Recent Advances in Imaging Macular Atrophy for Late-Stage Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Clinical Assessment
Clinical Presentation and Examinations
3. Assessment Using Imaging
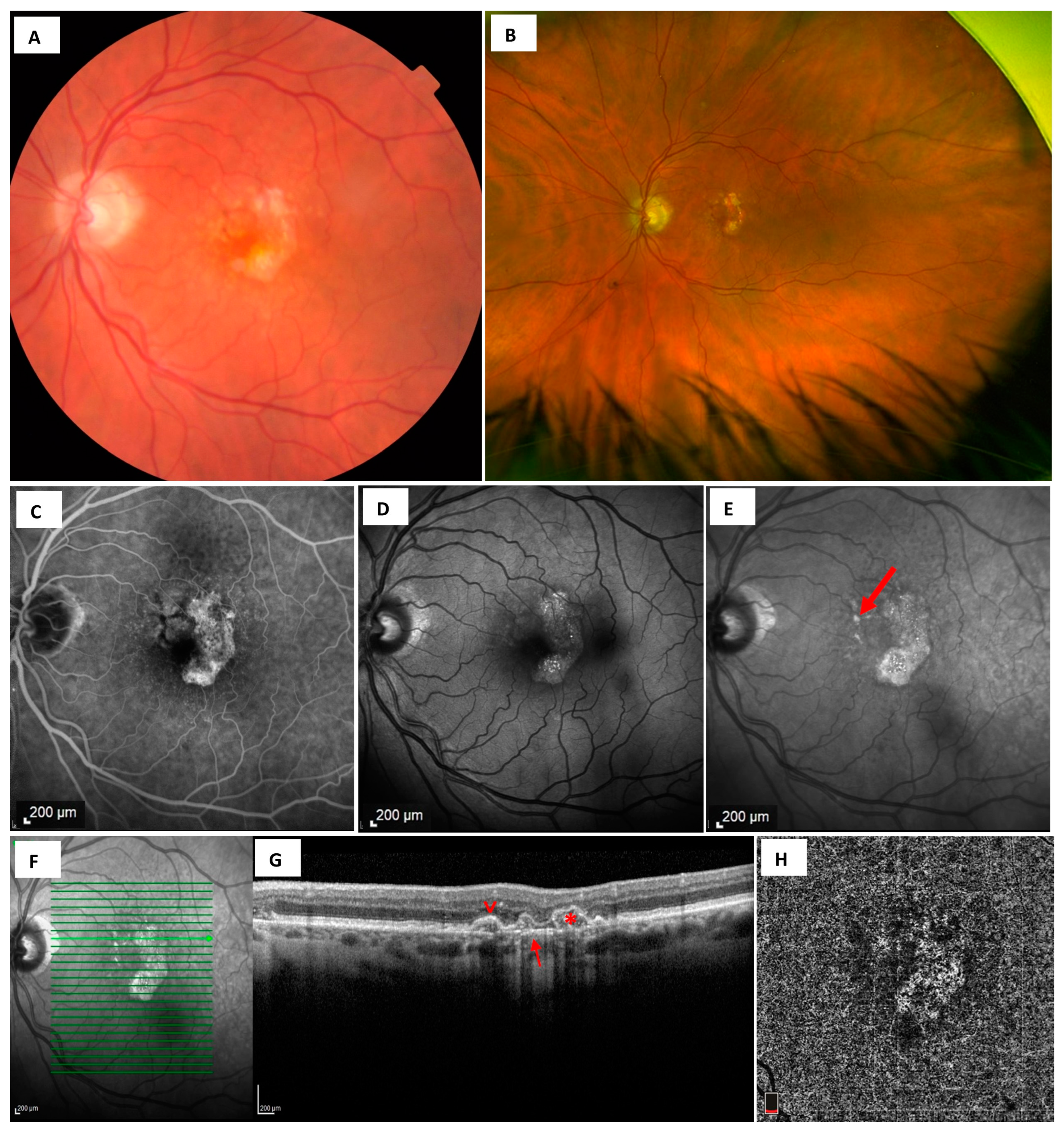
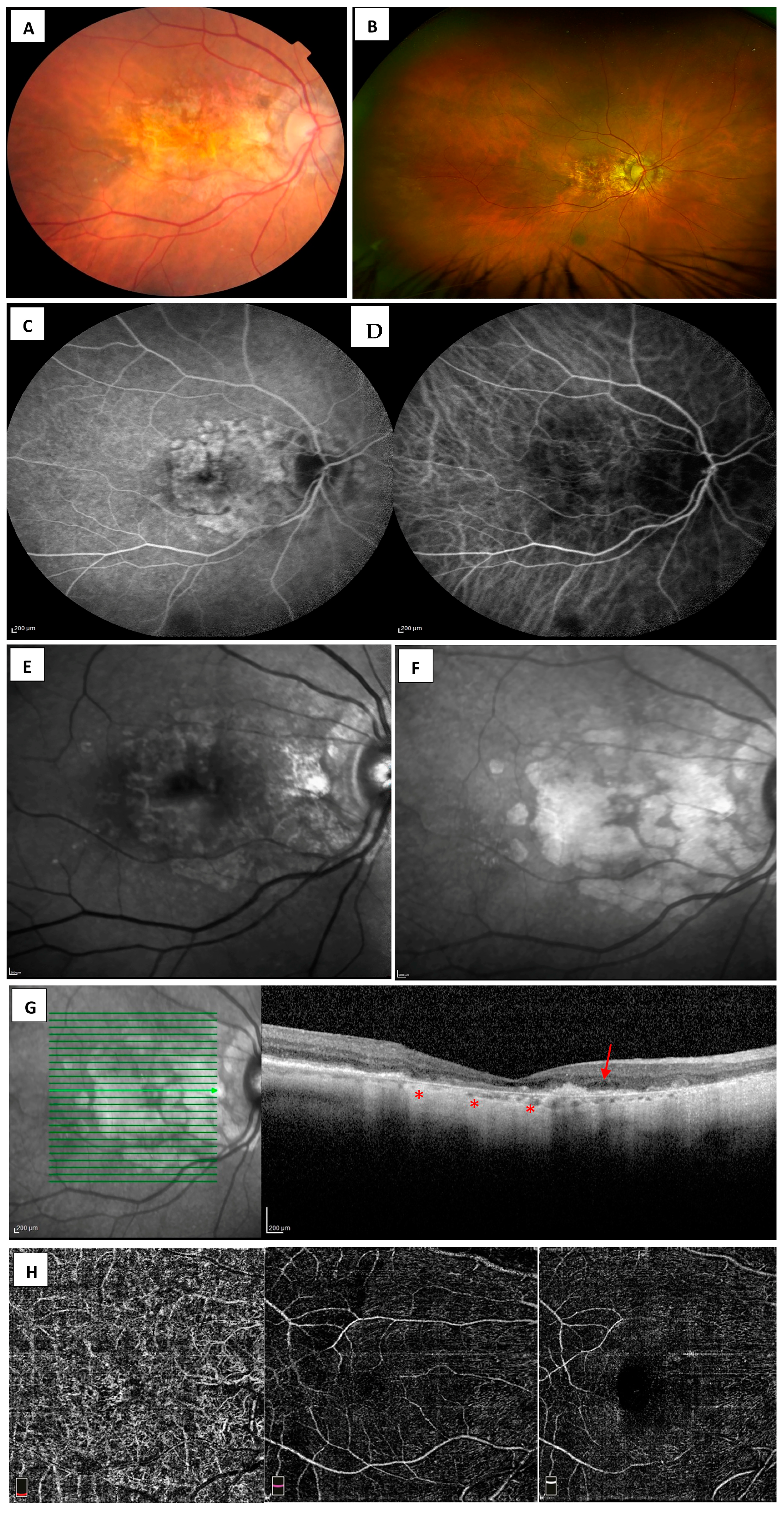
3.1. Fundus Photography
3.2. Dye-Based Angiography-Fluorescein and Indocyanine Green Angiography
3.3. Fundus Autofluorescence
3.4. Optical Coherence Tomography
3.5. Optical Coherence Tomography Angiography
4. Current Approach and Future Directions
4.1. Imaging Algorithm
4.2. New Modalities for Imaging MA-Microperimetry, Adaptive Optics, Home-Based OCT
Author Contributions
Funding
Conflicts of Interest
References
- Klaver, C.C.W. Age-Specific Prevalence and Causes of Blindness and Visual Impairment in an Older Population. Arch. Ophthalmol. 1998, 116, 653–658. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Christakis, P.G.; Agrón, E.; Klein, M.L.; Clemons, T.E.; Campbell, J.P.; Ferris, F.L.; Chew, E.Y.; Keenan, T.D. Incidence of Macular Atrophy after Untreated Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am. J. Ophthalmol. 2001, 132, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Campbell, T.G. Age-related macular degeneration. Lancet 2023, 401, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Heimes, B.; Lommatzsch, A.; Zeimer, M.; Gutfleisch, M.; Spital, G.; Dietzel, M.; Pauleikhoff, D. Long-term visual course after anti-VEGF therapy for exudative AMD in clinical practice evaluation of the German reinjection scheme. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wightman, A.J.; Guymer, R.H. Reticular pseudodrusen: Current understanding. Clin. Exp. Optom. 2019, 102, 455–462. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Sadda, S.; Staurenghi, G.; Chew, E.Y.; Fleckenstein, M.; Holz, F.G. Geographic Atrophy. Retina 2016, 36, 2250–2264. [Google Scholar] [CrossRef]
- Capuano, V.; Miere, A.; Querques, L.; Sacconi, R.; Carnevali, A.; Amoroso, F.; Bandello, F.; Souied, E.H.; Querques, G. Treatment-Naïve Quiescent Choroidal Neovascularization in Geographic Atrophy Secondary to Nonexudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2017, 182, 45–55. [Google Scholar] [CrossRef]
- Pfau, M.; Möller, P.T.; Künzel, S.H.; von der Emde, L.; Lindner, M.; Thiele, S.; Dysli, C.; Nadal, J.; Schmid, M.; Schmitz-Valckenberg, S.; et al. Type 1 Choroidal Neovascularization Is Associated with Reduced Localized Progression of Atrophy in Age-Related Macular Degeneration. Ophthalmol. Retin. 2020, 4, 238–248. [Google Scholar] [CrossRef]
- Sunness, J.S.; Rubin, G.S.; Zuckerbrod, A.; Applegate, C.A. Foveal-Sparing Scotomas in Advanced Dry Age-Related Macular Degeneration. J. Vis. Impair. Blind. 2008, 102, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Owsley, C.; Jackson, G.R.; White, M.; Feist, R.; Edwards, D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology 2001, 108, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Nolan, J.; Chakravarthy, U.; Beatty, S. Psychophysical function in age-related maculopathy. Surv. Ophthalmol. 2009, 54, 167–210. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, P.N.; Robman, L.D.; Varsamidis, M.; Aung, K.Z.; Makeyeva, G.A.; Guymer, R.H.; Vingrys, A.J. Visual function tests as potential biomarkers in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9457–9469. [Google Scholar] [CrossRef]
- Feigl, B.; Brown, B.; Lovie-Kitchin, J.; Swann, P. Cone-mediated multifocal electroretinogram in early age-related maculopathy and its relationships with subjective macular function tests. Curr. Eye Res. 2004, 29, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, L.; Wu, D.; Huang, S.; Wen, F.; Luo, G.; Long, S. The local cone and rod system function in early age-related macular degeneration. Doc. Ophthalmol. 2004, 109, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Tso, M.O.; Lam, T.T. Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br. J. Ophthalmol. 2001, 85, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Heinemann-Vernaleken, B.; Palmowski, A.M.; Allgayer, R.; Ruprecht, K.W. Comparison of different high resolution multifocal electroretinogram recordings in patients with age-related maculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 556–561. [Google Scholar] [CrossRef]
- Huang, S.; Wu, D.; Jiang, F.; Ma, J.; Wu, L.; Liang, J.; Luo, G. The multifocal electroretinogram in age-related maculopathies. Doc. Ophthalmol. 2000, 101, 115–124. [Google Scholar] [CrossRef]
- Gerth, C.; Hauser, D.; Delahunt, P.B.; Morse, L.S.; Werner, J.S. Assessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics in patients with large drusen. Arch. Ophthalmol. 2003, 121, 1404–1414. [Google Scholar] [CrossRef]
- Parisi, V.; Perillo, L.; Tedeschi, M.; Scassa, C.; Gallinaro, G.; Capaldo, N.; Varano, M. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina 2007, 27, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, A.M.; Sutter, E.E.; Bearse, M.A.; Fung, W. Multifocal electroretinogram (MF-ERG) in diagnosis of macular changes. Example: Senile macular degeneration. Ophthalmologe 1999, 96, 166–173. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Iarossi, G.; Fadda, A.; Merendino, E.; Valentini, P. Influence of short-term antioxidant supplementation on macular function in age-related maculopathy: A pilot study including electrophysiologic assessment. Ophthalmology 2003, 110, 51–60; discussion 61. [Google Scholar] [CrossRef]
