Machine Learning Radiomics Signature for Differentiating Lymphoma versus Benign Splenomegaly on CT
Abstract
:1. Introduction
2. Related Work
3. Materials and Methods
3.1. Study Patients
3.2. CT Image Acquisition
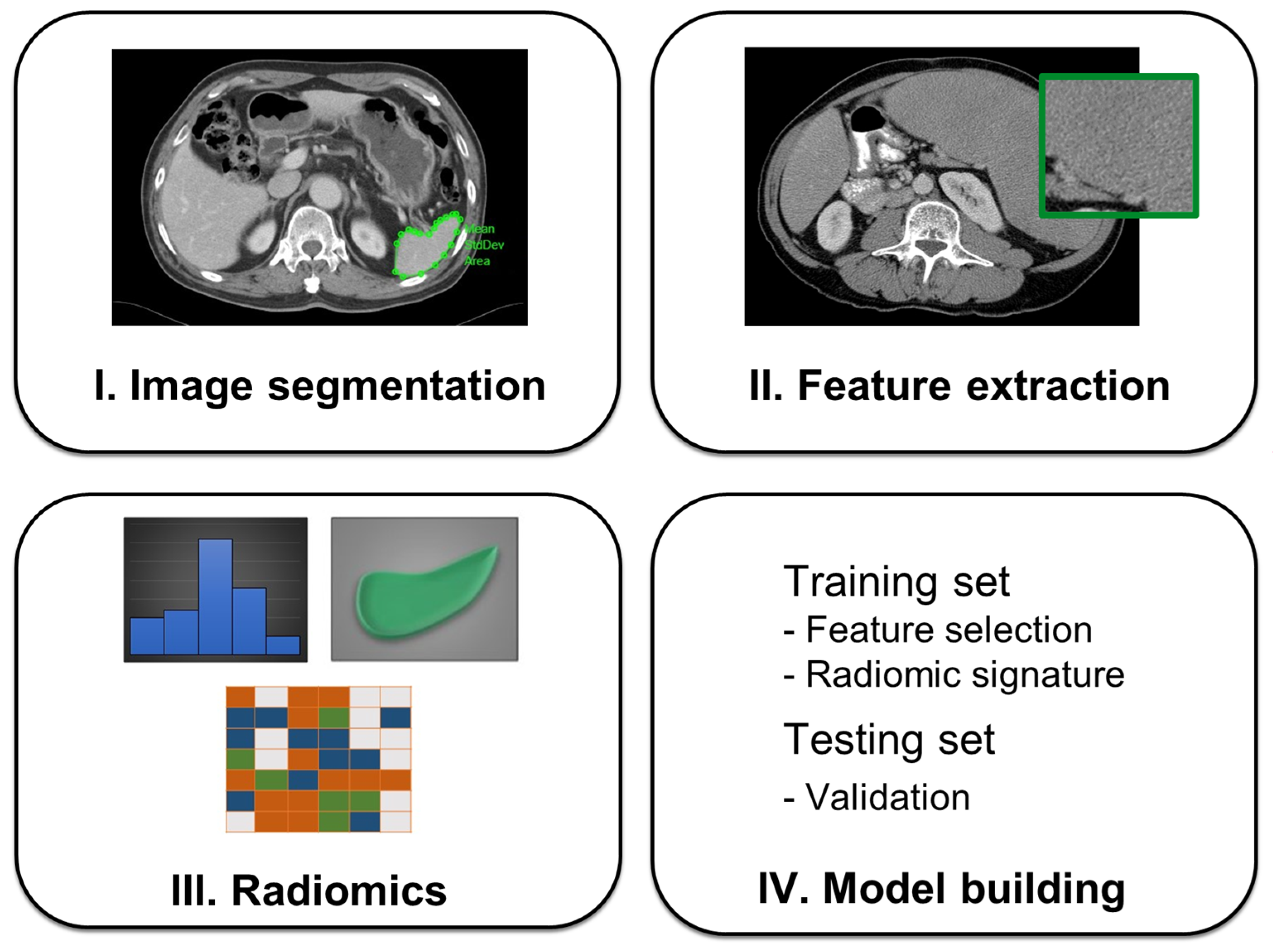
3.3. Radiomics Feature Extraction
3.4. Splenectomy Cause
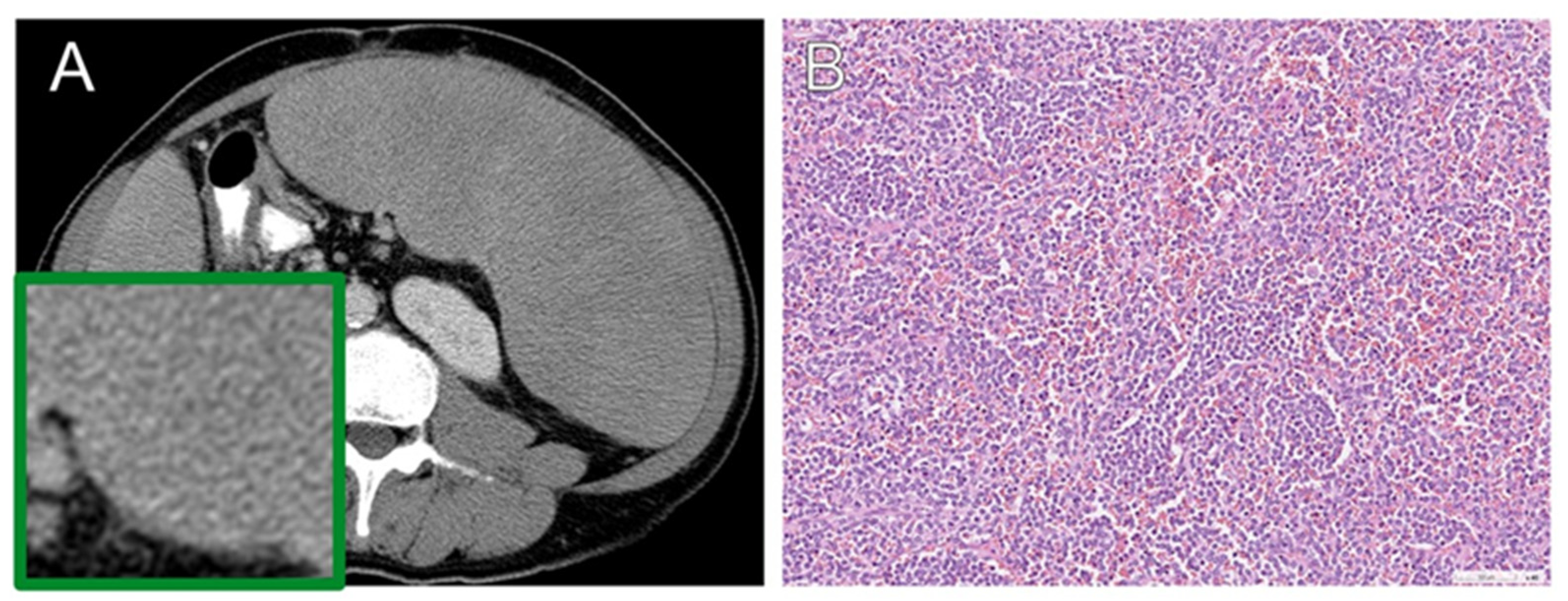
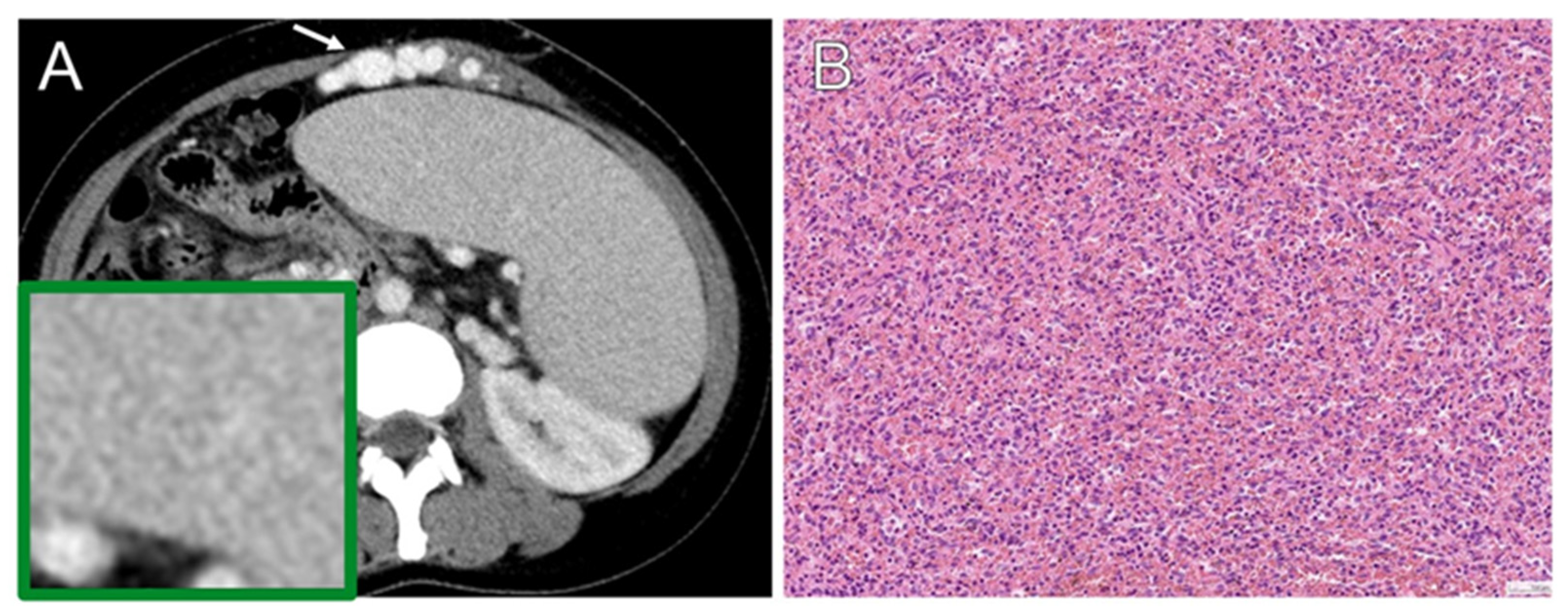
3.5. Histopathology
3.6. Complete Blood Cell Profile
3.7. Statistical Analysis
4. Results
4.1. Patient Characteristics
4.2. Radiomics Feature Comparison
4.3. Radiomics Feature Selection, Predictive Model Development, and Discriminative Ability Evaluation
4.4. Correlation between Radiomic Signature and CBC/DC Profile
5. Discussion
6. Study Limitations and Future Work
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sjoberg, B.P.; Menias, C.O.; Lubner, M.G.; Mellnick, V.M.; Pickhardt, P.J. Splenomegaly: A Combined Clinical and Radiologic Approach to the Differential Diagnosis. Gastroenterol. Clin. N. Am. 2018, 47, 643–666. [Google Scholar] [CrossRef] [PubMed]
- Saboo, S.S.; Krajewski, K.M.; O’Regan, K.N.; Giardino, A.; Brown, J.R.; Ramaiya, N.; Jagannathan, J.P. Spleen in haematological malignancies: Spectrum of imaging findings. Br. J. Radiol. 2012, 85, 81–92. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.V.; Colonne, C.K.; Yeo, J.H.; Fraser, S.T. Splenomegaly: Pathophysiological bases and therapeutic options. Int. J. Biochem. Cell Biol. 2018, 94, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Cho, J.S.; Shin, K.S.; Kim, S.S.; You, S.K.; Park, J.W.; Shin, H.S.; Yoon, Y.C. Diffuse Infiltrative Splenic Lymphoma: Diagnostic Efficacy of Arterial-Phase CT. Korean J. Radiol. 2016, 17, 734–741. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.; Kielar, A.Z.; Macdonald, D.B. Percutaneous image-guided biopsy of the spleen: Systematic review and meta-analysis of the complication rate and diagnostic accuracy. Radiology 2011, 260, 699–708. [Google Scholar] [CrossRef]
