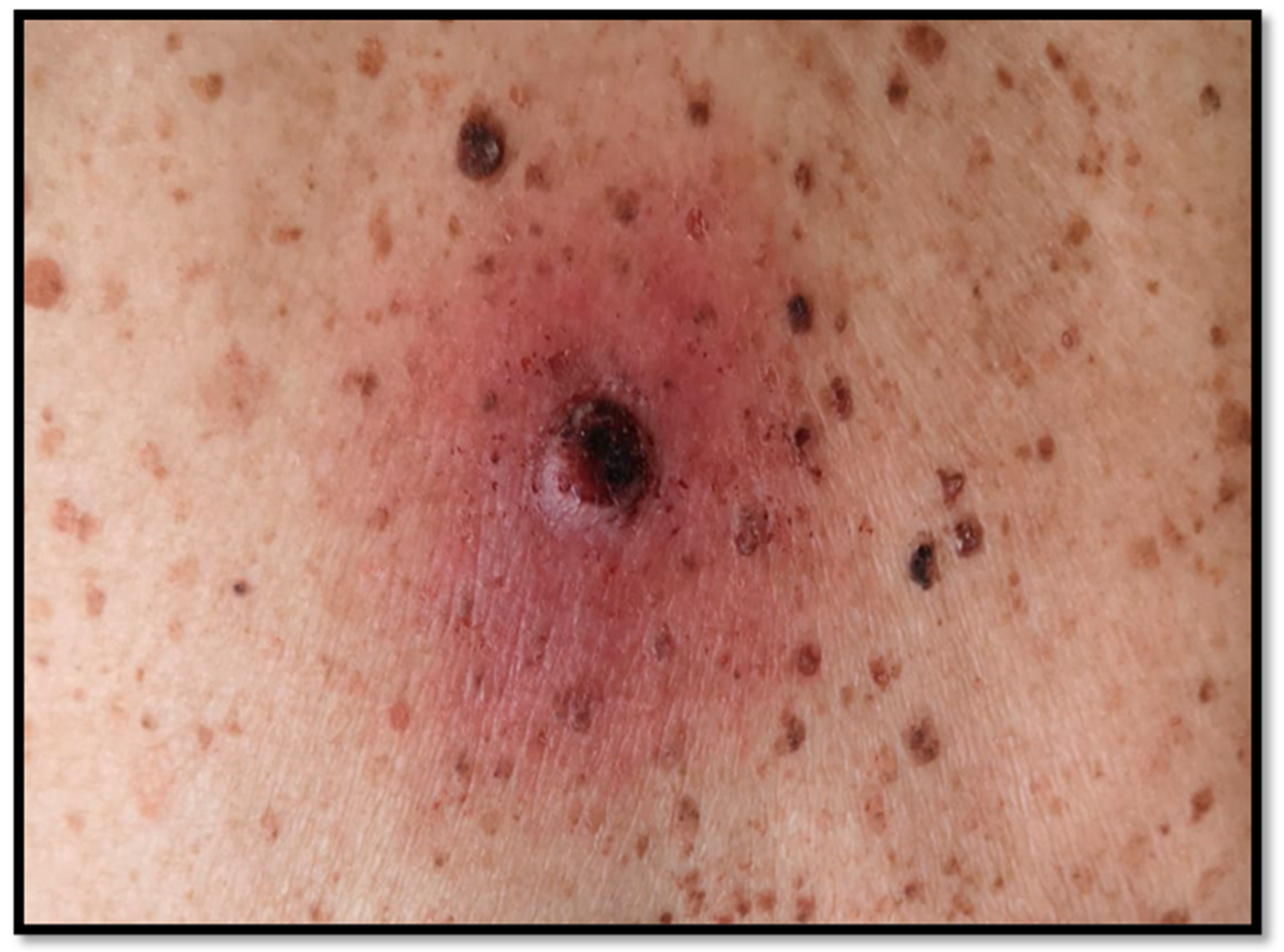
Partially Dedifferentiated Primitive Malignant Melanoma with Pseudo-Angiomatous Features: A Case Report with Review of the Literature
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Demarco, A.; Lospalluti, L.; Arezzo, F.; Resta, L.; Ingravallo, G. The Great Mime: Three Cases of Melanoma with Carcinoid-Like and Paraganglioma-Like Pattern with Emphasis on Differential Diagnosis. Dermatopathology 2021, 8, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- DeWane, M.E.; Kelsey, A.; Oliviero, M.; Rabinovitz, H.; Grant-Kels, J.M. Melanoma on chronically sun-damaged skin: Lentigo maligna and desmoplastic melanoma. J. Am. Acad. Dermatol. 2019, 81, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Haenssle, H.A.; Hoffmann, S.; Buhl, T.; Emmert, S.; Schön, M.P.; Bertsch, H.P.; Rosenberger, A. Assessment of melanoma histotypes and associated patient related factors: Basis for a predictive statistical model. JDDG J. Der Dtsch. Dermatol. Ges. 2015, 13, 37–44. [Google Scholar] [CrossRef]
- Bobos, M. Histopathologic classification and prognostic factors of melanoma: A 2021 update. G. Ital. Di Dermatol. E Venereol. 2021, 156, 300–321. [Google Scholar] [CrossRef]
- Adya, K.A.; Inamadar, A.C.; Palit, A.; Nikam, B.P. Acral Amelanotic Melanoma in Fitzpatrick Type IV Skin. Skinmed 2021, 19, 134–136. [Google Scholar]
- Limongelli, L.; Cascardi, E.; Capodiferro, S.; Favia, G.; Corsalini, M.; Tempesta, A.; Maiorano, E. Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics 2020, 10, 424. [Google Scholar] [CrossRef]
- Cazzato, G.; Cascardi, E.; Colagrande, A.; Belsito, V.; Lospalluti, L.; Foti, C.; Arezzo, F.; Dellino, M.; Casatta, N.; Lupo, C.; et al. PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience. Diagnostics 2022, 12, 2197. [Google Scholar] [CrossRef]
