Ultrasound Renal Score to Predict the Renal Disease Prognosis in Patients with Diabetic Kidney Disease: An Investigative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Parameters
2.3. Study Group Design and End-Points
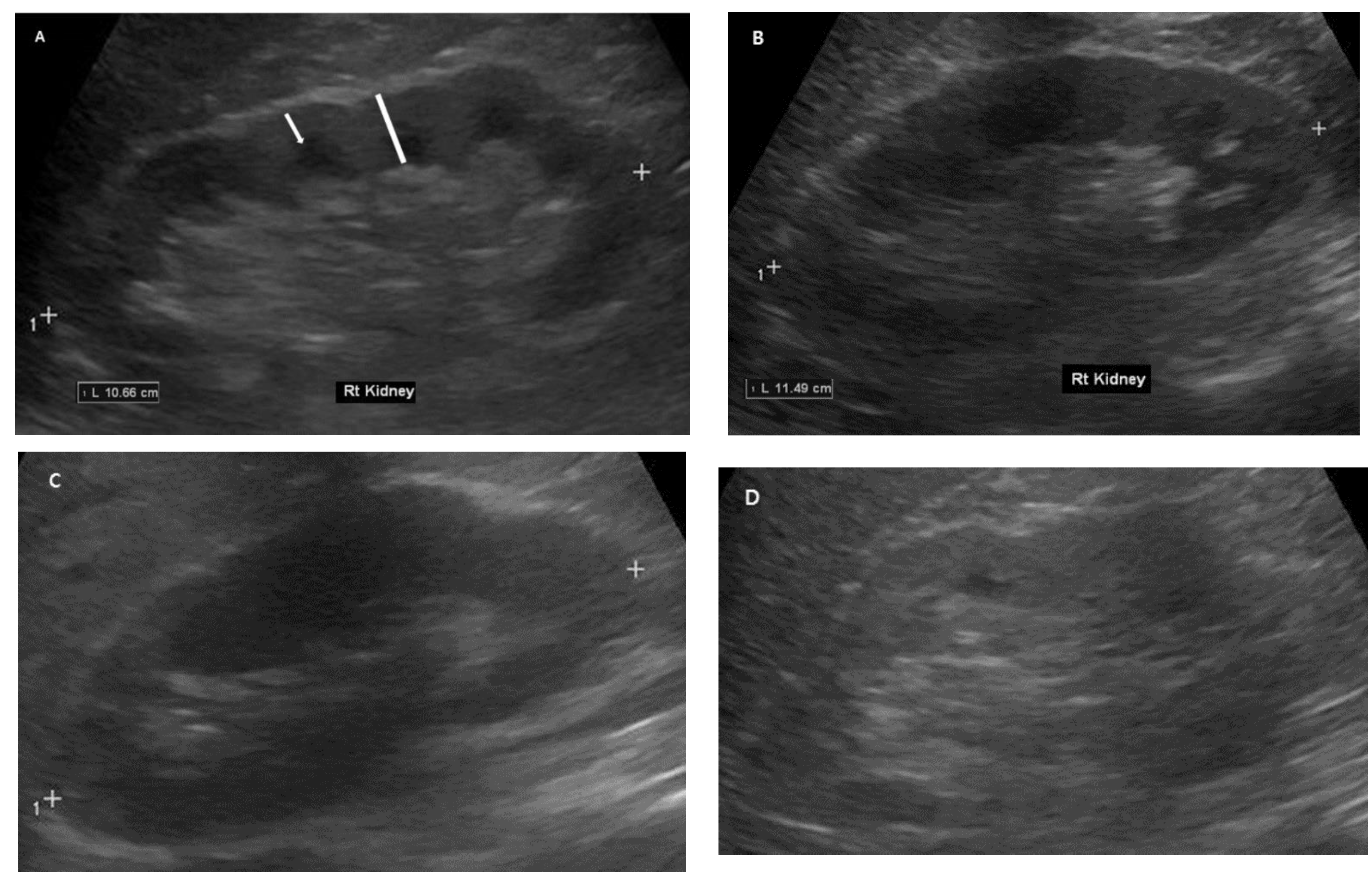
2.4. Renal Ultrasonography Examinations
2.5. Statistical Analysis
3. Results
3.1. Clinical Baseline Characteristics and Differences between Patients with and without DM
3.2. Baseline Renal Ultrasonography Findings and Differences between Patients with and without DM
3.3. Association between Renal Ultrasonography Measurements and Baseline Clinical Parameters
3.4. Association between Clinical and Ultrasonography Parameters and Renal Prognosis
3.5. Association between High Renal Scoring and Kidney Disease Progression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, G.; Bonfanti, R.; Meschi, F.; Bognetti, E.; Paesano, P.L.; Gianolli, L.; Querques, M.; Maestroni, A.; Calori, G.; Del Maschio, A.; et al. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes 2006, 55, 2620–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, R.A.; Wang, Y.; Zhu, V.; Rupnow, M.F. Chronic kidney disease in US adults with type 2 diabetes: An updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res. Notes 2014, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Plantinga, L.C.; Crews, D.C.; Coresh, J.; Miller, E.R., 3rd; Saran, R.; Yee, J.; Hedgeman, E.; Pavkov, M.; Eberhardt, M.S.; Williams, D.E.; et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin. J. Am. Soc. Nephrol. 2010, 5, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Zwi, L.J.; Collins, J.F.; Marshall, M.R.; Cundy, T. Presentation, pathology and prognosis of renal disease in type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000412. [Google Scholar] [CrossRef] [Green Version]
- Lucisano, G.; Comi, N.; Pelagi, E.; Cianfrone, P.; Fuiano, L.; Fuiano, G. Can renal sonography be a reliable diagnostic tool in the assessment of chronic kidney disease? J. Ultrasound Med. 2015, 34, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Romano, G.; Romano, M.; Rastelli, S.; Rapisarda, F.; Granata, A.; Fatuzzo, P.; Malaguarnera, M.; Castellino, P. Renal function and ultrasound imaging in elderly subjects. Sci. World J. 2014, 2014, 830649. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.C. Renal ultrasonography: A procedure for nephrologists. Am. J. Kidney Dis. 1997, 30, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, F.; Barozzi, L. The role of ultrasonography in the study of medical nephropathy. J Ultrasound 2007, 10, 161–167. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, W.C. Sonographic evaluation of renal failure. Am. J. Kidney Dis. 2000, 35, 1021–1038. [Google Scholar] [CrossRef]
- John, E.; Igbinedion, B.; Akhigbe, A. Comparative sonographic assessment of renal dimensions and clinicobiochemical parameters among diabetic and nondiabetic adults in Benin City, Nigeria. J. Med. Trop. 2018, 20, 17–23. [Google Scholar]
- Maksoud, A.A.A.; Sharara, S.M.; Nanda, A.; Khouzam, R.N. The renal resistive index as a new complementary tool to predict microvascular diabetic complications in children and adolescents: A groundbreaking finding. Ann. Transl. Med. 2019, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.A.A.; Eljack, A.H.; Gar-alnabi, M.E.M.; Mahmoud, M.Z.; Elseid, M.; Edam, G.A. Ultrasonographic Characteristics of Diabetes Impacts in Kidneys’ Morphology. Open J. Radiol. 2014, 4, 301–308. [Google Scholar] [CrossRef]
- Rigalleau, V.; Garcia, M.; Lasseur, C.; Laurent, F.; Montaudon, M.; Raffaitin, C.; Barthe, N.; Beauvieux, M.-C.; Vendrely, B.; Chauveau, P.; et al. Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC Nephrol. 2010, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Derchi, L.E.; Martinoli, C.; Saffioti, S.; Pontremoli, R.; De Micheli, A.; Bordone, C. Ultrasonographic imaging and Doppler analysis of renal changes in non-insulin-dependent diabetes mellitus. Acad. Radiol. 1994, 1, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Piras, D.; Masala, M.; Delitala, A.; Urru, S.A.M.; Curreli, N.; Balaci, L.; Ferreli, L.P.; Loi, F.; Atzeni, A.; Cabiddu, G.; et al. Kidney size in relation to ageing, gender, renal function, birthweight and chronic kidney disease risk factors in a general population. Nephrol. Dial. Transplant. 2020, 35, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Noortgate, N.; Velghe, A.; Petrovic, M.; Vandewiele, C.; Lameire, N.; Voet, D.; Afschrift, M. The role of ultrasonography in the assessment of renal function in the elderly. J. Nephrol. 2003, 16, 658–662. [Google Scholar]