- Singh, A.K.; Shankar, S.; Gervais, D.A.; Hahn, P.F.; Mueller, P.R. Image-guided percutaneous splenic interventions. Radiographics 2012, 32, 523–534. [Google Scholar] [CrossRef]
- Reinert, C.P.; Hinterleitner, C.; Fritz, J.; Nikolaou, K.; Horger, M. Diagnosis of diffuse spleen involvement in haematological malignancies using a spleen-to-liver attenuation ratio on contrast-enhanced CT images. Eur. Radiol. 2019, 29, 450–457. [Google Scholar] [CrossRef]
- Schaefer, N.G.; Hany, T.F.; Taverna, C.; Seifert, B.; Stumpe, K.D.; von Schulthess, G.K.; Goerres, G.W. Non-Hodgkin lymphoma and Hodgkin disease: Coregistered FDG PET and CT at staging and restaging--do we need contrast-enhanced CT? Radiology 2004, 232, 823–829. [Google Scholar] [CrossRef]
- Rao, L.; Wang, X.; Zong, Z.; Chen, Z.; Shi, X.; Yi, C.; Zhang, X.; Yang, Z. PET-CT for Evaluation of Spleen and Liver 18F-FDG Diffuse Uptake without Lymph Node Enlargement in Lymphoma. Medicine 2016, 95, e3750. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Rogasch, J.M.M.; Kim, D.; Rießelmann, J.; Furth, C.; Amthauer, H.; Hamm, B.; Steffen, I.G.; Elgeti, T.; Nagel, S.N. CT radiomics to predict Deauville score 4 positive and negative Hodgkin lymphoma manifestations. Sci. Rep. 2022, 12, 20008. [Google Scholar] [CrossRef]
- Starmans, M.P.A.; Miclea, R.L.; Vilgrain, V.; Ronot, M.; Purcell, Y.; Verbeek, J.; Niessen, W.J.; Ijzermans, J.N.M.; de Man, R.A.; Doukas, M.; et al. Automated Assessment of T2-Weighted MRI to Differentiate Malignant and Benign Primary Solid Liver Lesions in Noncirrhotic Livers Using Radiomics. Acad. Radiol. 2023. [Google Scholar] [CrossRef]