- Nicolson, N.G.; Han, D. Desmoplastic melanoma. J. Surg. Oncol. 2019, 119, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, N.M.; Singh, S.; Thomas, C.; Van Vliet, C.; Harvey, N.T.; Calonje, J.E.; Wood, B.A. Mitotically Active Nevus and Nevoid Melanoma: A Clinicopathological and Molecular Study. Am. J. Dermatopathol. 2020, 43, 182–190. [Google Scholar] [CrossRef]
- Cabrera, R.; Recule, F. Unusual Clinical Presentations of Malignant Melanoma: A Review of Clinical and Histologic Features with Special Emphasis on Dermatoscopic Findings. Am. J. Clin. Dermatol. 2018, 19, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, R.L.; Gupta, K. Unusual variants of malignant melanoma. Clin. Dermatol. 2009, 27, 564–587. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Lospalluti, L.; Colagrande, A.; Cimmino, A.; Romita, P.; Foti, C.; Demarco, A.; Arezzo, F.; Loizzi, V.; Cormio, G.; et al. Dedifferentiated Melanoma: A Diagnostic Histological Pitfall—Review of the Literature with Case Presentation. Dermatopathology 2021, 8, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Pisacane, A.; Cascardi, E.; Berrino, E.; Polidori, A.; Sarotto, I.; Casorzo, L.; Panero, M.; Boccaccio, C.; Verginelli, F.; Benvenuti, S.; et al. Real-world histopathological approach to malignancy of undefined primary origin (MUO) to diagnose cancers of unknown primary (CUPs). Virchows Arch. 2022, 1–13, Erratum in Virchows Arch. 2022, Epub ahead of print. [Google Scholar] [CrossRef]
- Massi, D.; Mihic-Probst, D.; Schadendorf, D.; Dummer, R.; Mandalà, M. Dedifferentiated melanomas: Morpho-phenotypic profile, genetic reprogramming and clinical implications. Cancer Treat. Rev. 2020, 88, 102060. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Shevchenko, A.; Lee, J.B. Evolution of a melanoma in situ to a sarcomatoid dedifferentiated melanoma. J. Cutan. Pathol. 2021, 48, 943–947. [Google Scholar] [CrossRef]
- Available online: http://www.prisma-statement.org/ (accessed on 2 December 2022).
