Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis
Abstract
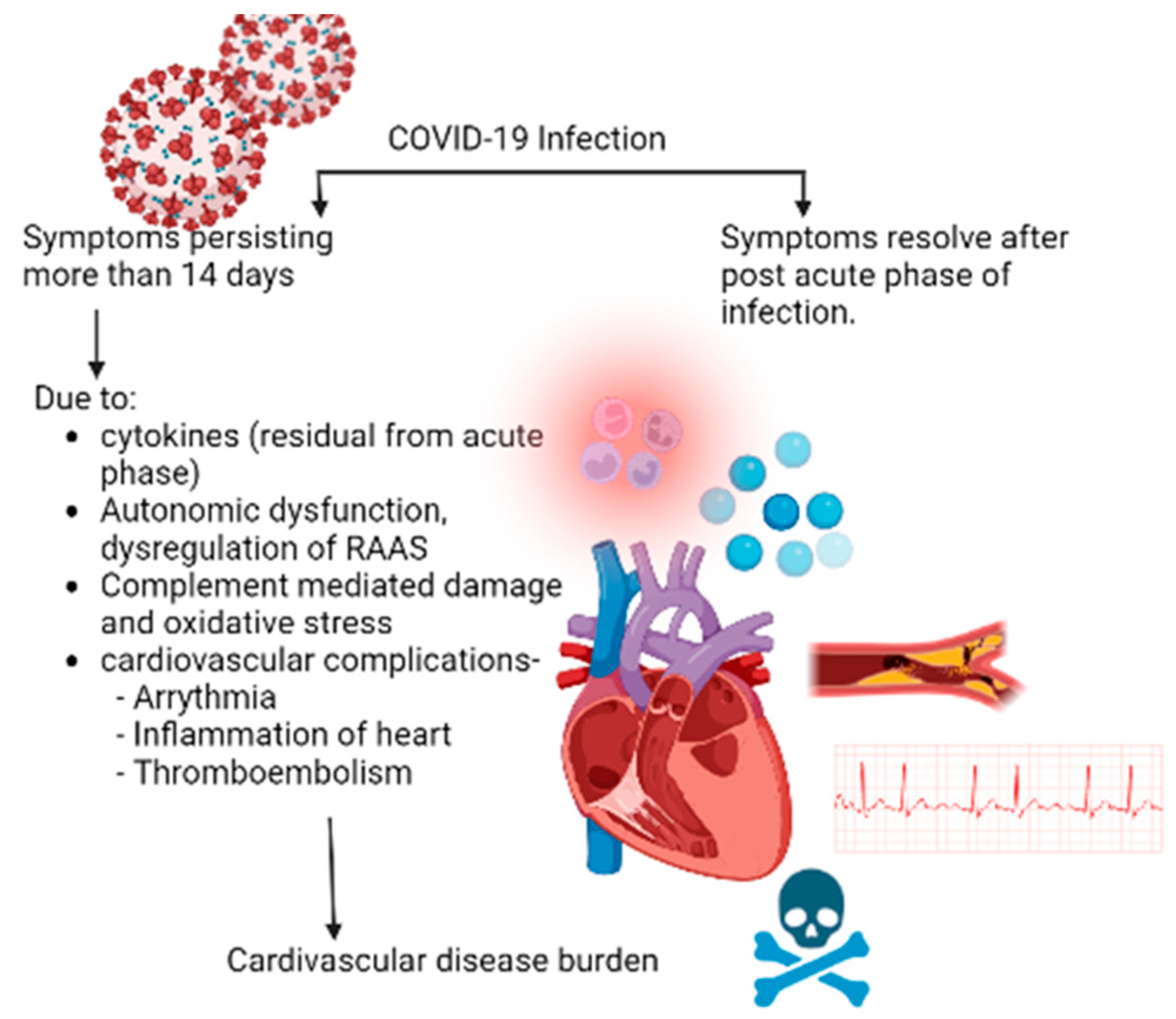
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Patient and Study Characteristics
3.3. Risk of Bias Assessment
3.4. Meta-Analysis
3.4.1. Electrophysiological Abnormalities (Arrhythmias)
3.4.2. Cardiac Tissue Abnormalities
3.4.3. Coronary Vessel Abnormalities
3.4.4. Thromboembolic Disorders
3.4.5. MACE and Mortality
3.5. Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Pace, N.L.; Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Curr. Cardiol. Rep. 2022, 24, 1711–1726. [Google Scholar] [CrossRef] [PubMed]
- CDC. Post-COVID Conditions: Information for Healthcare Providers. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed on 23 November 2022).
