Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression
Abstract
:1. Neurodegenerative Disorders
1.1. The Assessment of Neurodegenerative Disorders
1.2. Biomarkers in Blood and Cerebrospinal Fluid
1.3. Psychological and Neuropsychological Assessment
1.4. Neuroimaging
1.5. Connectivity
1.6. Electrical Activity of the Brain
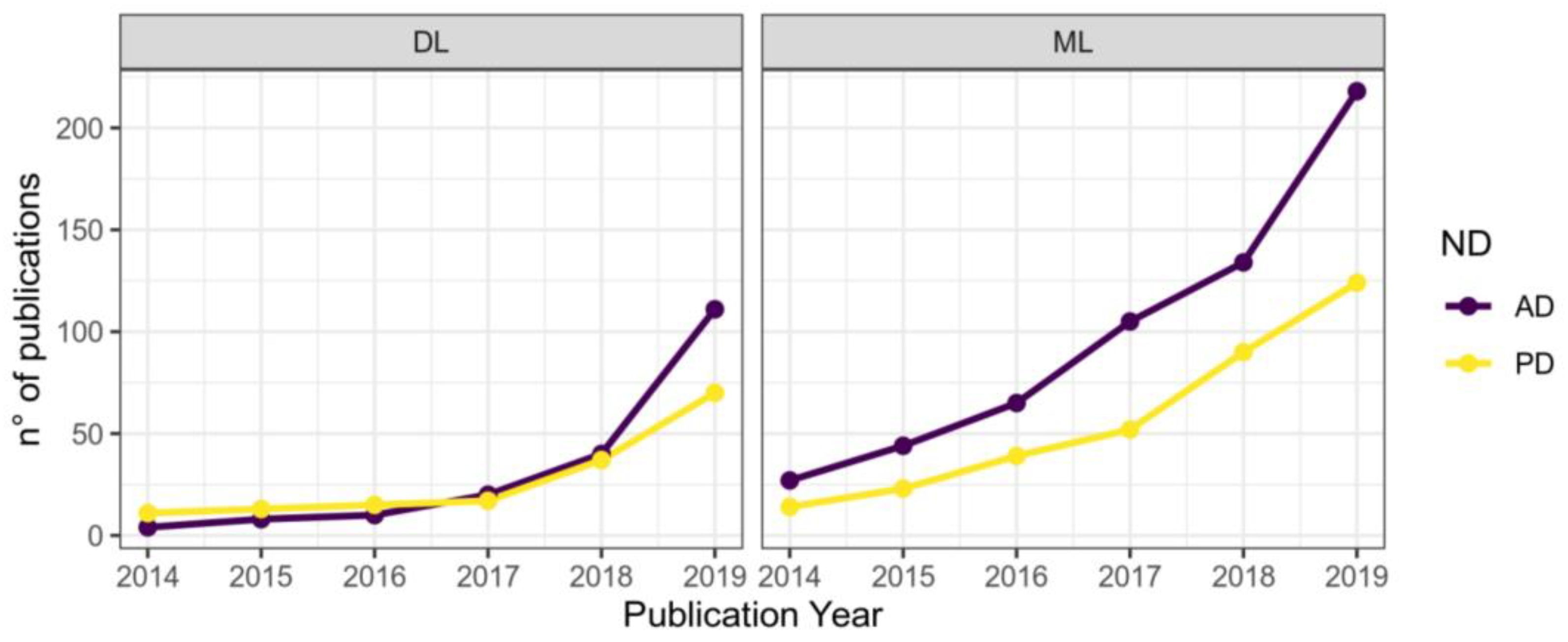
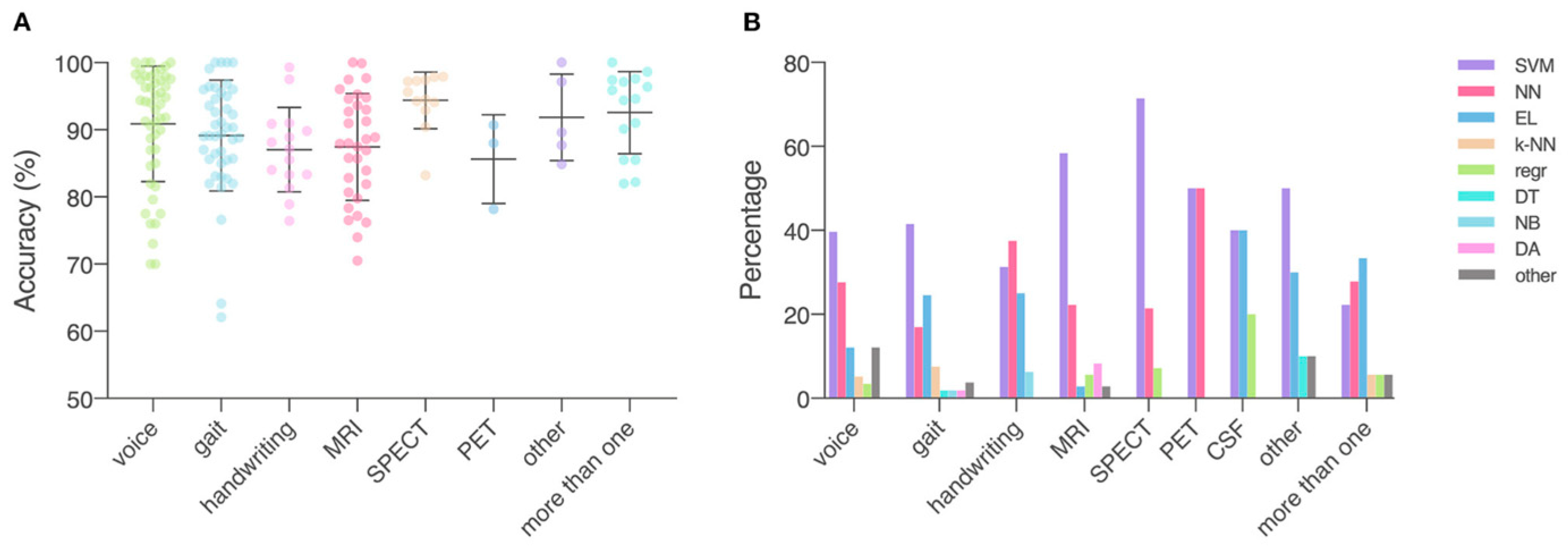
1.7. Application of Machine Learning
- 1.