- Lizarraga-Mollinedo, E.; Martinez-Calcerrada, J.M.; Padros-Fornieles, C.; Mas-Pares, B.; Xargay-Torrent, S.; Riera-Perez, E.; Prats-Puig, A.; Carreras-Badosa, G.; De Zegher, F.; Ibanez, L.; et al. Renal size and cardiovascular risk in prepubertal children. Sci. Rep. 2019, 9, 5265. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Yu, M.-C.; Fu, J.-F.; Hou, Y.-C.; Wang, I.-K.; Chih, Y.-H.; Weng, C.-H.; Huang, W.-H.; Hsu, C.-W.; Tam, F.W.K.; et al. Impact of kidney size on mortality in diabetic patients receiving peritoneal dialysis. Sci. Rep. 2021, 11, 8203. [Google Scholar] [CrossRef]
- El-Reshaid, W.; Abdul-Fattah, H. Sonographic assessment of renal size in healthy adults. Med. Princ. Pract. 2014, 23, 432–436. [Google Scholar] [CrossRef]
- Miletić, D.; Fuckar, Z.; Sustić, A.; Mozetic, V.; Stimac, D.; Zauhar, G. Sonographic measurement of absolute and relative renal length in adults. J. Clin. Ultrasound 1998, 26, 185–189. [Google Scholar] [CrossRef]
- Araújo, N.C.; Rebelo, M.A.P.; da Silveira Rioja, L.; Suassuna, J.H.R. Sonographically determined kidney measurements are better able to predict histological changes and a low CKD-EPI eGFR when weighted towards cortical echogenicity. BMC Nephrol. 2020, 21, 123. [Google Scholar] [CrossRef]
- Beland, M.D.; Walle, N.L.; Machan, J.T.; Cronan, J.J. Renal cortical thickness measured at ultrasound: Is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am. J. Roentgenol. 2010, 195, W146–W149. [Google Scholar] [CrossRef] [PubMed]
- Libório, A.B.; de Oliveira Neves, F.M.; Torres de Melo, C.B.; Leite, T.T.; de Almeida Leitão, R. Quantitative Renal Echogenicity as a Tool for Diagnosis of Advanced Chronic Kidney Disease in Patients with Glomerulopathies and no Liver Disease. Kidney Blood Press. Res. 2017, 42, 708–716. [Google Scholar] [CrossRef]
- Martino, P.; Galosi, A.B.; Bitelli, M.; Consonni, P.; Fiorini, F.; Granata, A.; Gunelli, R.; Liguori, G.; Palazzo, S.; Pavan, N.; et al. Practical recommendations for performing ultrasound scanning in the urological and andrological fields. Arch. Ital. Urol. Androl. 2014, 86, 56–78. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.R.; Atzingen, A.C.V.; Iared, W.; Bezerra, A.S.D.A.; Ammirati, A.L.; Canziani, M.E.F.; D’Ippolito, G. Value of renal cortical thickness as a predictor of renal function impairment in chronic renal disease patients. Radiol. Bras. 2015, 48, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, M.; Aras, B.; Güneyli, S.; Yılmaz, M. Clinical significance of renal cortical thickness in patients with chronic kidney disease. Ultrasonography 2018, 37, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, S.D.; Beale, A.M.; Cattell, W.R.; Webb, J.A. What is the value of measuring renal parenchymal thickness before renal biopsy? Clin. Radiol. 