Striatal Patchwork of D1-like and D2-like Receptors Binding Densities in Rats with Genetic Audiogenic and Absence Epilepsies
Abstract
1. Introduction
2. Materials and Methods
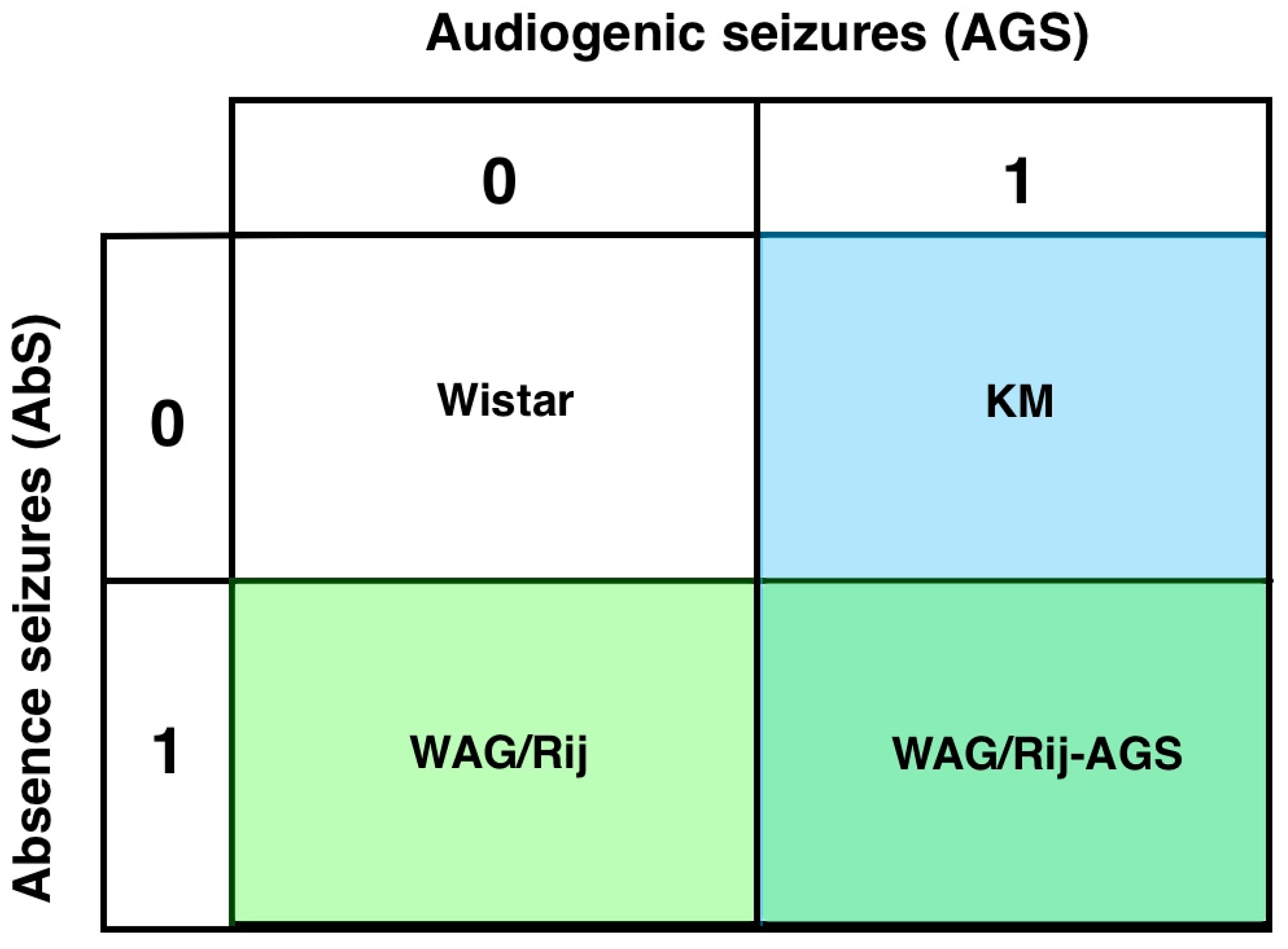
