Prediction of Increased Intracranial Pressure in Traumatic Brain Injury Using Quantitative Electroencephalogram in a Porcine Experimental Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
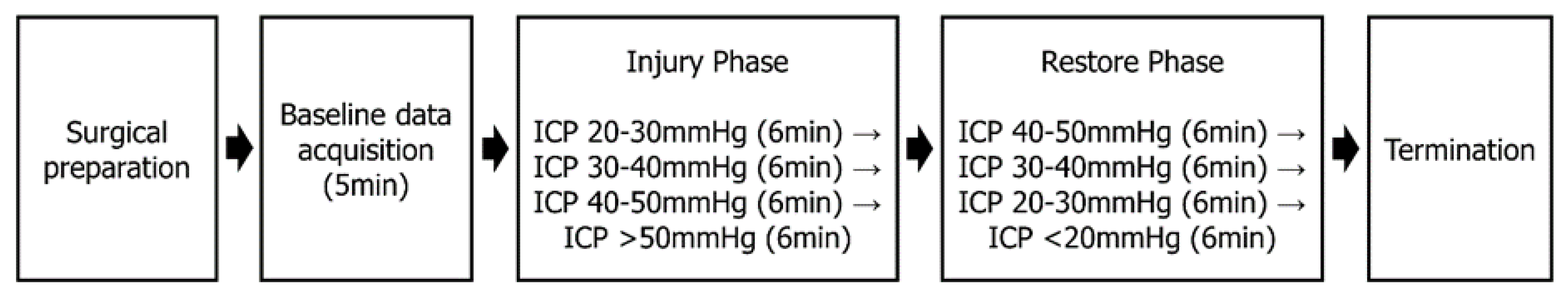
2.2. Study Design and Setting
2.3. Experimental Animal and Housing
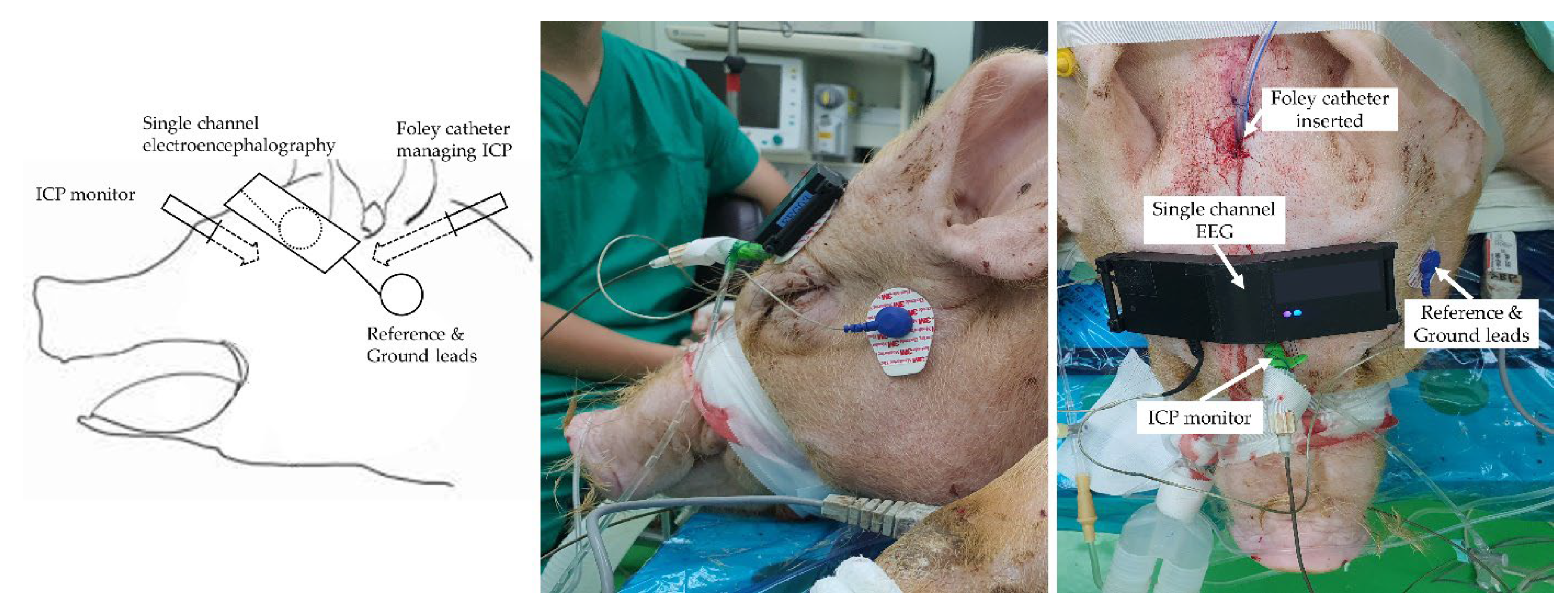
2.4. Surgical Procedure and Haemodynamic Measurements
2.5. EEG Signal Acquisition
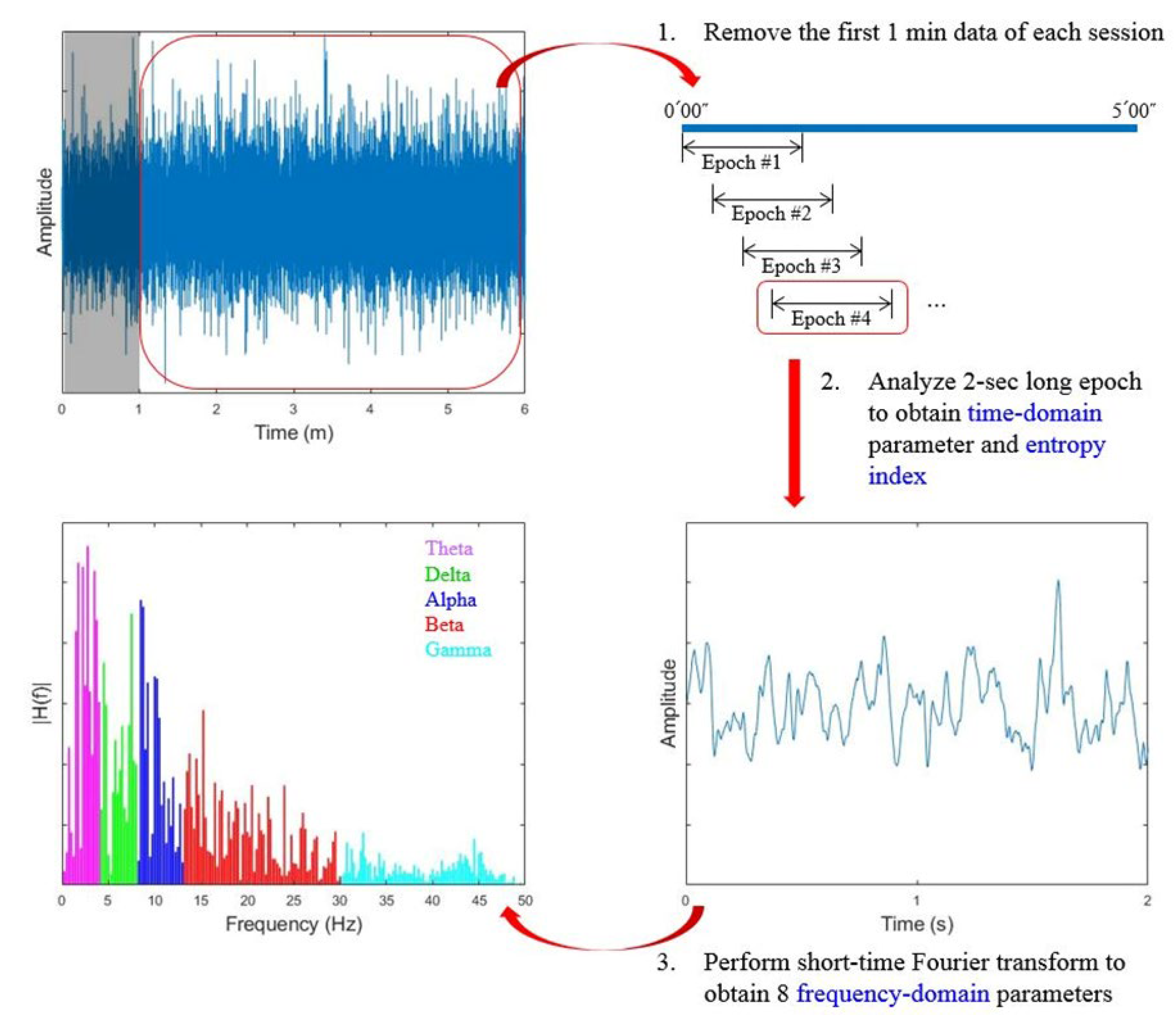
2.6. Data Processing
2.7. Prediction Model Development and Validation
2.8. Statistical Analysis
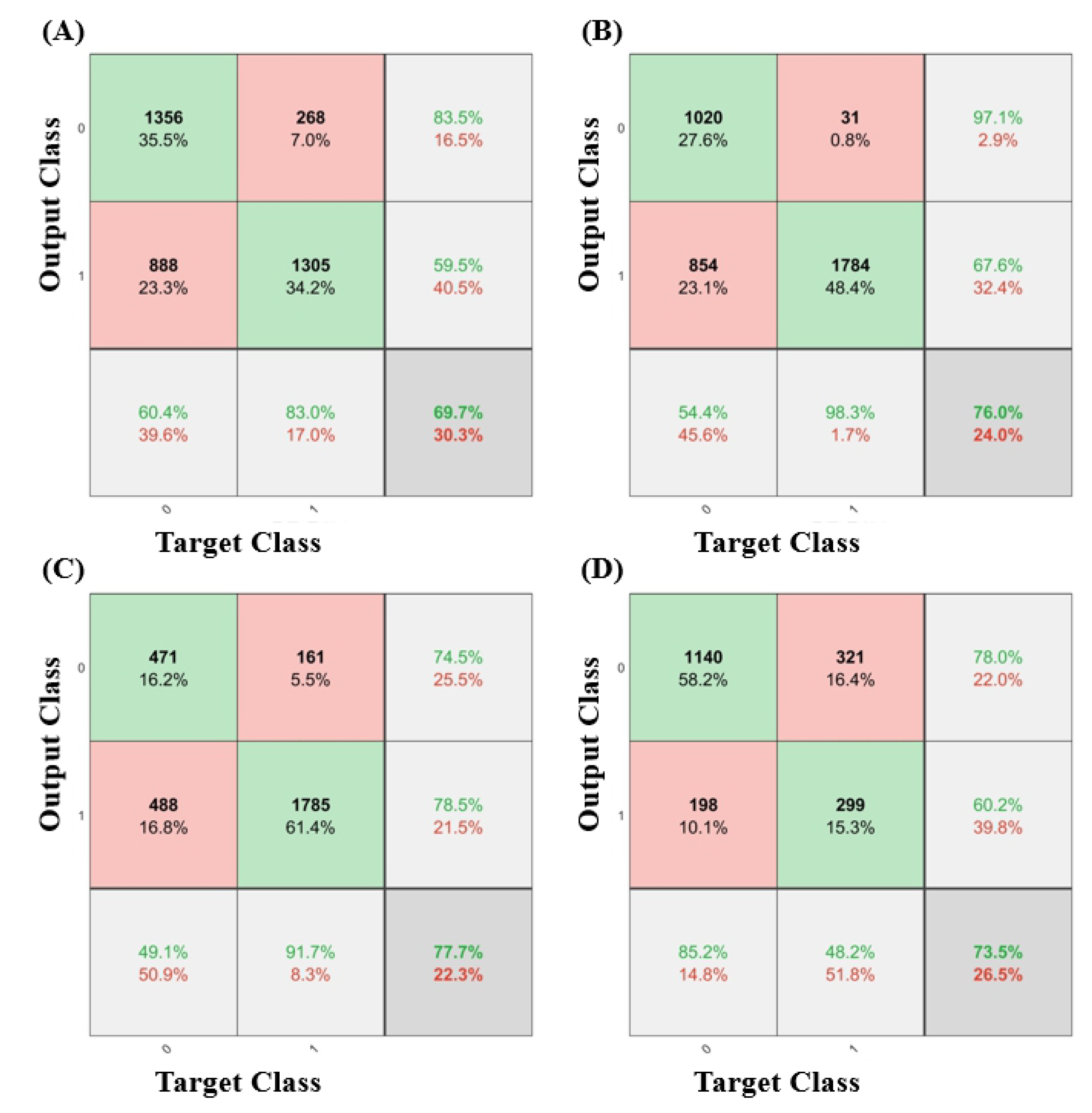
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| EEG Parameters | Definition | Domain |
|---|---|---|
| Magnitude of EEG | Maximal amplitude during the epoch | Time |
| DeltaR | log(P8–20 Hz/P1–4 Hz) | Frequency |
| DTABR | log(P1–8 Hz/P8–30 Hz) | Frequency |
| ThetaPR | P4–8 Hz/P0.5–47 Hz | Frequency |
| GammaPR | P30–47 Hz/P0.5–47 Hz | Frequency |
| Log energy entropy | Entropy | |
| SD_theta | Standard deviation of the amplitude in the theta band (4−8 Hz) | Frequency |
| SD_alpha | Standard deviation of the amplitude in the alpha band (8−13 Hz) | Frequency |
| SD_beta | Standard deviation of the amplitude in the beta band (13−30 Hz) | Frequency |
| SD_gamma | Standard deviation of the amplitude in the theta band (30−47 Hz) | Frequency |
References
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, Z.; Jafari, A.; Hatam, N.; Enaami, M. The economic burden of traumatic brain injury due to fatal traffic accidents in shahid rajaei trauma hospital, shiraz, iran. Arch. Trauma Res. 2015, 4, e22594. [Google Scholar] [CrossRef] [PubMed]