- Nieri, A.; Manco, L.; Bauckneht, M.; Urso, L.; Caracciolo, M.; Donegani, M.I.; Borgia, F.; Vega, K.; Colella, A.; Ippolito, C.; et al. [18F]FDG PET-TC radiomics and machine learning in the evaluation of prostate incidental uptake. Expert. Rev. Med. Devices 2023, 20, 1183–1191. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, X.; Zhao, J.; Peng, Y.; Yao, X.; Hu, X.; Cui, J.; Chen, H.; Chen, X.; Wu, J.; et al. A Machine Learning-Based Unenhanced Radiomics Approach to Distinguishing between Benign and Malignant Breast Lesions Using T2-Weighted and Diffusion-Weighted MRI. J. Magn. Reson. Imaging 2023. [Google Scholar] [CrossRef] [PubMed]
- Selvam, M.; Chandrasekharan, A.; Sadanandan, A.; Anand, V.K.; Murali, A.; Krishnamurthi, G. Radiomics as a non-invasive adjunct to Chest CT in distinguishing benign and malignant lung nodules. Sci. Rep. 2023, 13, 19062. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Qiu, J.; Xie, J.; Lu, W.; Ma, J.; Jia, M. Machine learning based on SPECT/CT to differentiate bone metastasis and benign bone lesions in lung malignancy patients. Med. Phys. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Hectors, S.; Taouli, B. Radiomics of hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 111–123. [Google Scholar] [CrossRef]
- Bhandari, A.; Ibrahim, M.; Sharma, C.; Liong, R.; Gustafson, S.; Prior, M. CT-based radiomics for differentiating renal tumours: A systematic review. Abdom. Radiol. 2021, 46, 2052–2063. [Google Scholar] [CrossRef]
- Reinert, C.P.; Kloth, C.; Fritz, J.; Nikolaou, K.; Horger, M. Discriminatory CT-textural features in splenic infiltration of lymphoma versus splenomegaly in liver cirrhosis versus normal spleens in controls and evaluation of their role for longitudinal lymphoma monitoring. Eur. J. Radiol. 2018, 104, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Zhang, W.; Yang, X.; Zhu, C.; Pan, B.; Zeng, Y.; Xu, J.; Chen, X.; Shen, X. Use of radiomics to extract splenic features to predict prognosis of patients with gastric cancer. Eur. J. Surg. Oncol. 2020, 46, 1932–1940. [Google Scholar] [CrossRef]
