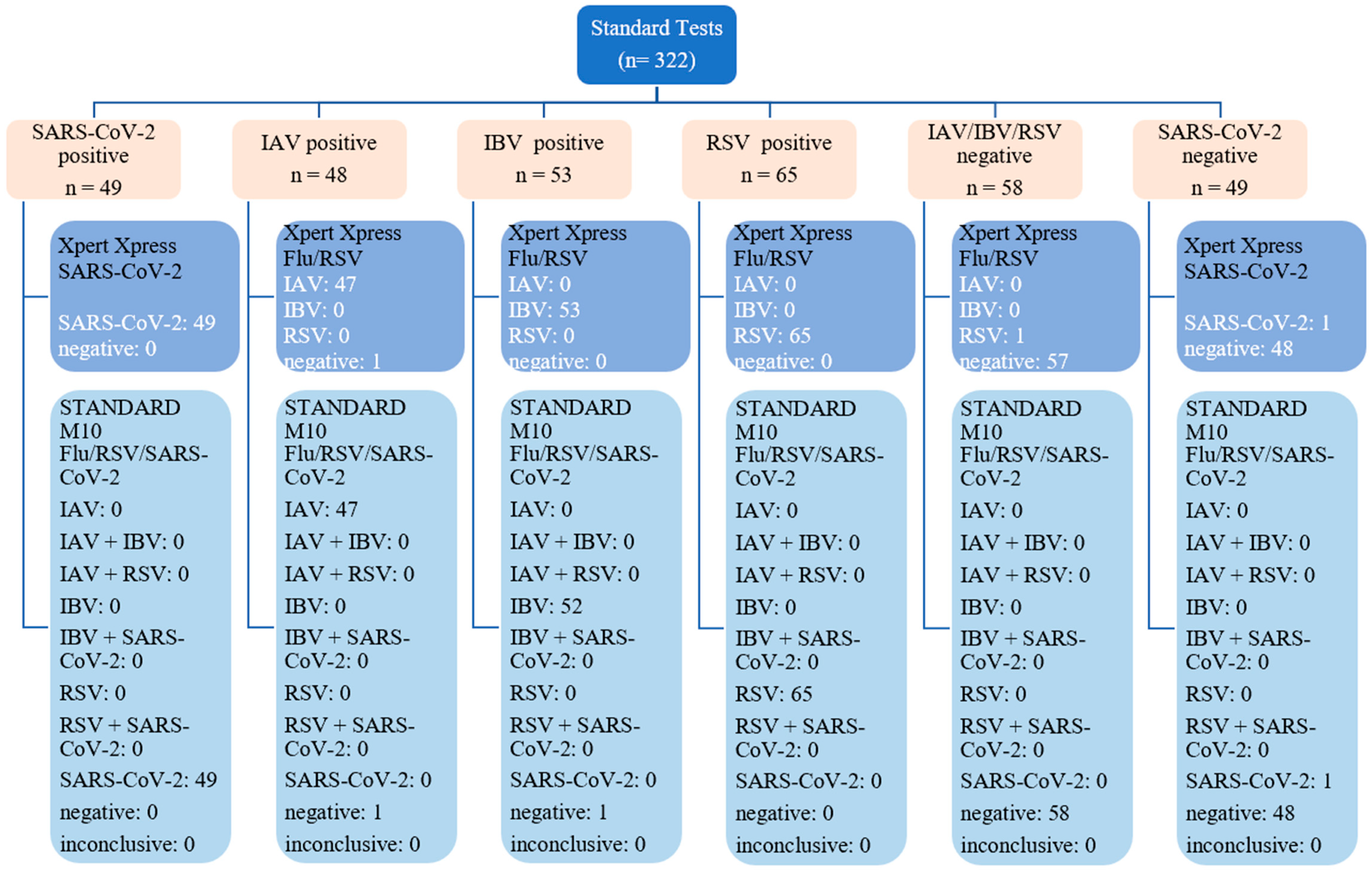
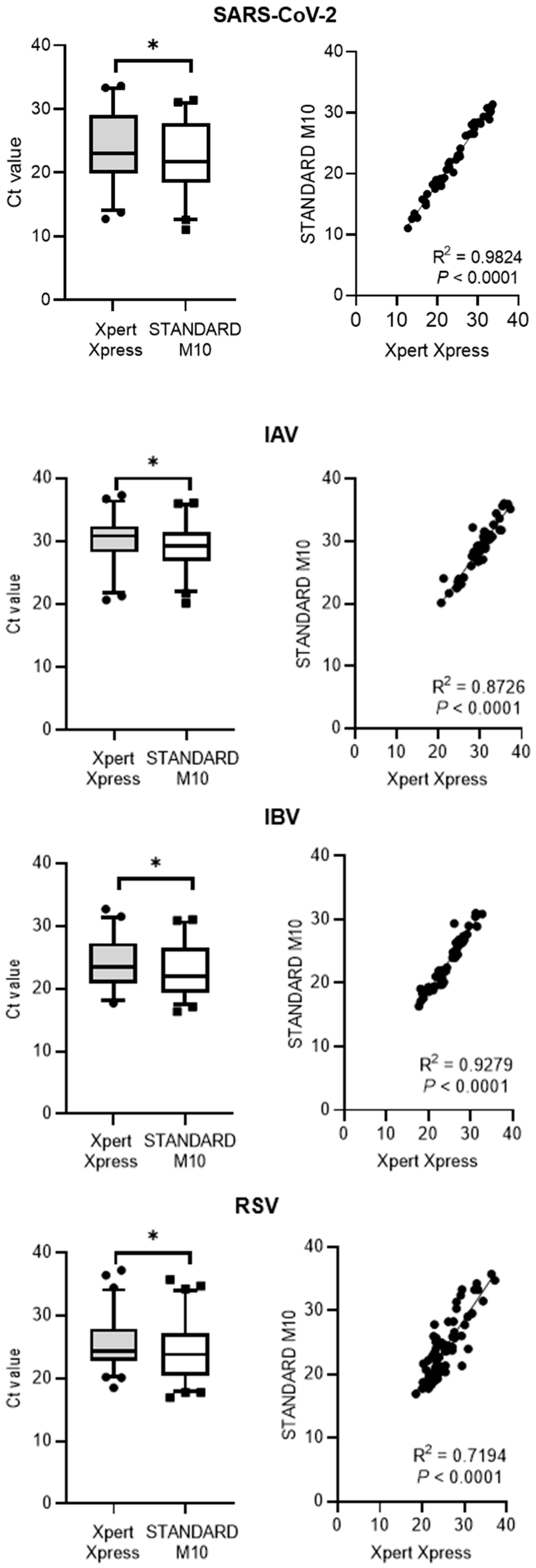
3.2. The Correlation between STANDARD M10 and Either Xpert Xpress SARS-CoV-2 or Xpert Xpress Flu/RSV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmerman, R.K.; Balasubramani, G.K.; D’Agostino, H.; Clarke, L.; Yassin, M.; Middleton, D.B.; Silveira, F.P.; Wheeler, N.; Landis, J.; Peterson, A.; et al. Population-based Hospitalization Burden Estimates for Respiratory Viruses, 2015–2019. Influenza Other Respir. Viruses 2022, 16, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Avni, Y.S.; O’Rourke, N.; Michael, T. Prevalence of Common Infectious Diseases after COVID-19 Vaccination and Easing of Pandemic Restrictions in Israel. JAMA Netw. Open 2022, 5, e2146175. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.-S.; Sam, I.-C.; Hooi, P.S.; Quek, K.F.; Chan, Y.F. Epidemiology and Seasonality of Respiratory Viral Infections in Hospitalized Children in Kuala Lumpur, Malaysia: A Retrospective Study of 27 Years. BMC Pediatr. 2012, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Loeffelholz, M.J.; Alland, D.; Butler-Wu, S.M.; Pandey, U.; Perno, C.F.; Nava, A.; Carroll, K.C.; Mostafa, H.H.; Davies, E.; McEwan, A.; et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-COV-2 Test. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.Y.; Jeong, H.; Jung, J.; Kim, E.S.; Park, K.U.; Kim, H.B.; Park, J.S.; Song, K.H. Performance of STANDARD M10 SARS-CoV-2 Assay for the Diagnosis of COVID-19 from a Nasopharyngeal Swab. Infect. Chemother. 2022, 54, 360. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.M.; Tan, X.H.; Hooi, P.S.; Lee, L.M.; Sam, I.-C.; Chan, Y.F. Evaluation of Rapid Influenza Diagnostic Tests for Influenza A and B in the Tropics. J. Med. Virol. 2019, 91, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Bruzzone, B.; Trombetta, C.-S.; De Pace, V.; Ricucci, V.; Varesano, S.; Garzillo, G.; Ogliastro, M.; Orsi, A.; Icardi, G. Rapid Differential Diagnosis of SARS-CoV-2, Influenza A/B and Respiratory Syncytial Viruses: Validation of a Novel RT-PCR Assay. J. Clin. Virol. 2023, 161, 105402. [Google Scholar] [CrossRef] [PubMed]
- Cepheid. GeneXpert®: Xpert® Xpress SARS-CoV-2, 2022. Instructions for Use: For Use with GeneXpert Dx System or GeneXpert Infinity System. Available online: https://www.cepheid.com/content/dam/www-cepheid-com/documents/package-insert-files/xpress-sars-cov-2/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3562%20Rev.%20G.pdf (accessed on 15 July 2023).