- Manskow, U.S.; Friborg, O.; Roe, C.; Braine, M.; Damsgard, E.; Anke, A. Patterns of change and stability in caregiver burden and life satisfaction from 1 to 2 years after severe traumatic brain injury: A Norwegian longitudinal study. NeuroRehabilitation 2017, 40, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bayen, E.; Jourdan, C.; Ghout, I.; Darnoux, E.; Azerad, S.; Vallat-Azouvi, C.; Weiss, J.J.; Aegerter, P.; Pradat-Diehl, P.; Joel, M.E.; et al. Objective and Subjective Burden of Informal Caregivers 4 Years after a Severe Traumatic Brain Injury: Results from the PariS-TBI Study. J. Head Trauma Rehabil. 2016, 31, E59–E67. [Google Scholar] [CrossRef]
- Kelly, D.F.; Kordestani, R.K.; Martin, N.A.; Nguyen, T.; Hovda, D.A.; Bergsneider, M.; McArthur, D.L.; Becker, D.P. Hyperemia following traumatic brain injury: Relationship to intracranial hypertension and outcome. J. Neurosurg. 1996, 85, 762–771. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Charry, J.D.; Rubiano, A.M.; Nikas, C.V.; Ortiz, J.C.; Puyana, J.C.; Carney, N.; Adelson, P.D. Results of early cranial decompression as an initial approach for damage control therapy in severe traumatic brain injury in a hospital with limited resources. J. Neurosci. Rural. Pract. 2016, 7, 7–12. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Gillman, L.M.; Teitelbaum, J.; West, M. Early Implementation of THAM for ICP Control: Therapeutic Hypothermia Avoidance and Reduction in Hypertonics/Hyperosmotics. Case Rep. Crit. Care 2014, 2014, 139342. [Google Scholar] [CrossRef]
- Anstey, J.R.; Taccone, F.S.; Udy, A.A.; Citerio, G.; Duranteau, J.; Ichai, C.; Badenes, R.; Prowle, J.R.; Ercole, A.; Oddo, M.; et al. Early Osmotherapy in Severe Traumatic Brain Injury: An International Multicenter Study. J. Neurotrauma 2020, 37, 178–184. [Google Scholar] [CrossRef]