- Neveu, M.M.; Tufail, A.; Dowler, J.G.; Holder, G.E. A comparison of pattern and multifocal electroretinography in the evaluation of age-related macular degeneration and its treatment with photodynamic therapy. Doc. Ophthalmol. 2006, 113, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Brown, M.C.; Hagan, R.P.; Fisher, A.C.; Grierson, I.; Harding, S.P. Deficits in the electroretinogram in neovascular age-related macular degeneration and changes during photodynamic therapy. Doc. Ophthalmol. 2007, 115, 69–76. [Google Scholar] [CrossRef]
- Feigl, B.; Lovie-Kitchin, J.; Brown, B. Objective functional assessment of age-related maculopathy: A special application for the multifocal electroretinogram. Clin. Exp. Optom. 2005, 88, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Pieramici, D.; Chakravarthy, U.; Patel, S.S.; Gupta, S.; Lotery, A.; Lad, E.M.; Silverman, D.; Henry, E.C.; Anderesi, M.; et al. Visual Function Decline Resulting from Geographic Atrophy. Ophthalmol. Retin. 2020, 4, 673–688. [Google Scholar] [CrossRef]
- Sarks, S.H. Ageing and degeneration in the macular region: A clinico-pathological study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef]
- Wu, Z.; Ayton, L.N.; Luu, C.D.; Baird, P.N.; Guymer, R.H. Reticular Pseudodrusen in Intermediate Age-Related Macular Degeneration: Prevalence, Detection, Clinical, Environmental, and Genetic Associations. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1310–1316. [Google Scholar] [CrossRef]
- Sunness, J.S.; Gonzalez-Baron, J.; Applegate, C.A.; Bressler, N.M.; Tian, Y.; Hawkins, B.; Barron, Y.; Bergman, A. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999, 106, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.S.; Fleckenstein, M.; Schmitz-Valckenberg, S.; Holz, F.G. Clinical Application of Multicolor Imaging Technology. Ophthalmologica 2016, 236, 8–18. [Google Scholar] [CrossRef]
- Pang, C.E.; Freund, K.B. Ghost maculopathy: An artifact on near-infrared reflectance and multicolor imaging masquerading as chorioretinal pathology. Am. J. Ophthalmol. 2014, 158, 171–178.e2. [Google Scholar] [CrossRef] [PubMed]
- Ben Moussa, N.; Georges, A.; Capuano, V.; Merle, B.; Souied, E.H.; Querques, G. MultiColor imaging in the evaluation of geographic atrophy due to age-related macular degeneration. Br. J. Ophthalmol. 2015, 99, 842–847. [Google Scholar] [CrossRef] [PubMed]
- De Bats, F.; Mathis, T.; Mauget-Faÿsse, M.; Joubert, F.; Denis, P.; Kodjikian, L. Prevalence of Reticular Pseudodrusen in Age-Related Macular Degeneration Using Multimodal Imaging. Retina 2016, 36, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Alten, F.; Clemens, C.R.; Heiduschka, P.; Eter, N. Characterisation of reticular pseudodrusen and their central target aspect in multi-spectral, confocal scanning laser ophthalmoscopy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the OPTOS PEripheral RetinA (OPERA) Study (Ancillary Study of Age-Related Eye Disease Study 2); Domalpally, A.; Clemons, T.E.; Danis, R.P.; Sadda, S.R.; Cukras, C.A.; Toth, C.A.; Friberg, T.R.; Chew, E.Y. Peripheral Retinal Changes Associated with Age-Related Macular Degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology 2017, 124, 479–487. [Google Scholar] [CrossRef]
- Lengyel, I.; Csutak, A.; Florea, D.; Leung, I.; Bird, A.C.; Jonasson, F.; Peto, T. A Population-Based Ultra-Widefield Digital Image Grading Study for Age-Related Macular Degeneration-Like Lesions at the Peripheral Retina. Ophthalmology 2015, 122, 1340–1347. [Google Scholar] [CrossRef]
- Forshaw, T.R.J.; Minör, Å.S.; Subhi, Y.; Sørensen, T.L. Peripheral Retinal Lesions in Eyes with Age-Related Macular Degeneration Using Ultra-Widefield Imaging: A Systematic Review with Meta-analyses. Ophthalmol. Retin. 2019, 3, 734–743. [Google Scholar] [CrossRef]
- Hill, D.W. Fluorescein angiography in fundus diagnosis. Br. Med. Bull. 1970, 26, 161–165. [Google Scholar] [CrossRef]
- Tomi, A.; Marin, I. Angiofluorographic aspects in age-related macular degeneration. J. Med. Life 2014, 7, 4–17. [Google Scholar]