1994, 49, 45–49. [Google Scholar] [CrossRef]
- Kaplon, D.M.; Lasser, M.S.; Sigman, M.; Haleblian, G.E.; Pareek, G. Renal parenchyma thickness: A rapid estimation of renal function on computed tomography. Int. Braz. J. Urol. 2009, 35, 3–8. [Google Scholar] [CrossRef]
- Granata, A.; Galeano, D.; Fiorini, F. Acute and Chronic Nephropathy. In Atlas of Ultrasonography in Urology, Andrology, and Nephrology; Martino, P., Galosi, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–26. [Google Scholar]
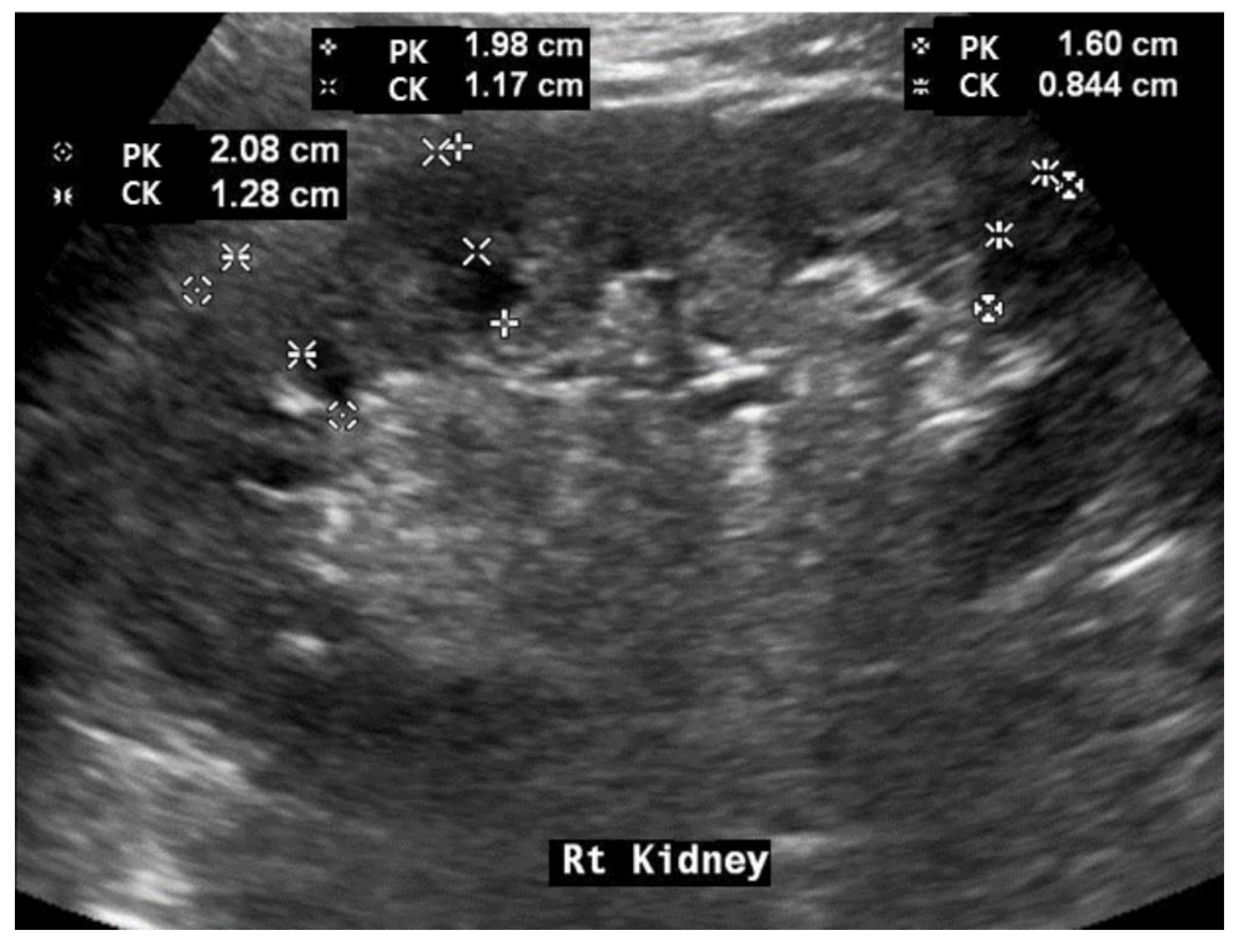

| Value | Scoring | Value | Scoring | |
|---|---|---|---|---|
| RK (cm) | 11.6 | Height (m) | 1.698 | |
| CK (cm) | 1.1 | PK (cm) | 1.89 | |
| RH: RK/Height | 6.83 (Median: 6.427) | CKH, 10 × CK (RH) | 1.61 (Median: 0.99) | |
| value | Scoring | |||
| Echogenicity | Normal (0) | Slightly increased (1) | Increased (2) | 0 |
| Capsular irregularity | Normal (0) | Slightly irregular (1) | Irregular (2) | 0 |
| CK/PK | Above (0) | Median | Below (1) | 0 |
| RH | Above (0) | Median | Below (1) | 0 |
| CKH | Above (0) | Median | Below (1) | 0 |
| Total scoring | 0 | |||
| Total (N = 252) | Non-DM (149) | DM (103) | p-Value | |
|---|---|---|---|---|
| Age (years) | 59.72 ± 9.66 (27–81) | 58.15 ± 10.24 | 61.99 ± 8.27 | 0.001 |
| Male sex, n (%) | 142 (56.1%) | 80 (53.7%) | 62 (60.2%) | 0.186 |
| HTN, n (%) | 166 (65.6%) | 80 (53.7%) | 86 (83.5%) | 0.000 |
| BMI | 25.1 ± 3.73 (16.2–39.1) | 24.72 ± 3.80 | 25.56 ± 3.60 | 0.100 |
| CKD stage | 55:104:93 (21.8:41.3:36.9, %) | 43:62:44 (28.9:41.6:29.5, %) | 12:42:49 (11.7:40.8:47.6, %) | 0.000 |
| Laboratory parameters | ||||
| HbA1c | 6.87 ± 1.76 (4.4–13.2) | 5.49 ± 0.62 | 7.34 ± 1.78 | 0.000 |
| Hemoglobin (g/dL) | 13.2 ± 1.8 (9.3–18.7) | 13.7 ± 1.68 | 12.5 ± 1.61 | 0.000 |
| Albumin (mg/dL) | 3.97 ± 0.59 (1.6–4.9) | 4.01 ± 0.64 | 3.91 ± 0.53 | 0.194 |
| Serum creatinine (mg/dL) | 1.12 ± 0.34 (0.52–2.13) | 1.05 ± 0.32 | 1.21 ± 0.34 | 0.000 |
| eGFR (mL/min per 1.73 m2) | 71.1 ± 20.7 (40.4–119.5) | 75.2 ± 20.8 | 65.3 ± 19.1 | 0.000 |