- Yin, Y.; Yakar, D.; Dierckx, R.A.J.O.; Mouridsen, K.B.; Kwee, T.C.; de Haas, R.J. Combining Hepatic and Splenic CT Radiomic Features Improves Radiomic Analysis Performance for Liver Fibrosis Staging. Diagnostics 2022, 12, 550. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Fan, C.; Zhang, Y.; Zhang, S.; Wang, Z.; Huang, T.; Ding, Z.; Hu, K.; Li, L.; et al. A novel machine learning-based radiomic model for diagnosing high bleeding risk esophageal varices in cirrhotic patients. Hepatol. Int. 2022, 16, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Liang, P.; Huang, C.; Chen, X.; Cheng, M.; Zhu, B.; Liu, M.; Yue, S.; Gao, J. Are radiomic spleen features useful for assessing the differentiation status of advanced gastric cancer? Front. Oncol. 2023, 13, 1167602. [Google Scholar] [CrossRef]
- Tseng, Y.; Ma, L.; Li, S.; Luo, T.; Luo, J.; Zhang, W.; Wang, J.; Chen, S. Application of CT-based radiomics in predicting portal pressure and patient outcome in portal hypertension. Eur. J. Radiol. 2020, 126, 108927. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Zhang, W.; Chen, W.; Zheng, J.; Yang, X.; Sun, J.; Sun, X.; Chen, X.; Shen, X. Establishment of the Radiologic Tumor Invasion Index Based on Radiomics Splenic Features and Clinical Factors to Predict Serous Invasion of Gastric Cancer. Front. Oncol. 2021, 11, 682456. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Wei, Y.; Feng, X.; Kang, B.; Wang, X.; Qi, J.; Zhao, X.; Zhu, Q. CT-Based Radiomics Score Can Accurately Predict Esophageal Variceal Rebleeding in Cirrhotic Patients. Front. Med. 2021, 8, 745931. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Gao, J.; Gan, W.; Xie, W.B. Clinical-radiomics nomogram for predicting esophagogastric variceal bleeding risk noninvasively in patients with cirrhosis. World J. Gastroenterol. 2023, 29, 1076–1089. [Google Scholar] [CrossRef]