- Novotny, H.R.; Alvis, D.L. A Method of Photographing Fluorescence in Circulating Blood in the Human Retina. Circulation 1961, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wald, K.J.; Elsner, A.E.; Wolf, S.; Staurenghi, G.; Weiter, J.J. Indocyanine green videoangiography for the imaging of choroidal neovascularization associated with macular degeneration. Int. Ophthalmol. Clin. 1994, 34, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Sherif-Adel, S.; Gelisken, F.; Kreissig, I. Indocyanine green angiography and transmission defects. Acta Ophthalmol. Scand. 2009, 75, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, A.; Alagoz, C.; Garip, R.; Alkin, Z.; Perente, I.; Yazici, A.T.; Taskapili, M. The role of indocyanine green angiography imaging in further differential diagnosis of patients with nAMD who are morphologically poor responders to ranibizumab in a real-life setting. Eye 2016, 30, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Rebelo Gomes, E.; Carneiro, Â. Immediate Reactions to Fluorescein and Indocyanine Green in Retinal Angiography: Review of Literature and Proposal for Patient’s Evaluation. Clin. Ophthalmol. 2020, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Schmitz-Valckenberg, S.; Boyer, D.; Heier, J.; Wolf-Schnurrbusch, U.; Staurenghi, G.; Schmidt-Erfurth, U.; Holz, F.G. Randomized Trial to Evaluate Tandospirone in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The GATE Study. Am. J. Ophthalmol. 2015, 160, 1226–1234. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Sahel, J.-A.; Danis, R.; Fleckenstein, M.; Jaffe, G.J.; Wolf, S.; Pruente, C.; Holz, F.G. Natural History of Geographic Atrophy Progression Secondary to Age-Related Macular Degeneration (Geographic Atrophy Progression Study). Ophthalmology 2016, 123, 361–368. [Google Scholar] [CrossRef]
- Hopkins, J.; Walsh, A.; Chakravarthy, U. Fundus autofluorescence in age-related macular degeneration: An epiphenomenon? Investig. Ophthalmol. Vis. Sci. 2006, 47, 2269–2271. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Fleckenstein, M.; Scholl, H.P.N.; Holz, F.G. Fundus autofluorescence and progression of age-related macular degeneration. Surv. Ophthalmol. 2009, 54, 96–117. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A.; Zweifel, S.A. Drusen, an old but new frontier. Retina 2010, 30, 1163–1165. [Google Scholar] [CrossRef]
- Curcio, C.A. Antecedents of Soft Drusen, the Specific Deposits of Age-Related Macular Degeneration, in the Biology of Human Macula. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD182–AMD194. [Google Scholar] [CrossRef]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.-Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. Age-Related Eye Disease Study (AREDS) Research Group. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef]
- Davis, M.D.; Gangnon, R.E.; Lee, L.-Y.; Hubbard, L.D.; Klein, B.E.K.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C. Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005, 123, 1484–1498. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Chen, L.; Messinger, J.D.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Stages of Drusen-Associated Atrophy in Age-Related Macular Degeneration Visible via Histologically Validated Fundus Autofluorescence. Ophthalmol. Retin. 2021, 5, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Curcio, C.A.; Mullins, R.F.; Spaide, R.F. REFRACTILE DRUSEN: Clinical Imaging and Candidate Histology. Retina 2015, 35, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Balaratnasingam, C.; Cherepanoff, S.; Dolz-Marco, R.; Killingsworth, M.; Chen, F.K.; Mendis, R.; Mrejen, S.; Too, L.K.; Gal-Or, O.; Curcio, C.A.; et al. Cuticular Drusen: Clinical Phenotypes and Natural History Defined Using Multimodal Imaging. Ophthalmology 2018, 125, 100–118. [Google Scholar] [CrossRef]
- Lima, L.H.; Laud, K.; Freund, K.B.; Yannuzzi, L.A.; Spaide, R.F. Acquired vitelliform lesion associated with large drusen. Retina 2012, 32, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Parikh, R.; Gal-Or, O.; Balaratnasingam, C.; Leong, B.C.; Tanaka, K.; Cherepanoff, S.; Spaide, R.F.; Freund, K.B.; Yannuzzi, L.A. CUTICULAR DRUSEN: Risk of Geographic Atrophy and Macular Neovascularization. Retina 2020, 40, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Drusen characterization with multimodal imaging. Retina 2010, 30, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Shijo, T.; Sakurada, Y.; Tanaka, K.; Miki, A.; Yoneyama, S.; Machida, Y.; Chubachi, A.; Wakatsuki, Y.; Sugiyama, A.; Onoe, H.; et al. Drusenoid Pigment Epithelial Detachment: Genetic and Clinical Characteristics. Int. J. Mol. Sci. 2021, 22, 4074. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Sakurada, Y.; Mabuchi, F.; Imasawa, M.; Sugiyama, A.; Kubota, T.; Iijima, H. Genetic and clinical factors associated with reticular pseudodrusen in exudative age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Arakawa, N.; Ooto, S.; Nakata, I.; Yamashiro, K.; Tsujikawa, A.; Oishi, A.; Yoshimura, N. Prevalence and genomic association of reticular pseudodrusen in age-related macular degeneration. Am. J. Ophthalmol. 2013, 155, 260–269.e2. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, S.A.; Imamura, Y.; Spaide, T.C.; Fujiwara, T.; Spaide, R.F. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010, 117, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Daniel, E.; Maguire, M.G.; Grunwald, J.E.; Martin, E.R.; Martin, D.F.; Ying, G.-S. Pseudodrusen and Incidence of Late Age-Related Macular Degeneration in Fellow Eyes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sato, T.; Spaide, R.F. Pseudodrusen subtypes as delineated by multimodal imaging of the fundus. Am. J. Ophthalmol. 2014, 157, 1005–1012. [Google Scholar] [CrossRef]