| Glucose (mg/dL) | 132.1 ± 66.3 (42–536) | 106.6 ± 22.3 | 169.3 ± 87.8 | 0.000 |
| UPCR (g/g) | 1.17 ± 2.20 (0.01–14.20) | 0.75 ± 1.21 | 1.62 ± 2.82 | 0.031 |
| LDL (mg/dL) | 110.1 ± 48.3 (20–119) | 123.6 ± 55.2 | 91.7 ± 28.5 | 0.000 |
| ΔeGFR | −8.7 ± 25.3 (−109.6–101.1) | −1.5 ± 22.6 | −19.2 ± 25.3 | 0.000 |
| Slope of ΔeGFR (/y) | −2.12 ± 6.18 (−24.7–25.27) | −0.31 ± 5.56 | −4.73 ± 6.11 | 0.000 |
| Total (N = 239) | Non-DM (149) | DM (103) | p-Value | |
|---|---|---|---|---|
| RL (cm) | 10.45 ± 0.94 (7.8–13.4) | 10.37 ± 0.95 | 10.55 ± 0.93 | 0.141 |
| CK (cm) | 0.66 ± 0.17 (0.31–1.41) | 0.65 ± 0.16 | 0.69 ± 0.19 | 0.049 |
| PK (cm) | 1.42 ± 0.24 (0.84–2.45) | 1.38 ± 0.23 | 1.49 ± 0.27 | 0.001 |
| CK/PK | 0.46 ± 0.65 (0.29–0.70) | 0.47 ± 0.06 | 0.46 ± 0.07 | 0.447 |
| CK/RK | 0.06 ± 0.01 (0.04–0.12) | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.074 |
| PK/RK | 0.14 ± 0.02 (0.08–0.21) | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.005 |
| RH | 6.44 ± 0.53 (5.28–8.99) | 6.41 ± 0.54 | 6.48 ± 0.51 | 0.150 |
| CKH | 1.03 ± 0.26 (0.52–2.11) | 1.38 ± 0.23 | 1.06 ± 0.28 | 0.147 |
| PKH | 2.22 ± 0.37 (1.45–3.47) | 2.16 ± 0.36 | 2.29 ± 0.37 | 0.007 |
| Irregularity (%) | 0 (71.0%), 1 (22.6%) 3 (6.3%) | 0 (75.8%), 1 (17.4%) 3 (6.7%) | 0 (64.1%), 1 (30.1%) 3 (6.5%) | |
| Echogenicity (%) | 0 (59.1%), 1 (29.8%) 3 (11.1%) | 0 (57.7%), 1 (32.2%) 3 (10.1%) | 0 (61.2%), 1 (26.2%) 3 (12.6%) |
| Non-Progression (N = 219) | Progression (N = 33) | p-Value | |
|---|---|---|---|
| Age (years) | 59.94 ± 9.53 | 58.30 ± 10.49 | 0.366 |
| Male sex, n (%) | 13 (39.4%) | 97 (44.3%) | 0.707 |
| DM, n (%) | 76 (34.7%) | 27 (81.8%) | 0.000 |
| HTN, n (%) | 140 (63.9%) | 26 (78.8%) | 0.093 |
| BMI | 25.19 ± 3.82 | 24.39 ± 3.05 | 0.278 |
| Laboratory parameters | |||
| HbA1c | 6.52 ± 1.50 | 8.26 ± 2.04 | 0.000 |
| Hemoglobin (g/dL) | 13.31 ± 1.79 | 12.38 ± 1.83 | 0.004 |
| Albumin (mg/dL) | 4.04 ± 0.58 | 3.54 ± 0.56 | 0.000 |
| Serum creatinine (mg/dL) | 1.09 ± 0.33 | 1.32 ±0.36 | 0.000 |
| eGFR (mL/min per 1.73 m2) | 72.62 ± 20.53 | 60.21 ± 17.67 | 0.001 |
| UPCR (g/g) | 0.86 ± 1.82 | 3.21 ± 3.20 | 0.001 |
| LDL (mg/dL) | 112.23 ± 50.03 | 96.83 ± 34.05 | 0.157 |
| Ultrasound parameter | |||
| RL (cm) | 10.43 ± 0.94 | 10.54 ± 0.97 | 0.538 |
| RH | 6.45 ± 0.54 | 6.36 ± 0.47 | 0.373 |
| CKH | 0.41 ± 0.10 | 0.38 ± 0.12 | 0.170 |