- Increasing the accuracy and coverage of pre-clinical diagnostics. Identification of the pathological process before the onset of clinically observable symptoms will allow treatment to be started in advance, slowing down the progression of the disease and improving the quality of life of the patient.
- 2.
- Differential diagnosis of various neurodegenerative disorders and comorbidities. Increasing the accuracy of diagnosis will improve the selection of treatment and correction of symptoms.
- 3.
- Increasing the accuracy of forecasts for the progression of the disease after its onset. The application of machine learning methods to the analysis of longitudinal data will make it possible to build dynamic models for symptom monitoring.
1.8. The Treatment of Neurodegenerative Disorders
1.9. Pharmacological Therapy
1.10. Cognitive Training
1.11. Physical Exercises
1.12. Ergotherapy
1.13. Brain Stimulation
1.14. Prevention of the Associated Psychological Problems
1.15. Application of Machine Learning
2. Depressive Disorders
2.1. Diagnostics of Depressive Disorders
2.1.1. Use of Standardized Questionnaires
2.1.2. Analysis of the Functional Activity of the Brain (EEG)
2.1.3. Application of Machine Learning
2.2. Treatment of Depressive Disorders
2.2.1. Pharmacological Therapy
2.2.2. Psychosocial Interventions
2.2.3. Biofeedback
2.2.4. Brain Stimulation
2.2.5. Application of Machine Learning
3. The Use of Auxiliary Indicators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Institute of Health. Degenerative Nerve Diseases. Available online: https://medlineplus.gov/degenerativenervediseases.html (accessed on 2 November 2022).
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- World Health Organization. Global Health Estimates: Leading Causes of DALYs. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys (accessed on 2 November 2022).
- Termine, A.; Fabrizio, C.; Strafella, C.; Caputo, V.; Petrosini, L.; Caltagirone, C.; Giardina, E.; Cascella, R. Multi-Layer Picture of Neurodegenerative Diseases: Lessons from the Use of Big Data through Artificial Intelligence. J. Pers. Med. 2021, 11, 280. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Wu, Y.; Le, W.; Jankovic, J. Preclinical biomarkers of Parkinson disease. Arch. Neurol. 2011, 68, 22–30. [Google Scholar] [CrossRef] [PubMed]
- NICE. Dementia: Assessment, Management and Support for People Living with Dementia and Their Carers. Available online: https://www.nice.org.uk/guidance/ng97/chapter/Recommendations#diagnosis (accessed on 2 November 2022).
- Tagaris, A.; Kollias, D.; Stafylopatis, A.; Tagaris, G.; Kollias, S. Machine learning for neurodegenerative disorder diagnosis—Survey of practices and launch of benchmark dataset. Int. J. Artif. Intell. Tools 2018, 27, 1850011. [Google Scholar] [CrossRef]
- Sunderland, T.; Linker, G.; Mirza, N.; Putnam, K.T.; Friedman, D.L.; Kimmel, L.H.; Bergeson, J.; Manetti, G.J.; Zimmermann, M.; Tang, B. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. Jama 2003, 289, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Tariciotti, L.; Casadei, M.; Honig, L.S.; Teich, A.F.; McKhann II, G.M.; Tosto, G.; Mayeux, R. Clinical Experience with Cerebrospinal Fluid Aβ 42, Total and Phosphorylated Tau in the Evaluation of 1,016 Individuals for Suspected Dementia. J. Alzheimer’s Dis. 2018, 65, 1417–1425. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Thijssen, E.H.; Verberk, I.M. Plasma p-tau217: From ‘new kid’to most promising candidate for Alzheimer’s disease blood test. Brain 2020, 143, 3170–3172. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. Jama 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Grasso, M.; Piscopo, P.; Confaloni, A.; Denti, M.A. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules 2014, 19, 6891–6910. [Google Scholar] [CrossRef] [PubMed]
- Foran, A.; Mathias, J.; Bowden, S. Effectiveness of sorting tests for detecting cognitive decline in older adults with dementia and other common neurodegenerative disorders: A meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 442–454. [Google Scholar] [CrossRef]
- Donohue, M.C.; Sperling, R.A.; Salmon, D.P.; Rentz, D.M.; Raman, R.; Thomas, R.G.; Weiner, M.; Aisen, P.S. The preclinical Alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurol. 2014, 71, 961–970. [Google Scholar] [CrossRef]
- Hedderich, D.M.; Dieckmeyer, M.; Andrisan, T.; Ortner, M.; Grundl, L.; Schön, S.; Suppa, P.; Finck, T.; Kreiser, K.; Zimmer, C. Normative brain volume reports may improve differential diagnosis of dementing neurodegenerative diseases in clinical practice. Eur. Radiol. 2020, 30, 2821–2829. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies—Still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef]