- Shijo, T.; Sakurada, Y.; Yoneyama, S.; Sugiyama, A.; Kikushima, W.; Tanabe, N.; Iijima, H. Prevalence and characteristics of pseudodrusen subtypes in advanced age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1125–1131. [Google Scholar] [CrossRef]
- Lee, M.Y.; Yoon, J.; Ham, D.-I. Clinical features of reticular pseudodrusen according to the fundus distribution. Br. J. Ophthalmol. 2012, 96, 1222–1226. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, Y.S.; Kim, J.W.; Lee, T.G.; Kim, C.G. Prevalence of Subtypes of Reticular Pseudodrusen in Newly Diagnosed Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy in Korean Patients. Retina 2015, 35, 2604–2612. [Google Scholar] [CrossRef]
- Spaide, R.F. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina 2013, 33, 1800–1808. [Google Scholar] [CrossRef]
- Ueda-Arakawa, N.; Ooto, S.; Tsujikawa, A.; Yamashiro, K.; Oishi, A.; Yoshimura, N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina 2013, 33, 490–497. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Brinkmann, C.K.; Alten, F.; Herrmann, P.; Stratmann, N.K.; Göbel, A.P.; Fleckenstein, M.; Diller, M.; Jaffe, G.J.; Holz, F.G. Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7640–7646. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Schnurrbusch, U.E.K.; Wittwer, V.V.; Ghanem, R.; Niederhaeuser, M.; Enzmann, V.; Framme, C.; Wolf, S. Blue-light versus green-light autofluorescence: Lesion size of areas of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9497–9502. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Querques, G.; Querques, L.; Leveziel, N.; Benhamou, N.; Souied, E.H. Multimodal evaluation of foveal sparing in patients with geographicatrophy due to age-related macular degeneration. Retina 2013, 33, 482–489. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Martens, C.; Kosanetzky, S.; Brinkmann, C.K.; Hageman, G.S.; Holz, F.G. Fundus autofluorescence and spectral-domain optical coherence tomography characteristics in a rapidly progressing form of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Hassenstein, A.; Meyer, C.H. Clinical use and research applications of Heidelberg retinal angiography and spectral-domain optical coherence tomography—A review. Clin. Exp. Ophthalmol. 2009, 37, 130–143. [Google Scholar] [CrossRef]
- Charbel Issa, P.; Finger, R.P.; Holz, F.G.; Scholl, H.P.N. Multimodal Imaging Including Spectral Domain OCT and Confocal Near Infrared Reflectance for Characterization of Outer Retinal Pathology in Pseudoxanthoma Elasticum. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5913–5918. [Google Scholar] [CrossRef]
- Weinberger, A.W.A.; Lappas, A.; Kirschkamp, T.; Mazinani, B.A.E.; Huth, J.K.; Mohammadi, B.; Walter, P. Fundus Near Infrared Fluorescence Correlates with Fundus Near Infrared Reflectance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3098–3108. [Google Scholar] [CrossRef]
- Sakurada, Y.; Tanaka, K.; Fragiotta, S. Differentiating drusen and drusenoid deposits subtypes on multimodal imaging and risk of advanced age-related macular degeneration. Jpn. J. Ophthalmol. 2023, 67, 1–13. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Alten, F.; Steinberg, J.S.; Jaffe, G.J.; Fleckenstein, M.; Mukesh, B.N.; Hohman, T.C.; Holz, F.G. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5009–5015. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Sohrab, M.A.; Busuioc, M.; Barile, G. Reticular macular disease. Am. J. Ophthalmol. 2009, 148, 733–743.e2. [Google Scholar] [CrossRef] [PubMed]
- Youngquist, R.C.; Carr, S.; Davies, D.E. Optical coherence-domain reflectometry: A new optical evaluation technique. Opt. Lett. 1987, 12, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on, O.C.T. Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2016, 50, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D. Drusen and disciform macular detachment and degeneration. Arch. Ophthalmol. 1973, 90, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.L.; Ferris, F.L.; Armstrong, J.; Hwang, T.S.; Chew, E.Y.; Bressler, S.B.; Chandra, S.R. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology 2008, 115, 1026–1031. [Google Scholar] [CrossRef]
- Christenbury, J.G.; Folgar, F.A.; O’Connell, R.V.; Chiu, S.J.; Farsiu, S.; Toth, C.A. Age-Related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 2013, 120, 1038–1045. [Google Scholar] [CrossRef]
- Ouyang, Y.; Heussen, F.M.; Hariri, A.; Keane, P.A.; Sadda, S.R. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology 2013, 120, 2656–2665. [Google Scholar] [CrossRef]
- Wu, Z.; Luu, C.D.; Ayton, L.N.; Goh, J.K.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology 2014, 121, 2415–2422. [Google Scholar] [CrossRef]