- Li, P.; Wu, L.; Li, Z.; Li, J.; Ye, W.; Shi, Z.; Xu, Z.; Zhu, C.; Ye, H.; Liu, Z.; et al. Spleen Radiomics Signature: A Potential Biomarker for Prediction of Early and Late Recurrences of Hepatocellular Carcinoma After Resection. Front. Oncol. 2021, 11, 716849. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, Y.; Yu, D.; Liu, Z.; Gao, Y.; Qiao, J. A Multi-Organ Fusion and LightGBM Based Radiomics Algorithm for High-Risk Esophageal Varices Prediction in Cirrhotic Patients. IEEE Access 2021, 9, 15041–15052. [Google Scholar] [CrossRef]
- Batur, A.; Kılınçer, A.; Ateş, F.; Aktuğ Demir, N.; Ergün, R. Evaluation of systemic involvement of Coronavirus disease 2019 through spleen; size and texture analysis. Turk. J. Med. Sci. 2021, 51, 972–980. [Google Scholar] [CrossRef]
- Enke, J.S.; Moltz, J.H.; D’Anastasi, M.; Kunz, W.G.; Schmidt, C.; Maurus, S.; Mühlberg, A.; Katzmann, A.; Sühling, M.; Hahn, H.; et al. Radiomics Features of the Spleen as Surrogates for CT-Based Lymphoma Diagnosis and Subtype Differentiation. Cancers 2022, 14, 713. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Lu, X.; Kan, Y.; Wang, W.; Zhang, S.; Liu, L.; Zhang, H.; Li, J.; Yang, J. Development and Validation of a Nomogram Based on 18F-FDG PET/CT Radiomics to Predict the Overall Survival in Adult Hemophagocytic Lymphohistiocytosis. Front. Med. 2021, 8, 792677. [Google Scholar] [CrossRef]
- Indiran, V.; Vinod Singh, N.; Ramachandra Prasad, T.; Maduraimuthu, P. Does coronal oblique length of spleen on CT reflect splenic index? Abdom. Radiol. 2017, 42, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.; Clements, J.; Cody, D.; Gress, D.; Kanal, K.; Kofler, J.; McNitt-Gray, M.F.; Norweck, J.; Pfeiffer, D.; Ruckdeschel, T.G.; et al. Computed Tomography—Quality Control Manual; American Colloges of Radiology: Reston, VA, USA, 2017. [Google Scholar]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- WHO. Classification of Tumours Editorial Board. Hematolymphoid Tumors [Internet; Beta Version Ahead of Print. (In Progress)]. Lyon. (France): International Agency for Research on Cancer; [Cited 2022 Aug 29], 5th ed.; WHO Classification of Tumors Series; WHO: Geneva, Switzerland, 2022.