- Benussi, A.; Grassi, M.; Palluzzi, F.; Koch, G.; Di Lazzaro, V.; Nardone, R.; Cantoni, V.; Dell’Era, V.; Premi, E.; Martorana, A. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Ann. Neurol. 2020, 87, 394–404. [Google Scholar] [CrossRef]
- Ahmadlou, M.; Adeli, H.; Adeli, A. Fractality and a wavelet-chaos-methodology for EEG-based diagnosis of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011, 25, 85–92. [Google Scholar] [CrossRef]
- Kulkarni, N.; Bairagi, V. EEG-Based Diagnosis of Alzheimer Disease: A Review and Novel Approaches for Feature Extraction and Classification Techniques; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Ehrenberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.J.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N. Relevance of biomarkers across different neurodegenerative diseases. Alzheimer’s Res. Ther. 2020, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M. The development of pre-clinical assessment and preventive treatment of neurogenerative disorders. Zhurnal Nevrol. I Psihiatr. Im. CC Korsakova 2015, 115, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Wang, S.; Dong, Z. Classification of Alzheimer disease based on structural magnetic resonance imaging by kernel support vector machine decision tree. Prog. Electromagn. Res. 2014, 144, 171–184. [Google Scholar] [CrossRef]
- Aung, Y.Y.; Wong, D.C.; Ting, D.S. The promise of artificial intelligence: A review of the opportunities and challenges of artificial intelligence in healthcare. Br. Med. Bull. 2021, 139, 4–15. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chiu, S.-I.; Chen, T.-F.; Jang, J.-S.R.; Chiu, M.-J. Classifications of neurodegenerative disorders using a multiplex blood biomarkers-based machine learning model. Int. J. Mol. Sci. 2020, 21, 6914. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Bellomo, G.; Parnetti, L.; Blennow, K.; Zetterberg, H.; Di Filippo, M. Neuroinflammation and Alzheimer’s Disease: A Machine Learning Approach to CSF Proteomics. Cells 2021, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Maass, F.; Michalke, B.; Willkommen, D.; Leha, A.; Schulte, C.; Tönges, L.; Mollenhauer, B.; Trenkwalder, C.; Rückamp, D.; Börger, M. Elemental fingerprint: Reassessment of a cerebrospinal fluid biomarker for Parkinson’s disease. Neurobiol. Dis. 2020, 134, 104677. [Google Scholar] [CrossRef]
- Young, A.L.; Marinescu, R.V.; Oxtoby, N.P.; Bocchetta, M.; Yong, K.; Firth, N.C.; Cash, D.M.; Thomas, D.L.; Dick, K.M.; Cardoso, J. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9, 4273. [Google Scholar] [CrossRef]
- Kwak, K.; Giovanello, K.S.; Bozoki, A.; Styner, M.; Dayan, E. Subtyping of mild cognitive impairment using a deep learning model based on brain atrophy patterns. Cell Rep. Med. 2021, 2, 100467. [Google Scholar] [CrossRef]
- Péran, P.; Barbagallo, G.; Nemmi, F.; Sierra, M.; Galitzky, M.; Traon, A.P.L.; Payoux, P.; Meissner, W.G.; Rascol, O. MRI supervised and unsupervised classification of Parkinson’s disease and multiple system atrophy. Mov. Disord. 2018, 33, 600–608. [Google Scholar] [CrossRef]
- Feng, X.; Provenzano, F.A.; Small, S.A. A deep learning MRI approach outperforms other biomarkers of prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 45. [Google Scholar] [CrossRef]
- Dyrba, M.; Hanzig, M.; Altenstein, S.; Bader, S.; Ballarini, T.; Brosseron, F.; Buerger, K.; Cantré, D.; Dechent, P.; Dobisch, L. Improving 3D convolutional neural network comprehensibility via interactive visualization of relevance maps: Evaluation in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 191. [Google Scholar] [CrossRef]
- Henschel, L.; Conjeti, S.; Estrada, S.; Diers, K.; Fischl, B.; Reuter, M. Fastsurfer-a fast and accurate deep learning based neuroimaging pipeline. NeuroImage 2020, 219, 117012. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Lampe, L.; Niehaus, S.; Huppertz, H.-J.; Merola, A.; Reinelt, J.; Mueller, K.; Anderl-Straub, S.; Fassbender, K.; Fliessbach, K.; Jahn, H. Comparative analysis of machine learning algorithms for multi-syndrome classification of neurodegenerative syndromes. Alzheimer’s Res. Ther. 2022, 14, 62. [Google Scholar] [CrossRef]
- Mei, J.; Desrosiers, C.; Frasnelli, J. Machine learning for the diagnosis of Parkinson’s disease: A review of literature. Front. Aging Neurosci. 2021, 13, 633752. [Google Scholar] [CrossRef] [PubMed]
- García-Fonseca, Á.; Martin-Jimenez, C.; Barreto, G.E.; Pachón, A.F.A.; González, J. The emerging role of long non-coding RNAs and microRNAs in neurodegenerative diseases: A perspective of machine learning. Biomolecules 2021, 11, 1132. [Google Scholar] [CrossRef]
- Sh, Y.; Liu, B.; Zhang, J.; Zhou, Y.; Hu, Z. Application of AI Modeling Technology Based on Fluid Biopsy to Diagnose AD. 2021. Available online: https://www.researchsquare.com/article/rs-731371/v1 (accessed on 8 December 2022).