- Lek, J.J.; Brassington, K.H.; Luu, C.D.; Chen, F.K.; Arnold, J.J.; Heriot, W.J.; Durkin, S.R.; Chakravarthy, U.; Guymer, R.H. Subthreshold Nanosecond Laser Intervention in Intermediate Age-Related Macular Degeneration: Study Design and Baseline Characteristics of the Laser in Early Stages of Age-Related Macular Degeneration Study (Report Number 1). Ophthalmol. Retin. 2017, 1, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Schaal, K.B.; Rosenfeld, P.J.; Gregori, G.; Yehoshua, Z.; Feuer, W.J. Anatomic Clinical Trial Endpoints for Nonexudative Age-Related Macular Degeneration. Ophthalmology 2016, 123, 1060–1079. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; van Lookeren Campagne, M. Geographic atrophy: Clinical features and potential therapeutic approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312.e1. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated Retinal Pigment Epithelium, an Optical Coherence Tomography Biomarker for Progression in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar]
- Ferrara, D.; Silver, R.E.; Louzada, R.N.; Novais, E.A.; Collins, G.K.; Seddon, J.M. Optical Coherence Tomography Features Preceding the Onset of Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3519–3529. [Google Scholar] [CrossRef]
- Veerappan, M.; El-Hage-Sleiman, A.-K.M.; Tai, V.; Chiu, S.J.; Winter, K.P.; Stinnett, S.S.; Hwang, T.S.; Hubbard, G.B.; Michelson, M.; Gunther, R.; et al. Optical Coherence Tomography Reflective Drusen Substructures Predict Progression to Geographic Atrophy in Age-related Macular Degeneration. Ophthalmology 2016, 123, 2554–2570. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Zhang, H.; Boyer, D.S.; Rosenfeld, P.J.; Feuer, W.J.; Gregori, G.; Sadda, S.R. Drusen Volume as a Predictor of Disease Progression in Patients with Late Age-Related Macular Degeneration in the Fellow Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1839–1846. [Google Scholar] [CrossRef]
- Schaal, K.B.; Gregori, G.; Rosenfeld, P.J. En Face Optical Coherence Tomography Imaging for the Detection of Nascent Geographic Atrophy. Am. J. Ophthalmol. 2017, 174, 145–154. [Google Scholar] [CrossRef]
- Monés, J.; Biarnés, M.; Trindade, F. Hyporeflective wedge-shaped band in geographic atrophy secondary to age-related macular degeneration: An underreported finding. Ophthalmology 2012, 119, 1412–1419. [Google Scholar] [CrossRef]
- Wu, Z.; Luu, C.D.; Ayton, L.N.; Goh, J.K.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Fundus autofluorescence characteristics of nascent geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Rosenfeld, P.J.; Curcio, C.A.; Holz, F.G.; Staurenghi, G.; Freund, K.B.; Schmitz-Valckenberg, S.; Sparrow, J.; Spaide, R.F.; Tufail, A.; et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology 2020, 127, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; de Sisternes, L.; Chen, Q.; Rubin, D.L.; Leng, T. Fully Automated Prediction of Geographic Atrophy Growth Using Quantitative Spectral-Domain Optical Coherence Tomography Biomarkers. Ophthalmology 2016, 123, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Litts, K.M.; Ach, T.; Hammack, K.M.; Sloan, K.R.; Zhang, Y.; Freund, K.B.; Curcio, C.A. Quantitative Analysis of Outer Retinal Tubulation in Age-Related Macular Degeneration from Spectral-Domain Optical Coherence Tomography and Histology. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2647–2656. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Auge, J.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Longitudinal analysis of reticular drusen associated with age-related macular degeneration using combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging. Ophthalmologica 2015, 233, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Deng, X.; Zhang, Q.; He, J.; Ye, P.; Liu, S.; Li, P.; Zhou, J.; Fang, X. Advances in swept-source optical coherence tomography and optical coherence tomography angiography. Adv. Ophthalmol. Pract. Res. 2023, 3, 67–79. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Hirata, M.; Tsujikawa, A.; Matsumoto, A.; Hangai, M.; Ooto, S.; Yamashiro, K.; Akiba, M.; Yoshimura, N. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4971–4978. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Mandai, M.; Yamanari, M.; Totani, K.; Nishida, M.; Sugita, S.; Maeda, T.; Koide, N.; Takagi, S.; Hirami, Y.; et al. Polarization-sensitive optical coherence tomography for estimating relative melanin content of autologous induced stem-cell derived retinal pigment epithelium. Sci. Rep. 2020, 10, 7656. [Google Scholar] [CrossRef]
- Baghaie, A.; Yu, Z.; D’Souza, R.M. State-of-the-art in retinal optical coherence tomography image analysis. Quant. Imaging Med. Surg. 2015, 5, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Fingler, J.; Kim, D.Y.; Zawadzki, R.J.; Morse, L.S.; Park, S.S.; Fraser, S.E.; Werner, J.S. Phase-variance optical coherence tomography: A technique for noninvasive angiography. Ophthalmology 2014, 121, 180–187. [Google Scholar] [CrossRef]
- Waheed, N.K.; Moult, E.M.; Fujimoto, J.G.; Rosenfeld, P.J. Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration. Dev. Ophthalmol. 2016, 56, 91–100. [Google Scholar] [CrossRef]
- Bischoff, P.M.; Flower, R.W. Ten years experience with choroidal angiography using indocyanine green dye: A new routine examination or an epilogue? Doc. Ophthalmol. 1985, 60, 235–291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zheng, Y.; von Kerczek, C.H.; Topoleski, L.D.T.; Flower, R.W. Feasibility of extracting velocity distribution in choriocapillaris in human eyes from ICG dye angiograms. J. Biomech. Eng. 2006, 128, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.W. Extraction of choriocapillaris hemodynamic data from ICG fluorescence angiograms. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2720–2729. [Google Scholar]