- Scala, I.; Rizzo, P.A.; Bellavia, S.; Brunetti, V.; Colò, F.; Broccolini, A.; Della Marca, G.; Calabresi, P.; Luigetti, M.; Frisullo, G. Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review. J. Clin. Med. 2022, 11, 3883. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering from COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.-Y.; Wang, S.-I.; Wei, J.C.-C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. eClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Rezel-Potts, E.; Douiri, A.; Sun, X.; Chowienczyk, P.J.; Shah, A.M.; Gulliford, M.C. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022, 19, e1004052. [Google Scholar] [CrossRef]
- Asarcikli, L.D.; Hayiroglu, M.İ.; Osken, A.; Keskin, K.; Kolak, Z.; Aksu, T. Heart rate variability and cardiac autonomic functions in post-COVID period. J. Interv. Card. Electrophysiol. 2022, 63, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Kunal, S.; Bansal, A.; Jain, J.; Poundrik, S.; Shetty, M.K.; Batra, V.; Chaturvedi, V.; Yusuf, J.; Mukhopadhyay, S.; et al. Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol. J. 2022, 22, 70–76. [Google Scholar] [CrossRef]
- Szekely, Y.; Lichter, Y.; Sadon, S.; Lupu, L.; Taieb, P.; Banai, A.; Sapir, O.; Granot, Y.; Hochstadt, A.; Friedman, S.; et al. Cardiorespiratory Abnormalities in Patients Recovering from Coronavirus Disease 2019. J. Am. Soc. Echocardiogr. 2021, 34, 1273–1284.e9. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 23 November 2022).
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Tacey, A.; Apostolopoulos, V.; Levinger, I.; Rimarova, K.; Egom, E.E.; Rodrigo, L.; Kruzliak, P.; et al. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin. Exp. Pharmacol. Physiol. 2020, 47, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, G.; Ricci, F.; Recce, V.; Serio, A.; Iannetti, G.; Chahal, A.A.; Ståhlberg, M.; Khanji, M.Y.; Fedorowski, A.; Gallina, S. Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? J. Cardiovasc. Dev. Dis. 2021, 8, 156. [Google Scholar] [CrossRef]
- Hartmann, C.; Miggiolaro, A.F.R.D.S.; Motta, J.d.S.J.; Carstens, L.B.; De Paula, C.B.V.; Grobe, S.F.; Nunes, L.H.D.S.; Marques, G.L.; Libby, P.; Moura, L.Z.; et al. The Pathogenesis of COVID-19 Myocardial Injury: An Immunohistochemical Study of Postmortem Biopsies. Front. Immunol. 2021, 12, 748417. [Google Scholar] [CrossRef]
- Zhao, X.; Nicholls, J.M.; Chen, Y.-G. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-β signaling. J. Biol. Chem. 2008, 283, 3272–3280. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Pathan, N.; A Hemingway, C.; Alizadeh, A.A.; Stephens, A.C.; Boldrick, J.C.; E Oragui, E.; McCabe, C.; Welch, S.B.; Whitney, A.; O’Gara, P.; et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004, 363, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Falasca, L.; Nardacci, R.; Colombo, D.; Lalle, E.; Di Caro, A.; Nicastri, E.; Antinori, A.; Petrosillo, N.; Marchioni, L.; Biava, G.; et al. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J. Infect Dis. 2020, 222, 1807–1815. [Google Scholar] [CrossRef]
- Cheng, R.; Leedy, D. COVID-19 and acute myocardial injury: The heart of the matter or an innocent bystander? Heart 2020, 106, 1122–1124. [Google Scholar] [CrossRef]