- Cepheid. GeneXpert®: Xpert® Xpress Flu/RSV, 2020. Instructions for Use: For Use with GeneXpert Xpress System (Point of Care System). Available online: https://www.cepheid.com/content/dam/www-cepheid-com/documents/package-insert-files/Xpress-Flu-RSV-US-IVD-ENGLISH-Package-Insert-301-7239-Rev.%20D.pdf (accessed on 15 July 2023).
- World Health Organization. WHO Information for the Molecular Detection of Influenza Viruses. 2017. Available online: https://www.who.int/influenza/gisrs_laboratory/WHO_information_for_the_molecular_detection_of_influenza_viruses_20171023_Final.pdf?ua=1 (accessed on 15 July 2023).
- Cobas®. Cobas® SARS-CoV-2, 2022. Qualitative Assay for Use on the Cobas® 6800/8800 Systems. Available online: https://www.fda.gov/media/136049/download (accessed on 15 July 2023).
- Seegene. Allplex™ SARS-CoV-2 Assay, 2022. Detection and Identification of 4 Target Genes for SARS-CoV-2 Using Multiplex Real-Time PCR. Available online: http://www.seegene.com/upload/images/sars_cov_2/allplex_sars_cov_2_assay_200917.pdf (accessed on 15 July 2023).
- World Health Organization. Regional Office for South-East Asia, 2017. Use of Cell Culture in Virology for Developing Countries in the South-East Asia Region. Available online: https://apps.who.int/iris/handle/10665/258809 (accessed on 15 July 2023).
- Renzoni, A.; Pérez, F.F.G.; Nsoga, M.T.N.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-COV-2. Diagnostics 2021, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Natori, Y.; Nagakubo, Y.; Hosaka, K.; Sueki, H.; Amemiya, K.; Hayakawa, M.; Mochizuki, H.; et al. Direct Comparison of Xpert Xpress, FilmArray Respiratory Panel, Lumipulse Antigen Test, and RT-QPCR in 165 Nasopharyngeal Swabs. BMC Infect. Dis. 2022, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, J.-U. Diagnostic Accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW Assay for Rapid Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. J. Med. Virol. 2021, 93, 4523–4531. [Google Scholar] [CrossRef] [PubMed]
- Kogoj, R.; Korva, M.; Knap, N.; Rus, K.R.; Pozvek, P.; Avšič-Županc, T.; Poljak, M. Comparative Evaluation of Six SARS-COV-2 Real-Time RT-PCR Diagnostic Approaches Shows Substantial Genomic Variant–Dependent Intra- and Inter-Test Variability, Poor Interchangeability of Cycle Threshold and Complementary Turn-Around Times. Pathogens 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Tirupathi, R.; Sule, A.A.; Aldali, J.A.; Mutair, A.A.; Alhumaid, S.; Muzaheed; Gupta, N.; Koritala, T.; Adhikari, R.; et al. Viral Dynamics and Real-Time RT-PCR Ct Values Correlation with Disease Severity in COVID-19. Diagnostics 2021, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
| Virus/Parameters % (95% CI) | Xpert Xpress SARS-CoV-2/Xpert Xpress Flu/RSV | STANDARD M10 |
|---|---|---|
| SARS-CoV-2 | ||
| Sensitivity | 100.0 (92.8–100.0) | 100.0 (92.8–100.0) |
| Specificity | 98.0 (89.2–100.0) | 98.0 (89.2–100.0) |
| PPV | 98.0 (89.4–100.0) | 98.0 (89.4–100.0) |