- Zhang, J.; Zhang, X.; Sh, Y.; Liu, B.; Hu, Z. Diagnostic AI Modeling and Pseudo Time Series Profiling of AD and PD Based on Individualized Serum Proteome Data. Front. Bioinform. 2021, 54, 764497. [Google Scholar] [CrossRef]
- Wingo, T.S.; Liu, Y.; Gerasimov, E.S.; Vattathil, S.M.; Wynne, M.E.; Liu, J.; Lori, A.; Faundez, V.; Bennett, D.A.; Seyfried, N.T. Shared mechanisms across the major psychiatric and neurodegenerative diseases. Nat. Commun. 2022, 13, 4314. [Google Scholar] [CrossRef]
- Su, C.; Tong, J.; Wang, F. Mining genetic and transcriptomic data using machine learning approaches in Parkinson’s disease. npj Park. Dis. 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Almubark, I.; Chang, L.-C.; Nguyen, T.; Turner, R.S.; Jiang, X. Early detection of Alzheimer’s disease using patient neuropsychological and cognitive data and machine learning techniques. In Proceedings of the 2019 IEEE International Conference on Big Data (Big Data), Los Angeles, CA, USA, 9–12 December 2019; pp. 5971–5973. [Google Scholar]
- Revathi, A.; Kaladevi, R.; Ramana, K.; Jhaveri, R.H.; Rudra Kumar, M.; Sankara Prasanna Kumar, M. Early detection of cognitive decline using machine learning algorithm and cognitive ability test. Secur. Commun. Netw. 2022, 2022, 4190023. [Google Scholar] [CrossRef]
- Bachli, M.B.; Sedeño, L.; Ochab, J.K.; Piguet, O.; Kumfor, F.; Reyes, P.; Torralva, T.; Roca, M.; Cardona, J.F.; Campo, C.G. Evaluating the reliability of neurocognitive biomarkers of neurodegenerative diseases across countries: A machine learning approach. Neuroimage 2020, 208, 116456. [Google Scholar] [CrossRef]
- García-Gutierrez, F.; Díaz-Álvarez, J.; Matias-Guiu, J.A.; Pytel, V.; Matías-Guiu, J.; Cabrera-Martín, M.N.; Ayala, J.L. GA-MADRID: Design and validation of a machine learning tool for the diagnosis of Alzheimer’s disease and frontotemporal dementia using genetic algorithms. Med. Biol. Eng. Comput. 2022, 60, 2737–2756. [Google Scholar] [CrossRef]
- Manevich, T.M. Methods of cognitive rehabilitation of patients with neurodegenerative disorders. Nevrol. Zhurnal 2018, 23, 63–70. [Google Scholar] [CrossRef]
- Osadchij, A. Basis therapy for Alzheimer’s disease: Modern trends. Ukraїns’kij Med. Chasopis 2019, 1, 79–82. [Google Scholar] [CrossRef]
- NICE. Parkinson’s Disease in Adults. Available online: https://www.nice.org.uk/guidance/ng71/chapter/recommendations (accessed on 2 November 2022).
- Wolinsky, F.D.; Mahncke, H.W.; Kosinski, M.; Unverzagt, F.W.; Smith, D.M.; Jones, R.N.; Stoddard, A.; Tennstedt, S.L. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv. Res. 2009, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Kallio, E.-L.; Öhman, H.; Kautiainen, H.; Hietanen, M.; Pitkälä, K. Cognitive training interventions for patients with Alzheimer’s disease: A systematic review. J. Alzheimer’s Dis. 2017, 56, 1349–1372. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gonzalez, D.; Hernandez-Martinez, A.; Valenzuela, P.L.; Morales, J.S.; Soriano-Maldonado, A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 128, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Manadas, B.; Melo, C.; Gomes, J.; Mendes, C.; Graos, M.; Carvalho, R.; Carvalho, A.; Duarte, C. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Smallfield, S.; Heckenlaible, C. Effectiveness of occupational therapy interventions to enhance occupational performance for adults with Alzheimer’s disease and related major neurocognitive disorders: A systematic review. Am. J. Occup. Ther. 2017, 71, 7105180010p1–77105180010p9. [Google Scholar] [CrossRef]
- Yuan, X. Risk, risk assessment, and community corrections in China. Int. J. Offender Ther. Comp. Criminol. 2019, 63, 2466–2482. [Google Scholar] [CrossRef]
- von Gunten, A.; Pocnet, C.; Rossier, J. The impact of personality characteristics on the clinical expression in neurodegenerative disorders—A review. Brain Res. Bull. 2009, 80, 179–191. [Google Scholar] [CrossRef]
- Rama Raju, V.; Anji Reddy, D.; Narsimha, D.; Srinivas, K.; Kavitha Rani, B. Adaptive Closed-Loop Deep Brain Stimulator Coding Techniques for Target Detections in Parkinson’s. IETE J. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Cernera, S.; Alcantara, J.D.; Opri, E.; Cagle, J.N.; Eisinger, R.S.; Boogaart, Z.; Pramanik, L.; Kelberman, M.; Patel, B.; Foote, K.D. Wearable sensor-driven responsive deep brain stimulation for essential tremor. Brain Stimul. 2021, 14, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Donisi, L.; Cesarelli, G.; Balbi, P.; Provitera, V.; Lanzillo, B.; Coccia, A.; D’Addio, G. Positive impact of short-term gait rehabilitation in Parkinson patients: A combined approach based on statistics and machine learning. Math. Biosci. Eng. 2021, 18, 6995–7009. [Google Scholar] [CrossRef]
- Korhani, N.; Taati, B.; Iaboni, A.; Sabo, A.; Mehdizadeh, S.; Flint, A.; Mansfield, A. Ambient Monitoring of Gait and Machine Learning Models for Dynamic and Short-Term Falls Risk Assessment in People with Dementia. 2021. Available online: https://www.techrxiv.org/articles/preprint/Ambient_Monitoring_of_Gait_and_Machine_Learning_Models_for_Dynamic_and_Short-Term_Falls_Risk_Assessment_in_People_With_Dementia/16943395/1 (accessed on 8 December 2022).
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119. [Google Scholar] [CrossRef]
- World Health Organization. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 2 November 2022).