- Yu, L.; Chen, Z. Doppler variance imaging for three-dimensional retina and choroid angiography. J. Biomed. Opt. 2010, 15, 016029. [Google Scholar] [CrossRef]
- An, L.; Wang, R.K. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt. Express 2008, 16, 11438–11452. [Google Scholar] [CrossRef]
- Fingler, J.; Schwartz, D.; Yang, C.; Fraser, S.E. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt. Express 2007, 15, 12636–12653. [Google Scholar] [CrossRef]
- Makita, S.; Hong, Y.; Yamanari, M.; Yatagai, T.; Yasuno, Y. Optical coherence angiography. Opt. Express 2006, 14, 7821–7840. [Google Scholar] [CrossRef]
- Borrelli, E.; Shi, Y.; Uji, A.; Balasubramanian, S.; Nassisi, M.; Sarraf, D.; Sadda, S.R. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 196, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Alten, F.; Heiduschka, P.; Clemens, C.R.; Eter, N. Exploring choriocapillaris under reticular pseudodrusen using OCT-Angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Toma, C.; Villani, E.; Muraca, A.; Torti, E.; Florimbi, G.; Pezzotti, M.; Nucci, P.; De Cillà, S. Quantitative choriocapillaris evaluation in intermediate age-related macular degeneration by swept-source optical coherence tomography angiography. Acta Ophthalmol. 2019, 97, e919–e926. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Ahn, J.; Yun, C.; Kim, S.-W.; Oh, J. Variation of Retinal and Choroidal Vasculatures in Patients with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5246–5255. [Google Scholar] [CrossRef]
- Borrelli, E.; Souied, E.H.; Freund, K.B.; Querques, G.; Miere, A.; Gal-Or, O.; Sacconi, R.; Sadda, S.R.; Sarraf, D. Reduced Choriocapillaris Flow in Eyes with Type 3 Neovascularization and Age-Related Macular Degeneration. Retina 2018, 38, 1968–1976. [Google Scholar] [CrossRef]
- Müller, P.L.; Pfau, M.; Schmitz-Valckenberg, S.; Fleckenstein, M.; Holz, F.G. Optical Coherence Tomography-Angiography in Geographic Atrophy. Ophthalmologica 2021, 244, 42–50. [Google Scholar] [CrossRef]
- Thulliez, M.; Zhang, Q.; Shi, Y.; Zhou, H.; Chu, Z.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Gregori, G.; Wang, R.K.; et al. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol. Retin. 2019, 3, 478–488. [Google Scholar] [CrossRef]
- Alagorie, A.R.; Nassisi, M.; Verma, A.; Nittala, M.; Corradetti, G.; Velaga, S.; Sadda, S.R. Relationship between proximity of choriocapillaris flow deficits and enlargement rate of geographic atrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 995–1003. [Google Scholar] [CrossRef]
- Nassisi, M.; Baghdasaryan, E.; Borrelli, E.; Ip, M.; Sadda, S.R. Choriocapillaris flow impairment surrounding geographic atrophy correlates with disease progression. PLoS ONE 2019, 14, e0212563. [Google Scholar] [CrossRef] [PubMed]
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef]
- Choi, W.; Moult, E.M.; Waheed, N.K.; Adhi, M.; Lee, B.; Lu, C.D.; de Carlo, T.E.; Jayaraman, V.; Rosenfeld, P.J.; Duker, J.S.; et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology 2015, 122, 2532–2544. [Google Scholar] [CrossRef]
- McLeod, D.S.; Taomoto, M.; Otsuji, T.; Green, W.R.; Sunness, J.S.; Lutty, G.A. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1986–1993. [Google Scholar]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image Artifacts in Optical Coherence Tomography Angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration. Ophthalmology 2017, 124, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Santina, A.; Bousquet, E.; Sadda, S.R.; Sarraf, D. Pathways of Fluid Leakage in Age Related Macular Degeneration. Retina 2023, 43, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Engelbert, M. Differential diagnosis of neovascular age-related macular degeneration. Spektrum Augenheilkd. 2018, 32, 12–17. [Google Scholar] [CrossRef]
- Stone, E.M.; Andorf, J.L.; Whitmore, S.S.; DeLuca, A.P.; Giacalone, J.C.; Streb, L.M.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E.; Sheffield, V.C.; et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 2017, 124, 1314–1331. [Google Scholar] [CrossRef]
- Lindner, M.; Lambertus, S.; Mauschitz, M.M.; Bax, N.M.; Kersten, E.; Lüning, A.; Nadal, J.; Schmitz-Valckenberg, S.; Schmid, M.; Holz, F.G.; et al. Differential Disease Progression in Atrophic Age-Related Macular Degeneration and Late-Onset Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1001–1007. [Google Scholar] [CrossRef]
- Miere, A.; Capuano, V.; Kessler, A.; Zambrowski, O.; Jung, C.; Colantuono, D.; Pallone, C.; Semoun, O.; Petit, E.; Souied, E. Deep learning-based classification of retinal atrophy using fundus autofluorescence imaging. Comput. Biol. Med. 2021, 130, 104198. [Google Scholar] [CrossRef]