- Adler, M.J.; Beckstead, J.; White, C.R., Jr. Angiomatoid Melanoma: A Case of Metastatic Melanoma Mimicking a Vascular Malignancy. Am. J. Dermatopathol. 1997, 19, 606–609. [Google Scholar] [CrossRef]
- Baron, J.; Monzon, F.; Galaria, N.; Murphy, G.F. Angiomatoid melanoma: A novel pattern of differentiation in invasive periocular desmoplastic malignant melanoma. Hum. Pathol. 2000, 31, 1520–1522. [Google Scholar] [CrossRef]
- Zelger, B.G.; Zelger, B. Angiomatoid Metastatic Melanoma. Dermatol. Surg. 2004, 30, 336–340. [Google Scholar] [CrossRef]
- Ramos-Rodríguez, G.; Ortiz-Hidalgo, C. Primary angiomatoid melanoma as an exceptional morphologic pattern in cu-taneous melanoma. A case report and review of the literature. Actas Dermosifiliogr. 2015, 106, e13–e17. [Google Scholar] [CrossRef]
- Fonda-Pascual, P.; Moreno-Arrones, O.M.; Alegre-Sanchez, A.; Real, C.M.G.-D.; Miguel-Gomez, L.; Martin-Gonzalez, M. Primary cutaneous angiomatoid melanoma. JDDG J. Der Dtsch. Dermatol. Ges. 2018, 16, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’Er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular Channel Formation by Human Melanoma Cells in Vivo and in Vitro: Vasculogenic Mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef] [PubMed]


| Number of Case | Sex, Age | Localization | Histological Diagnosis | Immunohistochemical Features |
|---|---|---|---|---|
| 1 | M, 44 | Intravertebral Metastasis on forehead | MM Angiomatoid metastatic MM | S-100, HMB-45 and vimentin: positivity (+) |
| 2 | M, 84 | Periorbital region | Primitive DM with angiomatoid pattern | S-100 positivity (+) |
| 3 | F, 56 | Right arm Metastasis back | MM Angiomatoid MM | S-100 positivity (+), Melan-A and HMB-45 positivity (+) |
| 4 | M, 61 | Third finger of the left hand Axillary lymph node | MM Metastatic angiomatoid MM | S-100 positivity (+) |
| 5 | M, 59 | Right thigh | Angiomatoid MM | S-100 positivity (+), HMB-45 positivity (+) |
| 6 | F, 63 | Right scapular region | Angiomatoid MM | S-100, Melan-A e HMB-45 positivity (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrogio, F.; Colagrande, A.; Cascardi, E.; Grandolfo, M.; Filotico, R.; Foti, C.; Lupo, C.; Casatta, N.; Ingravallo, G.; Cazzato, G. Partially Dedifferentiated Primitive Malignant Melanoma with Pseudo-Angiomatous Features: A Case Report with Review of the Literature. Diagnostics 2023, 13, 495. https://doi.org/10.3390/diagnostics13030495
Ambrogio F, Colagrande A, Cascardi E, Grandolfo M, Filotico R, Foti C, Lupo C, Casatta N, Ingravallo G, Cazzato G. Partially Dedifferentiated Primitive Malignant Melanoma with Pseudo-Angiomatous Features: A Case Report with Review of the Literature. Diagnostics. 2023; 13(3):495. https://doi.org/10.3390/diagnostics13030495
Chicago/Turabian StyleAmbrogio, Francesca, Anna Colagrande, Eliano Cascardi, Mauro Grandolfo, Raffaele Filotico, Caterina Foti, Carmelo Lupo, Nadia Casatta, Giuseppe Ingravallo, and Gerardo Cazzato. 2023. "Partially Dedifferentiated Primitive Malignant Melanoma with Pseudo-Angiomatous Features: A Case Report with Review of the Literature" Diagnostics 13, no. 3: 495. https://doi.org/10.3390/diagnostics13030495
APA StyleAmbrogio, F., Colagrande, A., Cascardi, E., Grandolfo, M., Filotico, R., Foti, C., Lupo, C., Casatta, N., Ingravallo, G., & Cazzato, G. (2023). Partially Dedifferentiated Primitive Malignant Melanoma with Pseudo-Angiomatous Features: A Case Report with Review of the Literature. Diagnostics, 13(3), 495. https://doi.org/10.3390/diagnostics13030495