- Meizoso, J.P.; Valle, E.J.; Allen, C.J.; Ray, J.J.; Jouria, J.M.; Teisch, L.F.; Shatz, D.V.; Namias, N.; Schulman, C.I.; Proctor, K.G. Decreased mortality after prehospital interventions in severely injured trauma patients. J. Trauma Acute Care Surg. 2015, 79, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Almenawer, S.; Hawryluk, G. Timing of Decompressive Craniectomy for Ischemic Stroke and Traumatic Brain Injury: A Review. Front. Neurol. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Rennert, R.C.; Gabel, B.C.; Sack, J.A.; Karanjia, N.; Warnke, P.; Chen, C.C. ICP management in patients suffering from traumatic brain injury: A systematic review of randomized controlled trials. Acta Neurochir. 2017, 159, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Peitz, G.; Ares, W.; Hafeez, S.; Grandhi, R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg. Focus 2017, 43, E6. [Google Scholar] [CrossRef] [PubMed]
- Cardim, D.; Robba, C.; Czosnyka, M.; Savo, D.; Mazeraud, A.; Iaquaniello, C.; Banzato, E.; Rebora, P.; Citerio, G. Noninvasive Intracranial Pressure Estimation with Transcranial Doppler: A Prospective Observational Study. J. Neurosurg. Anesthesiol. 2020, 32, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Cardim, D.; Robba, C.; Bohdanowicz, M.; Donnelly, J.; Cabella, B.; Liu, X.; Cabeleira, M.; Smielewski, P.; Schmidt, B.; Czosnyka, M. Non-invasive Monitoring of Intracranial Pressure Using Transcranial Doppler Ultrasonography: Is It Possible? Neurocrit. Care 2016, 25, 473–491. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.S.; Yun, S.J. Optic nerve sheath diameter measurement for predicting raised intracranial pressure in adult patients with severe traumatic brain injury: A meta-analysis. J. Crit. Care 2020, 56, 182–187. [Google Scholar] [CrossRef]