- Hu, Z.; Medioni, G.G.; Hernandez, M.; Hariri, A.; Wu, X.; Sadda, S.R. Segmentation of the geographic atrophy in spectral-domain optical coherence tomography and fundus autofluorescence images. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8375–8383. [Google Scholar] [CrossRef] [PubMed]
- Ometto, G.; Montesano, G.; Sadeghi Afgeh, S.; Lazaridis, G.; Liu, X.; Keane, P.A.; Crabb, D.P.; Denniston, A.K. Merging Information from Infrared and Autofluorescence Fundus Images for Monitoring of Chorioretinal Atrophic Lesions. Transl. Vis. Sci. Technol. 2020, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Treder, M.; Lauermann, J.L.; Eter, N. Deep learning-based detection and classification of geographic atrophy using a deep convolutional neural network classifier. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2053–2060. [Google Scholar] [CrossRef]
- Liefers, B.; Taylor, P.; Alsaedi, A.; Bailey, C.; Balaskas, K.; Dhingra, N.; Egan, C.A.; Rodrigues, F.G.; Gonzalo, C.G.; Heeren, T.F.; et al. Quantification of Key Retinal Features in Early and Late Age-Related Macular Degeneration Using Deep Learning. Am. J. Ophthalmol. 2021, 226, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Chopra, R.; Spitz, T.; Winkens, J.; Obika, A.; Kelly, C.; Askham, H.; Lukic, M.; Huemer, J.; Fasler, K.; et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 2020, 26, 892–899. [Google Scholar] [CrossRef]
- Sarhan, M.H.; Nasseri, M.A.; Zapp, D.; Maier, M.; Lohmann, C.P.; Navab, N.; Eslami, A. Machine Learning Techniques for Ophthalmic Data Processing: A Review. IEEE J. Biomed. Health Inform. 2020, 24, 3338–3350. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, A.; Stinnett, S.S.; Petrowski, J.T.; Schuman, S.G.; Toth, C.A.; Cousins, S.W.; Lad, E.M. Visual Function Measures in Early and Intermediate Age-Related Macular Degeneration. Retina 2016, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ayton, L.N.; Luu, C.D.; Guymer, R.H. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015, 133, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Vujosevic, S.; Convento, E.; Manfre, A.; Cavarzeran, F.; Pilotto, E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br. J. Ophthalmol. 2007, 91, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Luu, C.D.; Dimitrov, P.N.; Robman, L.; Varsamidis, M.; Makeyeva, G.; Aung, K.-Z.; Vingrys, A.J.; Guymer, R.H. Role of flicker perimetry in predicting onset of late-stage age-related macular degeneration. Arch. Ophthalmol. 2012, 130, 690–699. [Google Scholar] [CrossRef]
- Wu, Z.; Ayton, L.N.; Guymer, R.H.; Luu, C.D. Comparison between multifocal electroretinography and microperimetry in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6431–6439. [Google Scholar] [CrossRef] [PubMed]
- Meleth, A.D.; Mettu, P.; Agrón, E.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L.; Wong, W.T. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1119–1126. [Google Scholar] [CrossRef]
- Querques, L.; Querques, G.; Forte, R.; Souied, E.H. Microperimetric correlations of autofluorescence and optical coherence tomography imaging in dry age-related macular degeneration. Am. J. Ophthalmol. 2012, 153, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Landa, G.; Su, E.; Garcia, P.M.T.; Seiple, W.H.; Rosen, R.B. Inner segment-outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina 2011, 31, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.H.; Tepelus, T.C.; Akil, H.; Nittala, M.G.; Sadda, S.R. Retinal Sensitivity at the Junctional Zone of Eyes with Geographic Atrophy Due to Age-Related Macular Degeneration. Am. J. Ophthalmol. 2016, 168, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Paques, M.; Meimon, S.; Rossant, F.; Rosenbaum, D.; Mrejen, S.; Sennlaub, F.; Grieve, K. Adaptive optics ophthalmoscopy: Application to age-related macular degeneration and vascular diseases. Prog. Retin. Eye Res. 2018, 66, 1–16. [Google Scholar] [CrossRef]
- Jonnal, R.S.; Kocaoglu, O.P.; Zawadzki, R.J.; Liu, Z.; Miller, D.T.; Werner, J.S. A Review of Adaptive Optics Optical Coherence Tomography: Technical Advances, Scientific Applications, and the Future. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT51–OCT68. [Google Scholar] [CrossRef]
- Rossi, E.A.; Granger, C.E.; Sharma, R.; Yang, Q.; Saito, K.; Schwarz, C.; Walters, S.; Nozato, K.; Zhang, J.; Kawakami, T.; et al. Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc. Natl. Acad. Sci. USA 2017, 114, 586–591. [Google Scholar] [CrossRef]
- Scoles, D.; Sulai, Y.N.; Dubra, A. In vivo dark-field imaging of the retinal pigment epithelium cell mosaic. Biomed. Opt. Express 2013, 4, 1710–1723. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Curcio, C.A. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Freund, K.B.; Curcio, C.A. The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Paques, M.; Simonutti, M.; Augustin, S.; Goupille, O.; El Mathari, B.; Sahel, J.-A. In vivo observation of the locomotion of microglial cells in the retina. Glia 2010, 58, 1663–1668. [Google Scholar] [CrossRef]
- Sennlaub, F.; Auvynet, C.; Calippe, B.; Lavalette, S.; Poupel, L.; Hu, S.J.; Dominguez, E.; Camelo, S.; Levy, O.; Guyon, E.; et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 2013, 5, 1775–1793. [Google Scholar] [CrossRef]