- Johnson, S.A.; Kumar, A.; Matasar, M.J.; Schöder, H.; Rademaker, J. Imaging for Staging and Response Assessment in Lymphoma. Radiology 2015, 276, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Olszewski, A.J. Diagnostic and therapeutic splenectomy for splenic lymphomas: Analysis of the National Cancer Data Base. Hematology 2019, 24, 378–386. [Google Scholar] [CrossRef]
- Vancauwenberghe, T.; Snoeckx, A.; Vanbeckevoort, D.; Dymarkowski, S.; Vanhoenacker, F.M. Imaging of the spleen: What the clinician needs to know. Singap. Med. J. 2015, 56, 133–144. [Google Scholar] [CrossRef]
- Chen, D.B.; Shen, D.H.; Yang, S.M.; Fang, X.Z. Clinicopathological features of splenic tumours of lymphoid tissue. Pathol. Res. Pr. 2018, 214, 1952–1958. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Wu, N.; Huang, W.; Lv, N. Imaging findings of primary splenic lymphoma: A review of 17 cases in which diagnosis was made at splenectomy. PLoS ONE 2013, 8, e80264. [Google Scholar] [CrossRef]
- Leite, L.A.; Domingues, A.L.; Lopes, E.P.; Ferreira, R.D.; Pimenta Filho, A.D.; Fonseca, C.S.; Santos, B.S.; Lima, V.L. Relationship between splenomegaly and hematologic findings in patients with hepatosplenic schistosomiasis. Rev. Bras. Hematol. Hemoter. 2013, 35, 332–336. [Google Scholar] [CrossRef]
- Ishigami, M.; Ishizu, Y.; Onishi, Y.; Kamei, H.; Kiuchi, T.; Itoh, A.; Hirooka, Y.; Katano, Y.; Goto, H. Long-term dynamics of hematological data and spleen volume in cirrhotic patients after liver transplantation-various dynamics depending on etiology. Springerplus 2013, 2, 374. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, T.B.; Domingues, A.L.; Luna, C.F.; Lopes, E.P. Correlation between platelet count and both liver fibrosis and spleen diameter in patients with schistosomiasis mansoni. Arq. Gastroenterol. 2014, 51, 34–38. [Google Scholar] [CrossRef]
- Ortega, C.; Eshet, Y.; Prica, A.; Anconina, R.; Johnson, S.; Constantini, D.; Keshavarzi, S.; Kulanthaivelu, R.; Metser, U.; Veit-Haibach, P. Combination of FDG PET/CT Radiomics and Clinical Parameters for Outcome Prediction in Patients with Hodgkin’s Lymphoma. Cancers 2023, 15, 2056. [Google Scholar] [CrossRef]
- Yan, J.; Chu-Shern, J.L.; Loi, H.Y.; Khor, L.K.; Sinha, A.K.; Quek, S.T.; Tham, I.W.; Townsend, D. Impact of Image Reconstruction Settings on Texture Features in 18F-FDG PET. J. Nucl. Med. 2015, 56, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T. Intravenous contrast medium administration and scan timing at CT: Considerations and approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Ahn, Y.; Yoon, J.S.; Lee, S.S.; Suk, H.I.; Son, J.H.; Sung, Y.S.; Lee, Y.; Kang, B.K.; Kim, H.S. Deep Learning Algorithm for Automated Segmentation and Volume Measurement of the Liver and Spleen Using Portal Venous Phase Computed Tomography Images. Korean J. Radiol. 2020, 21, 987–997. [Google Scholar] [CrossRef]