- Munawar, K.; Khan, M.T.; Hussain, S.W.; Qadeer, A.; Shad, Z.S.; Bano, S.; Abdullah, A. Optic Nerve Sheath Diameter Correlation with Elevated Intracranial Pressure Determined via Ultrasound. Cureus 2019, 11, e4145. [Google Scholar] [CrossRef]
- Singer, K.E.; Wallen, T.E.; Jalbert, T.; Wakefield, D.; Spuzzillo, A.; Sharma, S.; Earnest, R.; Heh, V.; Foreman, B.; Goodman, M.D. Efficacy of Noninvasive Technologies in Triaging Traumatic Brain Injury and Correlating with Intracranial Pressure: A Prospective Study. J. Surg. Res. 2021, 262, 27–37. [Google Scholar] [CrossRef]
- Laufs, H.; Kleinschmidt, A.; Beyerle, A.; Eger, E.; Salek-Haddadi, A.; Preibisch, C.; Krakow, K. EEG-correlated fMRI of human alpha activity. Neuroimage 2003, 19, 1463–1476. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Mao, S.; Dong, W.; Yang, H. A new method of intracranial pressure monitoring by EEG power spectrum analysis. Can. J. Neurol. Sci. 2012, 39, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Diedler, J.; Sykora, M.; Bast, T.; Poli, S.; Veltkamp, R.; Mellado, P.; Steiner, T.; Rupp, A. Quantitative EEG correlates of low cerebral perfusion in severe stroke. Neurocrit. Care 2009, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, A.; Perez-Romero, M.; Pastor, J.; Sola, R.G.; Vega-Zelaya, L.; Monasterio, F.; Torrecilla, C.; Vega, G.; Pulido, P.; Ortega, G.J. Identifying causal relationships between EEG activity and intracranial pressure changes in neurocritical care patients. J. Neural Eng. 2018, 15, 066029. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.; Kim, Y.T.; Won, K.; Czosnyka, M.; Kim, D.J. Prediction of Life-Threatening Intracranial Hypertension during the Acute Phase of Traumatic Brain Injury Using Machine Learning. IEEE J. Biomed. Health Inform. 2021, 25, 3967–3976. [Google Scholar] [CrossRef] [PubMed]