- Gocho, K.; Sarda, V.; Falah, S.; Sahel, J.-A.; Sennlaub, F.; Benchaboune, M.; Ullern, M.; Paques, M. Adaptive optics imaging of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Oakley, J.D.; Verdooner, S.; Russakoff, D.B.; Brucker, A.J.; Seaman, J.; Sahni, J.; Bianchi, C.D.; Cozzi, M.; Rogers, J.; Staurenghi, G. Quantitative Assessment of Automated Optical Coherence Tomography Image Analysis Using A Home-Based Device for Self-Monitoring Neovascular Age-Related Macular Degeneration. Retina 2023, 43, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.; Deonarain, D.M.; Gould, J.; Sothivannan, A.; Phillips, M.R.; Sarohia, G.S.; Sivaprasad, S.; Wykoff, C.C.; Cheung, C.M.G.; Sarraf, D.; et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 2023, 37, 6–16. [Google Scholar] [CrossRef]
- Patel, P.J.; Villavicencio, P.; Hanumunthadu, D. Systematic Review of Neovascular Age-Related Macular Degeneration Disease Activity Criteria Use to Shorten, Maintain or Extend Treatment Intervals with Anti-VEGF in Clinical Trials: Implications for Clinical Practice. Ophthalmol. Ther. 2023, 12, 2323–2346. [Google Scholar] [CrossRef]
- Cheng, A.M.; Joshi, S.; Banoub, R.G.; Saddemi, J.; Chalam, K.V. Faricimab Effectively Resolves Intraretinal Fluid and Preserves Vision in Refractory, Recalcitrant, and Nonresponsive Neovascular Age-Related Macular Degeneration. Cureus 2023, 15, e40100. [Google Scholar] [CrossRef]
- ElSheikh, R.H.; Chauhan, M.Z.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Neovascular Age-Related Macular Degeneration. Biomolecules 2022, 12, 1629. [Google Scholar] [CrossRef]
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-related Macular Degeneration. EBioMedicine 2016, 14, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Lai, C.-M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A.; et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet 2015, 386, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, R.; Schneider, M.; Nagy, Z.Z.; Sandor, G.L.; Kaan, K.; Asztalos, A.; Enyedi, L.; Pek, G.; Barcsay, G.; Szabo, A.; et al. Seven-year outcomes following intensive anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration. BMC Ophthalmol. 2023, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Pegcetacoplan (Syfovre) for geographic atrophy in age-related macular degeneration. Med. Lett. Drugs Ther. 2023, 65, 49–50. [CrossRef]
- Biarnés, M.; Garrell-Salat, X.; Gómez-Benlloch, A.; Guarro, M.; Londoño, G.; López, E.; Ruiz, S.; Vázquez, M.; Sararols, L. Methodological Appraisal of Phase 3 Clinical Trials in Geographic Atrophy. Biomedicines 2023, 11, 1548. [Google Scholar] [CrossRef]
- Cruz-Pimentel, M.; Wu, L. Complement Inhibitors for Advanced Dry Age-Related Macular Degeneration (Geographic Atrophy): Some Light at the End of the Tunnel? J. Clin. Med. 2023, 12, 5131. [Google Scholar] [CrossRef] [PubMed]
- Borchert, G.A.; Shamsnajafabadi, H.; Hu, M.L.; De Silva, S.R.; Downes, S.M.; MacLaren, R.E.; Xue, K.; Cehajic-Kapetanovic, J. The Role of Inflammation in Age-Related Macular Degeneration-Therapeutic Landscapes in Geographic Atrophy. Cells 2023, 12, 2092. [Google Scholar] [CrossRef]
| Techniques | Pros | Cons |
|---|---|---|
| Color fundus photography (CFP) |
|
|
|
| |
|
| |
| ||
| Multicolor imaging |
|
|
|
| |
| Ultra-widefield (UWF) |
|
|
|
| |
| ||
| Fundus fluorescein angiography (FA) |
|
|
|
| |
|
| |
|
| |
| ||
| Indocyanine green angiography (ICG-A) |
|
|
|
| |
| ||
| ||
| Fundus autofluorescence (FAF) |
|
|
|
| |
|
| |
| ||
| ||
| Near-infrared reflectance (NIR) |
|
|
|
| |
| ||
| ||
| Spectral-domain OCT (SD-OCT)/Swept-source OCT (SS-OCT) |
|
|
| ||
| ||
| ||
| Polarization-sensitive optical coherence tomography (PS-OCT) |
|
|
| ||
| Optical Doppler tomography (ODT) |
|
|
| ||
| Phase contrast optical coherence tomography (PC-OCT) |
|
|
| Optical coherence tomography angiography (OCTA) |
|
|
| ||
| ||
| ||
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.M.S.; Chalam, K.V.; Brar, V.S.; Yang, D.T.Y.; Bhatt, J.; Banoub, R.G.; Gupta, S.K. Recent Advances in Imaging Macular Atrophy for Late-Stage Age-Related Macular Degeneration. Diagnostics 2023, 13, 3635. https://doi.org/10.3390/diagnostics13243635
Cheng AMS, Chalam KV, Brar VS, Yang DTY, Bhatt J, Banoub RG, Gupta SK. Recent Advances in Imaging Macular Atrophy for Late-Stage Age-Related Macular Degeneration. Diagnostics. 2023; 13(24):3635. https://doi.org/10.3390/diagnostics13243635
Chicago/Turabian StyleCheng, Anny M. S., Kakarla V. Chalam, Vikram S. Brar, David T. Y. Yang, Jineel Bhatt, Raphael G. Banoub, and Shailesh K. Gupta. 2023. "Recent Advances in Imaging Macular Atrophy for Late-Stage Age-Related Macular Degeneration" Diagnostics 13, no. 24: 3635. https://doi.org/10.3390/diagnostics13243635
APA StyleCheng, A. M. S., Chalam, K. V., Brar, V. S., Yang, D. T. Y., Bhatt, J., Banoub, R. G., & Gupta, S. K. (2023). Recent Advances in Imaging Macular Atrophy for Late-Stage Age-Related Macular Degeneration. Diagnostics, 13(24), 3635. https://doi.org/10.3390/diagnostics13243635