- Peiris, J.; Chu, C.; Cheng, V.; Chan, K.; Hung, I.; Poon, L.; Law, K.; Tang, B.; Hon, T.; Chan, C.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Hayward, A.; Hemingway, H.; Denaxas, S.; Thomas, S.L.; Timmis, A.D.; Whitaker, H.; Smeeth, L. Influenza infection and risk of acute myocardial infarction in England and Wales: A CALIBER self-controlled case series study. J. Infect Dis. 2012, 206, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Y.; Geng, Y.J.; Huang, J.; Qian, H.Y. Pathogenesis and management of myocardial injury in coronavirus disease 2019. Eur. J. Heart Fail. 2020, 22, 1994–2006. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin. Rheumatol. 2020, 39, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Favaloro, E.J.; Lavie, C.J.; Henry, B.M. Coronavirus Disease 2019-Associated Coagulopathy. Mayo Clin. Proc. 2021, 96, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Dherange, P.; Lang, J.; Qian, P.; Oberfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19: A Review. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Kolettis, T.M. Coronary artery disease and ventricular tachyarrhythmia: Pathophysiology and treatment. Curr. Opin. Pharmacol. 2013, 13, 210–217. [Google Scholar] [CrossRef]
- Gettes, L.S. Electrolyte abnormalities underlying lethal and ventricular arrhythmias. Circulation 1992, 85 (Suppl. S1), 170–176. [Google Scholar]

| Study | Country | Study Design | Number of Participants (Cases) | Male (Percentage) | Age in Years; Median/Mean; Range | Evaluation Following Infection | Comorbidities Assessment |
|---|---|---|---|---|---|---|---|
| Ayoubkhani et al., 2021 [8] | England | Retrospective cohort study | 47,780 | 26245 (54.9) | Cases:64.5 ± 19.2 Controls: NA | Mean follow-up of 140 days, SD 50 days | Yes |
| Wang et al., 2022 [9] | United States | Retrospective cohort study | 690,892 | 298223 (43.2) | Cases: 43.2 ± 16.2 Controls: 44.5 ± 17.0 | After 30 days | Yes |
| Xie et al., 2022 [10] | United States | Retrospective cohort study | 153,760 | NA | Cases: 61.42 ± 15.64 Controls: 63.46 ± 16.23 | After 30 days | NA |
| Rezel-Potts et al., 2021 [11] | England | Prospective matched-cohort study | 428,650 | 167,867 (45) | Cases/Controls: 35 (22 to 50) | Post-acute: 5 to 12; Long: 13 to 52 weeks from index | NA |
| Asarcikli et al., 2022 [12] | Turkey | Retrospective observational study | 60 | 23 (38.3) | Cases: 30 (26–42) Controls: 39 (31–49) | After 12 weeks | None; healthy individuals |
| Shah et al., 2022 [13] | India | Prospective observational study | 92 | 54 (58.7) | Cases: 50.6 ± 12.1 Controls: 51.8 ± 4.2 | After 30 days | None; healthy individuals |
| Szekely et al., 2021 [14] | Israel | Prospective observational study | 71 | 47 (66) | Cases: 52.6 ± 16 Controls: 53.6 ± 16 | After 3 months | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, A.B.; Mehta, A.; Pokharel, P.; Mishra, A.; Adhikari, L.; Shrestha, S.; Yadav, R.S.; Khanal, S.; Sah, R.; Nowrouzi-Kia, B.; et al. Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 491. https://doi.org/10.3390/diagnostics13030491
Shrestha AB, Mehta A, Pokharel P, Mishra A, Adhikari L, Shrestha S, Yadav RS, Khanal S, Sah R, Nowrouzi-Kia B, et al. Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics. 2023; 13(3):491. https://doi.org/10.3390/diagnostics13030491
Chicago/Turabian StyleShrestha, Abhigan Babu, Aashna Mehta, Pashupati Pokharel, Aakash Mishra, Lukash Adhikari, Sajina Shrestha, Randhir Sagar Yadav, Surakshya Khanal, Ranjit Sah, Behdin Nowrouzi-Kia, and et al. 2023. "Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis" Diagnostics 13, no. 3: 491. https://doi.org/10.3390/diagnostics13030491
APA StyleShrestha, A. B., Mehta, A., Pokharel, P., Mishra, A., Adhikari, L., Shrestha, S., Yadav, R. S., Khanal, S., Sah, R., Nowrouzi-Kia, B., Padhi, B. K., & Chattu, V. K. (2023). Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics, 13(3), 491. https://doi.org/10.3390/diagnostics13030491