- Humpire-Mamani, G.E.; Bukala, J.; Scholten, E.T.; Prokop, M.; van Ginneken, B.; Jacobs, C. Fully Automatic Volume Measurement of the Spleen at CT Using Deep Learning. Radiol. Artif. Intell. 2020, 2, e190102. [Google Scholar] [CrossRef]

| Characteristics | Datum |
|---|---|
| Age (y) * | 54.3 ± 13.5 |
| Sex | |
| Male | 79 (57) |
| Female | 60 (43) |
| Pathological Diagnosis | |
| Lymphoma | |
| Splenic marginal zone lymphoma | 8 (5.8) |
| Diffuse large B cell lymphoma | 5 (3.6) |
| Follicular lymphoma | 2 (1.5) |
| Diffuse red pulp small B cell lymphoma | 1 (0.7) |
| Hepatosplenic T cell lymphoma | 1 (0.7) |
| Classical Hodgkin lymphoma | 1 (0.7) |
| Hairy cell leukemia variant | 1 (0.7) |
| Benign | |
| Congestion | 67 (48.2) |
| No cancer involvement | 53 (38.1) |
| (%) | Accuracy | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Training cohort | 98 (93–100) | 90 (56–100) | 100 (95–100) | 100 (66–100) | 98 (92–100) |
| Testing cohort | 97 (89–100) | 89 (52–100) | 98 (90–100) | 89 (52–100) | 90 (90–100) |
| Radiomic Signature | ||
|---|---|---|
| p Value | r Value | |
| WBC | 0.097 | 0.141 |
| RBC | 0.588 | 0.046 |
| Hemoglobin | 0.757 | −0.027 |
| Hematocrit | 0.899 | −0.011 |
| MCV | 0.178 | −0.115 |
| MCH | 0.168 | −0.118 |
| MCHC | 0.308 | −0.087 |
| RDW | 0.678 | 0.036 |
| Platelets | 0.021 * | −0.196 |
| Seg | 0.016 * | −0.212 |
| Lymph | 0.891 | 0.012 |
| Mono | 0.138 | 0.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.-A.; Lin, Y.-C.; Lin, Y.; Wu, R.-C.; Lu, H.-Y.; Yang, L.-Y.; Chiang, H.-J.; Juan, Y.-H.; Lai, Y.-C.; Lin, G. Machine Learning Radiomics Signature for Differentiating Lymphoma versus Benign Splenomegaly on CT. Diagnostics 2023, 13, 3632. https://doi.org/10.3390/diagnostics13243632
Cheng J-A, Lin Y-C, Lin Y, Wu R-C, Lu H-Y, Yang L-Y, Chiang H-J, Juan Y-H, Lai Y-C, Lin G. Machine Learning Radiomics Signature for Differentiating Lymphoma versus Benign Splenomegaly on CT. Diagnostics. 2023; 13(24):3632. https://doi.org/10.3390/diagnostics13243632
Chicago/Turabian StyleCheng, Jih-An, Yu-Chun Lin, Yenpo Lin, Ren-Chin Wu, Hsin-Ying Lu, Lan-Yan Yang, Hsin-Ju Chiang, Yu-Hsiang Juan, Ying-Chieh Lai, and Gigin Lin. 2023. "Machine Learning Radiomics Signature for Differentiating Lymphoma versus Benign Splenomegaly on CT" Diagnostics 13, no. 24: 3632. https://doi.org/10.3390/diagnostics13243632
APA StyleCheng, J.-A., Lin, Y.-C., Lin, Y., Wu, R.-C., Lu, H.-Y., Yang, L.-Y., Chiang, H.-J., Juan, Y.-H., Lai, Y.-C., & Lin, G. (2023). Machine Learning Radiomics Signature for Differentiating Lymphoma versus Benign Splenomegaly on CT. Diagnostics, 13(24), 3632. https://doi.org/10.3390/diagnostics13243632