- Carra, G.; Guiza, F.; Piper, I.; Citerio, G.; Maas, A.; Depreitere, B.; Meyfroidt, G.; CENTER-TBI High-Resolution ICU (HR ICU) Sub-Study Participants and Investigators. Development and External Validation of a Machine Learning Model for the Early Prediction of Doses of Harmful Intracranial Pressure in Patients with Severe Traumatic Brain Injury. J. Neurotrauma 2022. [Google Scholar] [CrossRef]
- Mangat, H.S.; Chiu, Y.L.; Gerber, L.M.; Alimi, M.; Ghajar, J.; Hartl, R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J. Neurosurg. 2015, 122, 202–210. [Google Scholar] [CrossRef]
- Bellner, J.; Romner, B.; Reinstrup, P.; Kristiansson, K.A.; Ryding, E.; Brandt, L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg. Neurol. 2004, 62, 45–51, discussion 51. [Google Scholar] [CrossRef]
- Homburg, A.M.; Jakobsen, M.; Enevoldsen, E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol. Scand. 1993, 87, 488–493. [Google Scholar] [CrossRef]
- Varsos, G.V.; Kolias, A.G.; Smielewski, P.; Brady, K.M.; Varsos, V.G.; Hutchinson, P.J.; Pickard, J.D.; Czosnyka, M. A noninvasive estimation of cerebral perfusion pressure using critical closing pressure. J. Neurosurg. 2015, 123, 638–648. [Google Scholar] [CrossRef]
- Sloan, M.A.; Alexandrov, A.V.; Tegeler, C.H.; Spencer, M.P.; Caplan, L.R.; Feldmann, E.; Wechsler, L.R.; Newell, D.W.; Gomez, C.R.; Babikian, V.L.; et al. Assessment: Transcranial Doppler ultrasonography: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004, 62, 1468–1481. [Google Scholar] [CrossRef]