- Fazel, S.; Runeson, B. Suicide. Reply. New Engl. J. Med. 2020, 382, e66. [Google Scholar] [CrossRef]
- Lang, U.E.; Borgwardt, S. Molecular mechanisms of depression: Perspectives on new treatment strategies. Cell. Physiol. Biochem. 2013, 31, 761–777. [Google Scholar] [CrossRef]
- Berger, A.-K.; Fratiglioni, L.; Forsell, Y.; Winblad, B.; Bäckman, L. The occurrence of depressive symptoms in the preclinical phase of AD: A population-based study. Neurology 1999, 53, 1998. [Google Scholar] [CrossRef]
- Elshanskij, S.P.; Anufriev, A.F.; Efimova, O.S.; Semenov, D.V. The characteristics of test-retest reliability of Beck Depression Inventory. Psihol. Sociol. I Pedagog. 2016, 55, 91–95. [Google Scholar]
- Assanovich, M.A. The optimization of Gamilton Depression Scale using the Rash model. Med. Psihol. V Ross. 2015, 31, 7. [Google Scholar]
- Uspenskij, J.P.; Gorbacheva, I.A.; Baryshnikova, N.V.; Akaeva, S.V.; Gnutov, A.A. The evaluation of the anxiety and depression level in patients with dyspepsia using the Hospital scale of anxiety and depression. Univ. Ter. Vestn. 2019, 1, 30–37. [Google Scholar]
- Varlamov, A.; Strelec, V. The analysis of EEG coherence in depression: The modern state of clinical application. Zhurnal Vyss. Nervn. Dejatel’nosti Im. IP Pavlov. 2013, 63, 613. [Google Scholar]
- Iznak, A.; Iznak, E.; Abramova, L.; Lozhnikov, M. Quantitative prognostic models for therapeutic response in patients with depression based on EEG parameters. Fiziol. Cheloveka 2019, 45, 36–43. [Google Scholar]
- Gao, S.; Calhoun, V.D.; Sui, J. Machine learning in major depression: From classification to treatment outcome prediction. CNS Neurosci. Ther. 2018, 24, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Hussain, S.A.; Aslan, S.; Raza, I.; Muzammel, M.; Othmani, A. EEG based Major Depressive disorder and Bipolar disorder detection using Neural Networks: A review. Comput. Methods Programs Biomed. 2021, 202, 106007. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Roy, N.; Islam, M.K.; Biswas, C.; Ahmed, H.U.; Amin, M.A.; Sarker, F.; Vaidyanathan, R.; Mamun, K.A. Exploration of EEG-based depression biomarkers identification techniques and their applications: A systematic review. IEEE Access 2022, 10, 16756–16781. [Google Scholar] [CrossRef]
- Chung, J.; Teo, J. Mental Health Prediction Using Machine Learning: Taxonomy, Applications, and Challenges. Appl. Comput. Intell. Soft Comput. 2022, 2022, 9970363. [Google Scholar] [CrossRef]
- Chen, Z.S.; Galatzer-Levy, I.R.; Bigio, B.; Nasca, C.; Zhang, Y. Modern Views of Machine Learning for Precision Psychiatry. arXiv 2022, arXiv:2204.01607. [Google Scholar] [CrossRef]
- Insel, T.R. The NIMH research domain criteria (RDoC) project: Precision medicine for psychiatry. Am. J. Psychiatry 2014, 171, 395–397. [Google Scholar] [CrossRef]
- Quaak, M.; van de Mortel, L.; Thomas, R.M.; van Wingen, G. Deep learning applications for the classification of psychiatric disorders using neuroimaging data: Systematic review and meta-analysis. NeuroImage: Clin. 2021, 30, 102584. [Google Scholar] [CrossRef]
- Mumtaz, W. MDD Patients and Healthy Controls EEG Data (New). Figshare, Dataset. 2016. Available online: https://figshare.com/articles/dataset/EEG_Data_New/4244171/2 (accessed on 8 December 2022).
- Cavanagh, F. EEG: Depression rest. OpenNeuro, Dataset 2021. Available online: https://openneuro.org/datasets/ds003478/versions/1.1.0 (accessed on 21 January 2021).
- Cai, H.; Gao, Y.; Sun, S.; Li, N.; Tian, F.; Xiao, H.; Li, J.; Yang, Z.; Li, X.; Zhao, Q. Modma dataset: A multi-modal open dataset for mental-disorder analysis. arXiv 2020, arXiv:2002.09283. [Google Scholar] [CrossRef]
- van Dijk, H.; van Wingen, G.; Denys, D.; Olbrich, S.; van Ruth, R.; Arns, M. The two decades brainclinics research archive for insights in neurophysiology (TDBRAIN) database. Sci. Data 2022, 9, 333. [Google Scholar] [CrossRef]
- Savinov, V.; Sapunov, V.; Shusharina, N.; Botman, S.; Kamyshov, G.; Tynterova, A. EEG-based depression classification using harmonized datasets. In Proceedings of the 2021 Third International Conference Neurotechnologies and Neurointerfaces (CNN), Kaliningrad, Russian, 13–15 September 2021; pp. 93–95. [Google Scholar]
- Rivera, M.J.; Teruel, M.A.; Maté, A.; Trujillo, J. Diagnosis and prognosis of mental disorders by means of EEG and deep learning: A systematic mapping study. Artif. Intell. Rev. 2021, 55, 1209–1251. [Google Scholar] [CrossRef]
- Liu, W.; Jia, K.; Wang, Z.; Ma, Z. A Depression Prediction Algorithm Based on Spatiotemporal Feature of EEG Signal. Brain Sci. 2022, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Shorten, C.; Khoshgoftaar, T.M. A survey on image data augmentation for deep learning. J. Big Data 2019, 6, 60. [Google Scholar] [CrossRef]
- Lashgari, E.; Liang, D.; Maoz, U. Data augmentation for deep-learning-based electroencephalography. J. Neurosci. Methods 2020, 346, 108885. [Google Scholar] [CrossRef]
- Mumtaz, W.; Qayyum, A. A deep learning framework for automatic diagnosis of unipolar depression. Int. J. Med. Inform. 2019, 132, 103983. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H.; Subha, D.P. Automated EEG-based screening of depression using deep convolutional neural network. Comput. Methods Programs Biomed. 2018, 161, 103–113. [Google Scholar] [CrossRef]
- Ay, B.; Yildirim, O.; Talo, M.; Baloglu, U.B.; Aydin, G.; Puthankattil, S.D.; Acharya, U.R. Automated depression detection using deep representation and sequence learning with EEG signals. J. Med. Syst. 2019, 43, 205. [Google Scholar] [CrossRef]