| PKH | 0.88 ± 0.15 | 0.87 ± 0.13 | 0.576 |
| Renal scoring system | 2.27 ± 1.644 | 3.03 ± 1.794 | 0.016 |
| Echogenicity | 0:62.1%, 1:29.2%, 2:8.7% | 0:39.4%, 1:33.3%, 2:27.3% | |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Scoring system | 1.599 (1.021–1.530) | 0.016 | 1.413 (1.032–1.933) | 0.031 |
| Age (Years) | 0.983 (0.946–1.020) | 0.365 | 0.919 (0.864–0.978) | 0.007 |
| DM (n, %) | 8.467 (3.350–21.40) | 0.000 | 4.917 (1.325–18.25) | 0.017 |
| HTN (n, %) | 2.096 (0.870–5.048) | 0.099 | 1.215 (0.290–5.081) | 0.790 |
| Hemoglobin (g/dL) | 0.717 (0.568–0.905) | 0.005 | 0.848 (0.605–1.188) | 0.337 |
| Albumin (mg/dL) | 0.339 (0.201–0.573) | 0.000 | 0.396 (0.135–1.163) | 0.396 |
| eGFR (mL/min/1.73 m2) | 0.964 (0.941–0.986) | 0.002 | 0.971 (0.938–1.002) | 0.094 |
| UPCR (g/g) | 1.388 (1.187–1.623) | 0.000 | 1.238 (0.962–1.593) | 0.097 |
| Autoimmune disease (n, %) | 0.364 (0.083–1.593) | 0.179 | 0.204 (0.025–1.674) | 0.204 |
| RH (cm) | 1.129 (0.768–1.659) | 0.536 | ||
| CK (cm) | 0.855 (0.101–7.207) | 0.886 | ||
| PK (cm) | 2.267 (0.556–9.241) | 0.253 | ||
| CKH | 0.608 (0.047–5.964) | 0.608 | ||
| PKH | 1.912 (0.377–9.711) | 0.434 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, Y.R.; Lee, E.J.; Kim, H.R.; Jeon, J.W.; Na, K.R.; Lee, K.W.; Choi, D.E. Ultrasound Renal Score to Predict the Renal Disease Prognosis in Patients with Diabetic Kidney Disease: An Investigative Study. Diagnostics 2023, 13, 515. https://doi.org/10.3390/diagnostics13030515
Ham YR, Lee EJ, Kim HR, Jeon JW, Na KR, Lee KW, Choi DE. Ultrasound Renal Score to Predict the Renal Disease Prognosis in Patients with Diabetic Kidney Disease: An Investigative Study. Diagnostics. 2023; 13(3):515. https://doi.org/10.3390/diagnostics13030515
Chicago/Turabian StyleHam, Young Rok, Eu Jin Lee, Hae Ri Kim, Jae Wan Jeon, Ki Ryang Na, Kang Wook Lee, and Dae Eun Choi. 2023. "Ultrasound Renal Score to Predict the Renal Disease Prognosis in Patients with Diabetic Kidney Disease: An Investigative Study" Diagnostics 13, no. 3: 515. https://doi.org/10.3390/diagnostics13030515
APA StyleHam, Y. R., Lee, E. J., Kim, H. R., Jeon, J. W., Na, K. R., Lee, K. W., & Choi, D. E. (2023). Ultrasound Renal Score to Predict the Renal Disease Prognosis in Patients with Diabetic Kidney Disease: An Investigative Study. Diagnostics, 13(3), 515. https://doi.org/10.3390/diagnostics13030515