- Borges, M.A.; Botos, H.J.; Bastos, R.F.; Godoy, M.F.; Marchi, N.S. Emergency EEG: Study of survival. Arq. Neuropsiquiatr. 2010, 68, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Su, Y.; Hussain, M.; Chen, W.; Ye, H.; Gao, D.; Tian, F. Poor outcome prediction by burst suppression ratio in adults with post-anoxic coma without hypothermia. Neurol. Res. 2014, 36, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.; Hong, K.J.; Shin, S.D.; Kim, H.C.; Park, Y.J.; Ro, Y.S.; Song, K.J.; Kim, K.H.; Choi, D.S.; et al. Prediction of cerebral perfusion pressure during CPR using electroencephalogram in a swine model of ventricular fibrillation. Am. J. Emerg. Med. 2021, 45, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, Y.Y.; Haupt, W.F.; Zhao, J.W.; Xiao, S.Y.; Li, H.L.; Pang, Y.; Yang, Q.L. Application of electrophysiologic techniques in poor outcome prediction among patients with severe focal and diffuse ischemic brain injury. J. Clin. Neurophysiol. 2011, 28, 497–503. [Google Scholar] [CrossRef] [PubMed]
| Study Group | Derivation Group (N = 21) | Validation Group (N = 9) | ||
|---|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | p-Value | |
| Bwt, Kg | 41.3 (2.3) | 44.8 (2.3) | <0.01 | |
| SBP, mmHg | 108.9 (14.5) | 105.3 (14.4) | <0.01 | |
| DBP, mmHg | 71.2 (13.9) | 65.4 (11.2) | <0.01 | |
| MAP, mmHg | 83.8 (13.9) | 78.7 (12.1) | <0.01 | |
| HR, beat/min | 99.1 (16.9) | 91.6 (11.0) | <0.01 | |
| BT, °C | 37.2 (1.1) | 36.2 (1.0) | <0.01 | |
| ICP, mmHg | 12.4 (6.0) | 15.5 (4.9) | <0.01 | |
| ABGA | pH | 7.56 (0.05) | 7.52 (0.03) | 0.1 |
| pCO2 | 33.66 (7.10) | 39.44 (3.85) | 0.03 | |
| pO2 | 156.92 (102.32) | 113.92 (54.99) | 0.25 | |
| SpO2 | 98.41 (2.77) | 97.96 (1.66) | 0.65 | |
| HCO3 | 29.84 (4.41) | 32.70 (3.75) | 0.1 | |
| Hb | 9.39 (1.38) | 9.52 (0.89) | 0.8 | |
| Na | 136.56 (4.73) | 139.91 (2.65) | 0.06 | |
| LA | 1.64 (0.72) | 1.87 (0.49) | 0.4 | |