- Sandheep, P.; Vineeth, S.; Poulose, M.; Subha, D. Performance analysis of deep learning CNN in classification of depression EEG signals. In Proceedings of the TENCON 2019-2019 IEEE Region 10 Conference (TENCON), Kochi, India, 17–20 October 2019; pp. 1339–1344. [Google Scholar]
- Loh, H.W.; Ooi, C.P.; Aydemir, E.; Tuncer, T.; Dogan, S.; Acharya, U.R. Decision support system for major depression detection using spectrogram and convolution neural network with EEG signals. Expert Syst. 2022, 39, e12773. [Google Scholar] [CrossRef]
- Seal, A.; Bajpai, R.; Agnihotri, J.; Yazidi, A.; Herrera-Viedma, E.; Krejcar, O. DeprNet: A deep convolution neural network framework for detecting depression using EEG. IEEE Trans. Instrum. Meas. 2021, 70, 2505413. [Google Scholar] [CrossRef]
- Sharma, G.; Parashar, A.; Joshi, A.M. DepHNN: A novel hybrid neural network for electroencephalogram (EEG)-based screening of depression. Biomed. Signal Process. Control 2021, 66, 102393. [Google Scholar] [CrossRef]
- Li, X.; La, R.; Wang, Y.; Niu, J.; Zeng, S.; Sun, S.; Zhu, J. EEG-based mild depression recognition using convolutional neural network. Med. Biol. Eng. Comput. 2019, 57, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, A.; Saeedi, M.; Maghsoudi, A.; Shalbaf, A. Major depressive disorder diagnosis based on effective connectivity in EEG signals: A convolutional neural network and long short-term memory approach. Cogn. Neurodynamics 2021, 15, 239–252. [Google Scholar] [CrossRef]
- Thoduparambil, P.P.; Dominic, A.; Varghese, S.M. EEG-based deep learning model for the automatic detection of clinical depression. Phys. Eng. Sci. Med. 2020, 43, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Chen, D.; Shah, T.; Liu, X.; Zhang, X.; Zhang, L.; Li, X. Cloud-aided online EEG classification system for brain healthcare: A case study of depression evaluation with a lightweight CNN. Softw. Pract. Exp. 2020, 50, 596–610. [Google Scholar] [CrossRef]
- Uyulan, C.; Ergüzel, T.T.; Unubol, H.; Cebi, M.; Sayar, G.H.; Nezhad Asad, M.; Tarhan, N. Major depressive disorder classification based on different convolutional neural network models: Deep learning approach. Clin. EEG Neurosci. 2021, 52, 38–51. [Google Scholar] [CrossRef]
- Savinov, V.; Sapunov, V.; Shusharina, N.; Botman, S.; Kamyshov, G. Research and selection of the optimal neural network architecture and parameters for depression classification using harmonized datasets. In Proceedings of the 2022 Fourth International Conference Neurotechnologies and Neurointerfaces (CNN), Kaliningrad, Russian, 14–16 September 2022; pp. 132–135. [Google Scholar]
- Acharya, U.R.; Sudarshan, V.K.; Adeli, H.; Santhosh, J.; Koh, J.E.; Puthankatti, S.D.; Adeli, A. A novel depression diagnosis index using nonlinear features in EEG signals. Eur. Neurol. 2015, 74, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Al-Azab, F.; Raahemi, B.; Richards, G.; Jaworska, N.; Smith, D.; de la Salle, S.; Blier, P.; Knott, V. Data mining EEG signals in depression for their diagnostic value. BMC Med. Inform. Decis. Mak. 2015, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- NICE. Depression in Adults: Recognition and Management. Available online: https://www.nice.org.uk/guidance/cg90 (accessed on 2 November 2022).
- Drobizhev, M.J.; Ovchinnikov, A.A.; Kikta, S.V. The mechanisms of action of antidepressants and pathogenesis of psychiatric disorders. What Are Commonalities? Soc. I Klin. Psihiatr. 2017, 27, 94–101. [Google Scholar]
- Kostjukova, E. The increased application of second-generation antipsychotics: From schizophrenia to bipolar disorders. Sovrem. Ter. Psihicheskih Rasstrojstv 2020, 3, 29–37. [Google Scholar]
- Dunlop, B.W. Evidence-based applications of combination psychotherapy and pharmacotherapy for depression. Focus 2016, 14, 156–173. [Google Scholar] [CrossRef]
- Bell, A.C.; D’Zurilla, T.J. Problem-solving therapy for depression: A meta-analysis. Clin. Psychol. Rev. 2009, 29, 348–353. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Jay Lynn, S.; Montgomery, G.H. An Introduction to the Science and Practice of Evidence-Based Psychotherapy; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–10. [Google Scholar]
- Harvey, S.B.; Øverland, S.; Hatch, S.L.; Wessely, S.; Mykletun, A.; Hotopf, M. Exercise and the prevention of depression: Results of the HUNT cohort study. Am. J. Psychiatry 2018, 175, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pizzoli, S.F.; Marzorati, C.; Gatti, D.; Monzani, D.; Mazzocco, K.; Pravettoni, G. A meta-analysis on heart rate variability biofeedback and depressive symptoms. Sci. Rep. 2021, 11, 6650. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.J.; Florisse, E.J.; van Lutterveld, R.; Kooyman, M.; Sommer, I.E. Efficacy of EEG neurofeedback in psychiatry: A comprehensive overview and meta-analysis. Transl. Brain Rhythm. 2016, 1, 19–29. [Google Scholar] [CrossRef]
- Fernández-Álvarez, J.; Grassi, M.; Colombo, D.; Botella, C.; Cipresso, P.; Perna, G.; Riva, G. Efficacy of bio-and neurofeedback for depression: A meta-analysis. Psychol. Med. 2022, 52, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Iznak, A.; Iznak, E.; Damjanovich, E.; Olejchik, I.; Bologov, P.; Kazachinskaja, I.; Medvedeva, T. Transcranial magnetic stimulation for complex therapy of pharmacoresistant depression: Clinical, psychological, and EEG markers. Fiziol. Cheloveka 2015, 41, 57. [Google Scholar]
- NICE. Repetitive Transcranial Magnetic Stimulation for Depression. Available online: https://www.nice.org.uk/guidance/ipg542 (accessed on 2 November 2022).