2.1. Animals
2.2. D1-like Receptor Autoradiography
2.3. D2-like Receptor Autoradiography
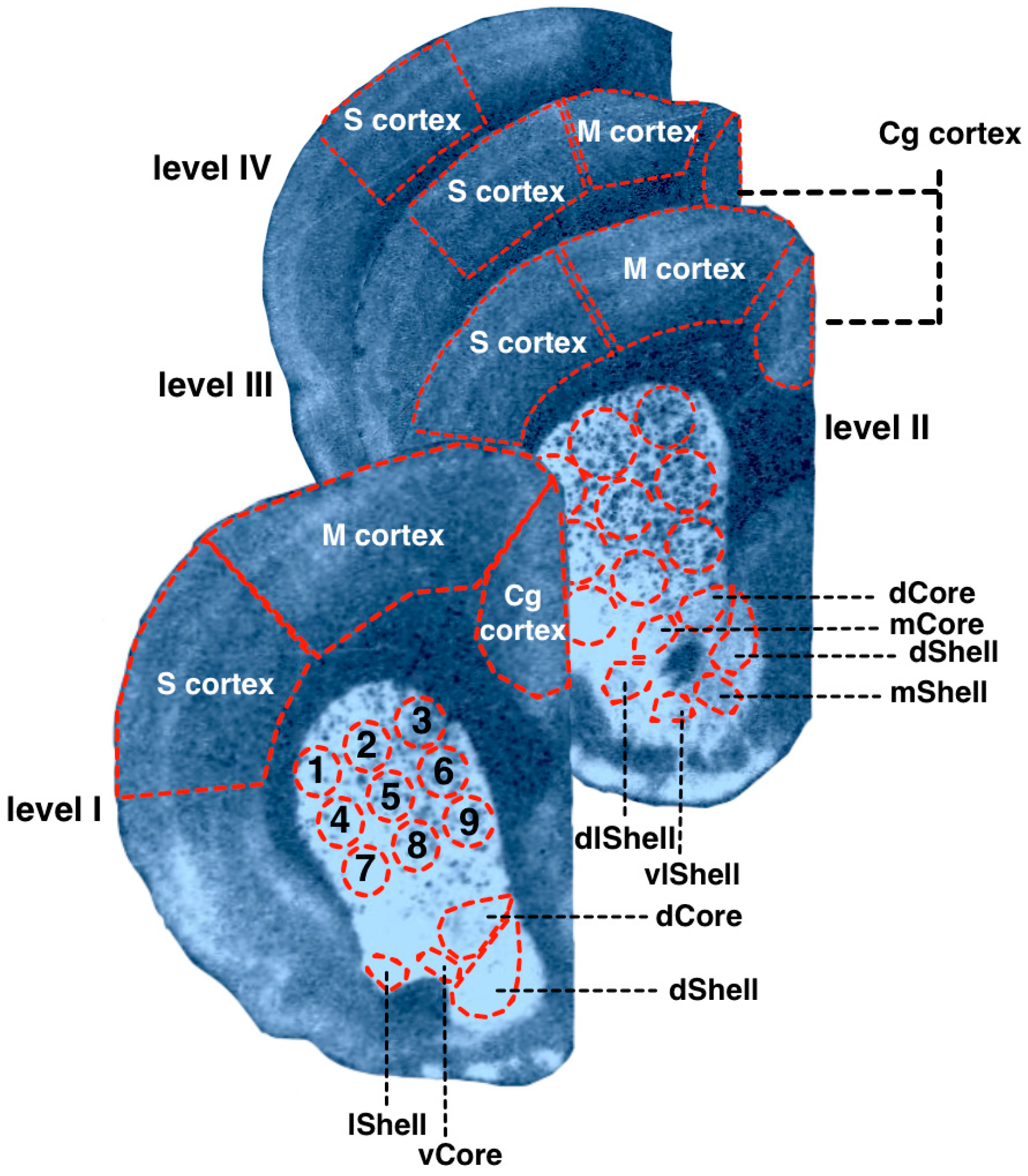
2.4. Measurements
2.5. Statistical Analyses
3. Results and Discussion