| Variables | ICP < 20 mmHg | ICP 20~30 mmHg | ICP 30~40 mmHg | ICP 40~50 mmHg | ICP ≥ 50 mmHg | p-Value | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Mean arterial pressure, mmHg | 90.2 (12.2) | 91.6 (19.6) | 101.1 (21.6) | 98.2 (24.4) | 120.1 (11.2) | <0.001 | |
| Heartrate, rate/min | 96.1 (16.9) | 103.2 (19.6) | 104.9 (20.4) | 114.8 (30.4) | 134.8 (52.0) | <0.001 | |
| EEG Indexes | Magnitude of EEG | 32.51 (17.14) | 33.54 (15.44) | 37.49 (18.20) | 37.68 (17.44) | 41.51 (9.83) | <0.001 |
| DELTAR | −0.81 (0.31) | −0.72 (0.42) | −0.87 (0.40) | −0.92 (0.33) | −0.39 (0.37) | <0.001 | |
| DTABR | 0.78 (0.29) | 0.70 (0.36) | 0.83 (0.35) | 0.86 (0.30) | 0.39 (0.33) | <0.001 | |
| THETAPR | 0.14 (0.05) | 0.14 (0.05) | 0.12 (0.05) | 0.11 (0.05) | 0.08 (0.02) | <0.001 | |
| GAMMAPR | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.02) | 0.07 (0.01) | <0.001 | |
| Log energy entropy | 1811.04 (475.04) | 1833.23 (455.48) | 1994.28 (508.89) | 2008.83 (470.69) | 1743.32 (282.71) | <0.001 | |
| SD_theta | 4.22 (2.56) | 4.24 (2.27) | 4.59 (2.62) | 4.28 (2.39) | 3.12 (1.18) | <0.001 | |
| SD_alpha | 2.95 (1.70) | 3.14 (1.58) | 3.21 (1.65) | 3.06 (1.57) | 3.52 (0.99) | <0.001 | |
| SD_beta | 3.19 (2.06) | 3.29 (1.52) | 3.41 (1.66) | 3.46 (1.72) | 8.10 (2.18) | <0.001 | |
| SD_gamma | 1.47 (0.90) | 1.57 (0.82) | 1.68 (0.95) | 1.81 (0.99) | 2.81 (0.63) | <0.001 | |
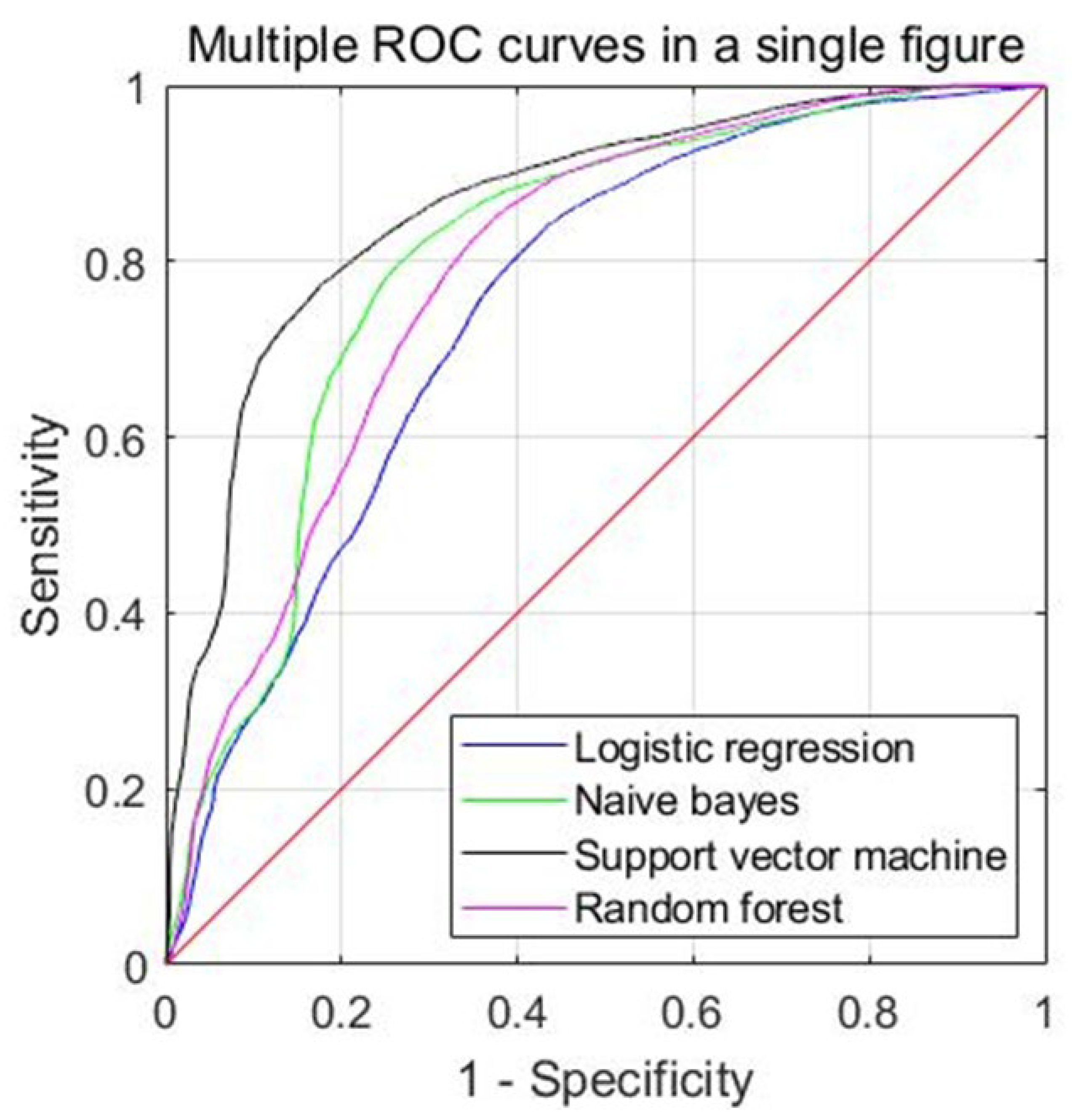
| Models | Accuracy | Sensitivity | Specificity | Precision | F1-Score | MCC | AUC |
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| LR | 0.706 (0.625–0.787) | 0.535 (0.374–0.696) | 0.822 (0.727–0.916) | 0.649 (0.464–0.834) | 0.506 (0.358–0.653) | 0.322 (0.176–0.469) | 0.748 (0.644–0.851) |
| NB | 0.749 (0.658–0.840) | 0.495 (0.316–0.675) | 0.874 (0.760–0.989) | 0.843 (0.730–0.956) | 0.572 (0.427–0.717) | 0.377 (0.218–0.536) | 0.824 (0.740–0.907) |