- Sajjadian, M.; Lam, R.W.; Milev, R.; Rotzinger, S.; Frey, B.N.; Soares, C.N.; Parikh, S.V.; Foster, J.A.; Turecki, G.; Müller, D.J. Machine learning in the prediction of depression treatment outcomes: A systematic review and meta-analysis. Psychol. Med. 2021, 51, 2742–2751. [Google Scholar] [CrossRef]
- Zhdanov, A.; Atluri, S.; Wong, W.; Vaghei, Y.; Daskalakis, Z.J.; Blumberger, D.M.; Frey, B.N.; Giacobbe, P.; Lam, R.W.; Milev, R. Use of machine learning for predicting escitalopram treatment outcome from electroencephalography recordings in adult patients with depression. JAMA Netw. Open 2020, 3, e1918377. [Google Scholar] [CrossRef]
- Jaworska, N.; De la Salle, S.; Ibrahim, M.-H.; Blier, P.; Knott, V. Leveraging machine learning approaches for predicting antidepressant treatment response using electroencephalography (EEG) and clinical data. Front. Psychiatry 2019, 9, 768. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, E.; Asgarinejad, M.; Saliminia, S.; Ashoori, S.; Seraji, M. Predicting clinical response to transcranial magnetic stimulation in major depression using time-frequency EEG signal processing. Biomed. Eng. Appl. Basis Commun. 2021, 33, 2150048. [Google Scholar] [CrossRef]
- Shahabi, M.S.; Shalbaf, A.; Maghsoudi, A. Prediction of drug response in major depressive disorder using ensemble of transfer learning with convolutional neural network based on EEG. Biocybern. Biomed. Eng. 2021, 41, 946–959. [Google Scholar] [CrossRef]
- Hasanzadeh, F.; Mohebbi, M.; Rostami, R. Prediction of rTMS treatment response in major depressive disorder using machine learning techniques and nonlinear features of EEG signal. J. Affect. Disord. 2019, 256, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Wu, H.; Jing, P.; Ji, Y. A novel deep learning approach for diagnosing Alzheimer’s disease based on eye-tracking data. Front. Hum. Neurosci. 2022, 16, 97277. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Ren, F.; Sun, X.; Yin, M.; Wu, J.; Ma, C.; Gao, Z. A multifrequency brain network-based deep learning framework for motor imagery decoding. Neural Plast. 2020, 2020, 8863223. [Google Scholar] [CrossRef]
- Trabassi, D.; Serrao, M.; Varrecchia, T.; Ranavolo, A.; Coppola, G.; De Icco, R.; Tassorelli, C.; Castiglia, S.F. Machine Learning Approach to Support the Detection of Parkinson’s Disease in IMU-Based Gait Analysis. Sensors 2022, 22, 3700. [Google Scholar] [CrossRef]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Schmidt, G.; Maetzler, W. A Deep Learning Approach for Gait Event Detection from a Single Shank-Worn IMU: Validation in Healthy and Neurological Cohorts. Sensors 2022, 22, 3859. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Karjadi, C.; Paschalidis, I.C.; Au, R.; Kolachalama, V.B. Detection of dementia on voice recordings using deep learning: A Framingham Heart Study. Alzheimer’s Res. Ther. 2021, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Fishbain, B.; Markus, A.; Richter-Levin, G.; Okon-Singer, H. Using machine learning-based analysis for behavioral differentiation between anxiety and depression. Sci. Rep. 2020, 10, 16381. [Google Scholar] [CrossRef]
- Richter, T.; Fishbain, B.; Fruchter, E.; Richter-Levin, G.; Okon-Singer, H. Machine learning-based diagnosis support system for differentiating between clinical anxiety and depression disorders. J. Psychiatr. Res. 2021, 141, 199–205. [Google Scholar] [CrossRef]
- Graham, S.A.; Lee, E.E.; Jeste, D.V.; Van Patten, R.; Twamley, E.W.; Nebeker, C.; Yamada, Y.; Kim, H.-C.; Depp, C.A. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020, 284, 112732. [Google Scholar] [CrossRef]
- Vinny, P.; Vishnu, V.; Srivastava, M.P. Artificial Intelligence shaping the future of neurology practice. Med. J. Armed India 2021, 77, 276–282. [Google Scholar] [CrossRef]
- Jayatilake, S.M.D.A.C.; Ganegoda, G.U. Involvement of machine learning tools in healthcare decision making. J. Healthc. Eng. 2021, 2021, 6679512. [Google Scholar] [CrossRef]
- Pandya, S.; Thakur, A.; Saxena, S.; Jassal, N.; Patel, C.; Modi, K.; Shah, P.; Joshi, R.; Gonge, S.; Kadam, K. A study of the recent trends of immunology: Key challenges, domains, applications, datasets, and future directions. Sensors 2021, 21, 7786. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.R.; Tang, Z.; Mew, N.C.; Das, S.; Athey, J.; McAleese, K.E.; Kofler, J.K.; Flanagan, M.E.; Borys, E.; White, C.L. Deep learning from multiple experts improves identification of amyloid neuropathologies. Acta Neuropathol. Commun. 2022, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Flint, C.; Cearns, M.; Opel, N.; Redlich, R.; Mehler, D.; Emden, D.; Winter, N.R.; Leenings, R.; Eickhoff, S.B.; Kircher, T. Systematic misestimation of machine learning performance in neuroimaging studies of depression. Neuropsychopharmacology 2021, 46, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Vinny, P.W.; Garg, R.; Srivastava, M.P.; Lal, V.; Vishnu, V.Y. Critical appraisal of a machine learning paper: A guide for the neurologist. Ann. Indian Acad. Neurol. 2021, 24, 481. [Google Scholar] [CrossRef]


| Features | Example(s) | Example Relevant in Mental Disorder(s) |
|---|---|---|
| Acoustic | ||
| Source of sound features | Jitter | Increase with depression severity |
| Filtering features by vocal and nasal tracks | First resonant peak in the spectrum | Increase with bipolar severity |
| Increase with bipolar severity | Mel frequency cepstral coefficients | A variety of disorders |