| NPV | 100.0 (92.6–100.0) | 100.0 (92.6–100.0) |
| Accuracy | 99.0 (94.5–100.0) | 99.0 (94.5–100.0) |
| IAV | ||
| Sensitivity | 97.9 (88.9–100.0) | 97.9 (88.9–100.0) |
| Specificity | 100.0 (93.8–100.0) | 100.0 (93.8–100.0) |
| PPV | 100.0 (92.5–100.0) | 100.0 (92.5–100.0) |
| NPV | 98.3 (90.9–100.0) | 98.3 (90.9–100.0) |
| Accuracy | 99.1 (94.9–100.0) | 99.1 (94.9–100.0) |
| IBV | ||
| Sensitivity | 100.0 (93.3–100.0) | 98.1 (89.9–100.0) |
| Specificity | 100.0 (93.8–100.0) | 100.0 (93.8–100.0) |
| PPV | 100.0 (93.3–100.0) | 100.0 (93.2–100.0) |
| NPV | 100.0 (93.8–100.0) | 98.3 (90.9–100.0) |
| Accuracy | 100.0 (96.7–100.0) | 99.1 (95.1–100.0) |
| RSV | ||
| Sensitivity | 100.0 (94.5–100.0) | 100.0 (94.5–100.0) |
| Specificity | 98.3 (90.8–100.0) | 100.0 (93.8–100.0) |
| PPV | 98.5 (91.8–100.0) | 100.0 (94.5–100.0) |
| NPV | 100.0 (93.7–100.0) | 100.0 (93.8–100.0) |
| Accuracy | 99.2 (95.6–100.0) | 100.0 (97.1–100.0) |
| Test Assay | Virus | Target Gene | Ct Value Range (Median ± Interquartile Range) |
|---|---|---|---|
| Xpert Xpress SARS-CoV-2 or Xpert Xpress Flu/RSV | SARS-CoV-2 | E | 12.10–32.40 (22.00 ± 8.30) |
| N2 | 13.40–34.90 (24.30 ± 9.20) | ||
| IAV | A1 | 19.80–36.70 (30.00 ± 4.05) | |
| A2 | 21.50–38.50 (31.60 ± 3.40) | ||
| IBV | IBV | 17.70–32.70 (23.60 ± 6.10) | |
| RSV | RSV | 18.50–37.20 (24.30 ± 4.80) | |
| STANDARD M10 | SARS-CoV-2 | ORF1ab | 11.60–31.02 (22.01 ± 8.56) |
| N | 10.52–31.77 (21.3 ± 9.22) | ||
| IAV | IAV | 20.14–35.95 (29.26 ± 4.25) | |
| IBV | IBV | 16.35–30.99 (21.93 ± 7.07) | |
| RSV | RSV | 16.92–35.71 (23.77 ± 6.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, A.; Sam, I.-C.; Ong, Y.J.; Theo, C.H.; Pukhari, M.H.; Chan, Y.F. Comparative Evaluation of a Standard M10 Assay with Xpert Xpress for the Rapid Molecular Diagnosis of SARS-CoV-2, Influenza A/B Virus, and Respiratory Syncytial Virus. Diagnostics 2023, 13, 3507. https://doi.org/10.3390/diagnostics13233507
Abdullah A, Sam I-C, Ong YJ, Theo CH, Pukhari MH, Chan YF. Comparative Evaluation of a Standard M10 Assay with Xpert Xpress for the Rapid Molecular Diagnosis of SARS-CoV-2, Influenza A/B Virus, and Respiratory Syncytial Virus. Diagnostics. 2023; 13(23):3507. https://doi.org/10.3390/diagnostics13233507
Chicago/Turabian StyleAbdullah, Azwani, I-Ching Sam, Yin Jie Ong, Chun Hao Theo, Muhammad Harith Pukhari, and Yoke Fun Chan. 2023. "Comparative Evaluation of a Standard M10 Assay with Xpert Xpress for the Rapid Molecular Diagnosis of SARS-CoV-2, Influenza A/B Virus, and Respiratory Syncytial Virus" Diagnostics 13, no. 23: 3507. https://doi.org/10.3390/diagnostics13233507
APA StyleAbdullah, A., Sam, I.-C., Ong, Y. J., Theo, C. H., Pukhari, M. H., & Chan, Y. F. (2023). Comparative Evaluation of a Standard M10 Assay with Xpert Xpress for the Rapid Molecular Diagnosis of SARS-CoV-2, Influenza A/B Virus, and Respiratory Syncytial Virus. Diagnostics, 13(23), 3507. https://doi.org/10.3390/diagnostics13233507