| SVM | 0.773 (0.694–0.853) | 0.506 (0.315–0.697) | 0.923 (0.857–0.988) | 0.870 (0.755–0.985) | 0.587 (0.412–0.761) | 0.425 (0.259–0.591) | 0.860 (0.781–0.938) |
| RF | 0.746 (0.673–0.818) | 0.654 (0.498–0.811) | 0.790 (0.671–0.910) | 0.670 (0.494–0.845) | 0.588 (0.428–0.747) | 0.403 (0.254–0.553) | 0.802 (0.710–0.894) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Kim, H.; Song, K.-J.; Shin, S.-D.; Kim, H.-C.; Lim, H.-J.; Kim, Y.; Kang, H.-J.; Hong, K.-J. Prediction of Increased Intracranial Pressure in Traumatic Brain Injury Using Quantitative Electroencephalogram in a Porcine Experimental Model. Diagnostics 2023, 13, 386. https://doi.org/10.3390/diagnostics13030386
Kim K-H, Kim H, Song K-J, Shin S-D, Kim H-C, Lim H-J, Kim Y, Kang H-J, Hong K-J. Prediction of Increased Intracranial Pressure in Traumatic Brain Injury Using Quantitative Electroencephalogram in a Porcine Experimental Model. Diagnostics. 2023; 13(3):386. https://doi.org/10.3390/diagnostics13030386
Chicago/Turabian StyleKim, Ki-Hong, Heejin Kim, Kyoung-Jun Song, Sang-Do Shin, Hee-Chan Kim, Hyouk-Jae Lim, Yoonjic Kim, Hyun-Jeong Kang, and Ki-Jeong Hong. 2023. "Prediction of Increased Intracranial Pressure in Traumatic Brain Injury Using Quantitative Electroencephalogram in a Porcine Experimental Model" Diagnostics 13, no. 3: 386. https://doi.org/10.3390/diagnostics13030386
APA StyleKim, K.-H., Kim, H., Song, K.-J., Shin, S.-D., Kim, H.-C., Lim, H.-J., Kim, Y., Kang, H.-J., & Hong, K.-J. (2023). Prediction of Increased Intracranial Pressure in Traumatic Brain Injury Using Quantitative Electroencephalogram in a Porcine Experimental Model. Diagnostics, 13(3), 386. https://doi.org/10.3390/diagnostics13030386