| Prosodic features of speech | Pause duration | Higher in SCZ |
| Video | ||
| Facial | Smile duration, eyebrow movement, disgust expression | Increased disgust expression in SI |
| Eyes | Gaze angle | More non-mutual gazes in MDD |
| Gait | Arm swing and stride | Reduced arm swing in MDD |
| Posture | Head pitch variance, upper body movements | Reduced head movement in SCZ Higher head movement in ASD |
| Language | ||
| Grandiosity | Unrealistic sense of superiority | Increased in bipolar |
| Semantic coherence | Flow of meaning | Decreased in psychosis |
| Rumination | Repetitive thought patterns | Increased in MDD |
| Self-focus | Self-referent information | Increased in stress |
| N | Country | No. Participants | No. with Depression Disorder | Source |
|---|---|---|---|---|
| 1 | Malaysia | 64 | 34 | [78] |
| 2 | USA | 121 | 46 | [79] |
| 3 | China | 53 | 24 | [80] |
| 4 | The Netherlands | 1274 | 426 | [81] |
| N | Architecture | Accuracy | No. Participants (Depression + Controls) | Source |
|---|---|---|---|---|
| 1 | 1D CNN (9 layers) | 98% | 33 + 30 | [87] |
| 2 | Hybrid 1D CNN + LSTM | 96% | 33 + 30 | [87] |
| 3 | 1D CNN (15 layers) | 94% | 15 + 15 | [88] |
| 4 | Hybrid 1D CNN + LSTM | 98% | 15 + 15 | [89] |
| 5 | 1D CNN (5 layers) | 96% | 30 + 30 | [90] |
| 6 | Hybrid 2D CNN + GRU | 89% | 24 + 29 16 + 16 | [84] |
| 7 | 2D CNN (8 layers) | 99% | 34 + 30 | [91] |
| 8 | 1D CNN (18 layers) | 99% | 15 + 18 | [92] |
| 9 | Hybrid CNN + LSTM (6 layers) | 99% | 21 + 24 | [93] |
| 10 | 2D CNN | 86% | 24 + 27 | [94] |
| 11 | Hybrid 1D CNN + LSTM | 99% | 34 + 30 | [95] |
| 12 | Hybrid 1D CNN + LSTM (12 layers) | 99% | 46 + 75 | [96] |
| 13 | 2D CNN (8 layers) | 99% | 34 + 30 | [97] |
| 14 | 2D CNN (ResNet-50) | 90% | 46 + 46 | [98] |
| 15 | 2D CNN (8 layers) | 68% | 122 + 123 | [99] |
| N | Type of Treatment | Accuracy | No. Participants | Source |
|---|---|---|---|---|
| 1 | Antidepressants | 99% | 17 | [97] |
| 2 | Antidepressants | 96% | 30 | [118] |
| 3 | Antidepressants | 79% | 122 | [115] |
| 4 | Antidepressants | 78% | 51 | [116] |
| 5 | TMS | 82% | 50 + 24 | [117] |
| 6 | TMS | 91% | 46 | [119] |
| Challenges | Potential Future Directions |
|---|---|
| Bias of sample size | Integrated multiple cohort modeling. |
| Handling whole spectrum genetic information | Engaging appropriate feature engineering tools such as genetic principal component analysis, multidimensional scaling, linear discriminant analysis, etc.; Incorporating appropriate deep learning model such as autoencoder. |
| Multifactorial modeling | Multivariate modeling; Incorporating kernel approaches and probability models. |
| Cohort diversity | Validation on an external cohort; Training model on data from multiple populations if possible; Engaging transfer learning. |
| Model interpretation | Using interpretable models such as Bayesian, rule-based (e.g., decision tree and random forest), logistic regression models, etc.; Incorporating or developing model interpretation methods for “black box” models, e.g., deep learning models. |
| Model evaluation | Evaluation using isolated validation dataset; Applying experimental test evaluation; Developing visualization tools for model evaluation. |
| Interdisciplinary issue | Deep interdisciplinary collaboration; Incorporating domain knowledge in model training. |
| № | Question |
|---|---|
| 1 | Was the study prospective or retrospective, observational, or randomized controlled trial? |
| 2 | Was the protocol published a priori? |
| 3 | Why was the dataset obtained, and what is its size? |
| 4 | What is the intended use of ML model in the context of the clinical pathway? |
| 5 | Does the dataset represent the disease spectrum in the target population? |
| 6 | How was the data split between training, validation and external testing? |
| 7 | How was the gold standard determined? |
| 8 | What was the type of ML employed? Which version of the model was used for the study? |
| 9 | Is the reported model “continuously evolving” or “continuously learning” by design? |
| 10 | Does the model suffer from a black box problem? |
| 11 | Which performance metric is being reported/optimized? How were performance errors identified and analyzed? |
| 12 | Is the model performance too good to be true? |
| 13 | Is the study repeatable and reproducible? Are source code and datasets available for scrutiny? |
| 13 | Does the AI intervention affect patient outcomes? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shusharina, N.; Yukhnenko, D.; Botman, S.; Sapunov, V.; Savinov, V.; Kamyshov, G.; Sayapin, D.; Voznyuk, I. Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics 2023, 13, 573. https://doi.org/10.3390/diagnostics13030573
Shusharina N, Yukhnenko D, Botman S, Sapunov V, Savinov V, Kamyshov G, Sayapin D, Voznyuk I. Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics. 2023; 13(3):573. https://doi.org/10.3390/diagnostics13030573
Chicago/Turabian StyleShusharina, Natalia, Denis Yukhnenko, Stepan Botman, Viktor Sapunov, Vladimir Savinov, Gleb Kamyshov, Dmitry Sayapin, and Igor Voznyuk. 2023. "Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression" Diagnostics 13, no. 3: 573. https://doi.org/10.3390/diagnostics13030573
APA StyleShusharina, N., Yukhnenko, D., Botman, S., Sapunov, V., Savinov, V., Kamyshov, G., Sayapin, D., & Voznyuk, I. (2023). Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics, 13(3), 573. https://doi.org/10.3390/diagnostics13030573