3.1. Distribution of D1DR and D2DR Binding Densities in Normal WS Rats
3.2. Effects of Epilepsies
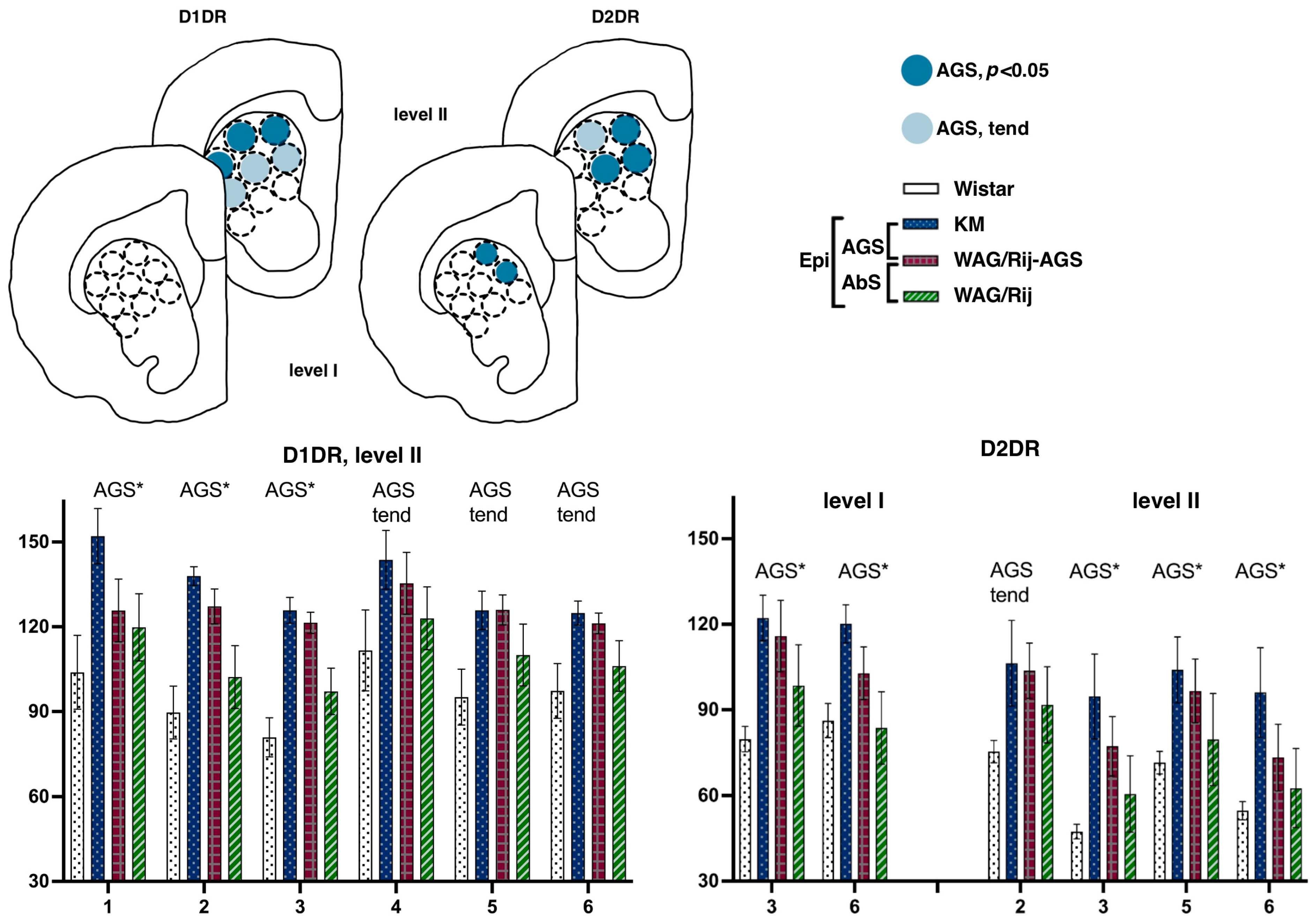
3.2.1. Dorsal Striatum
Striatal Binding Densities to D1DR
Striatal Binding Densities to D2DR
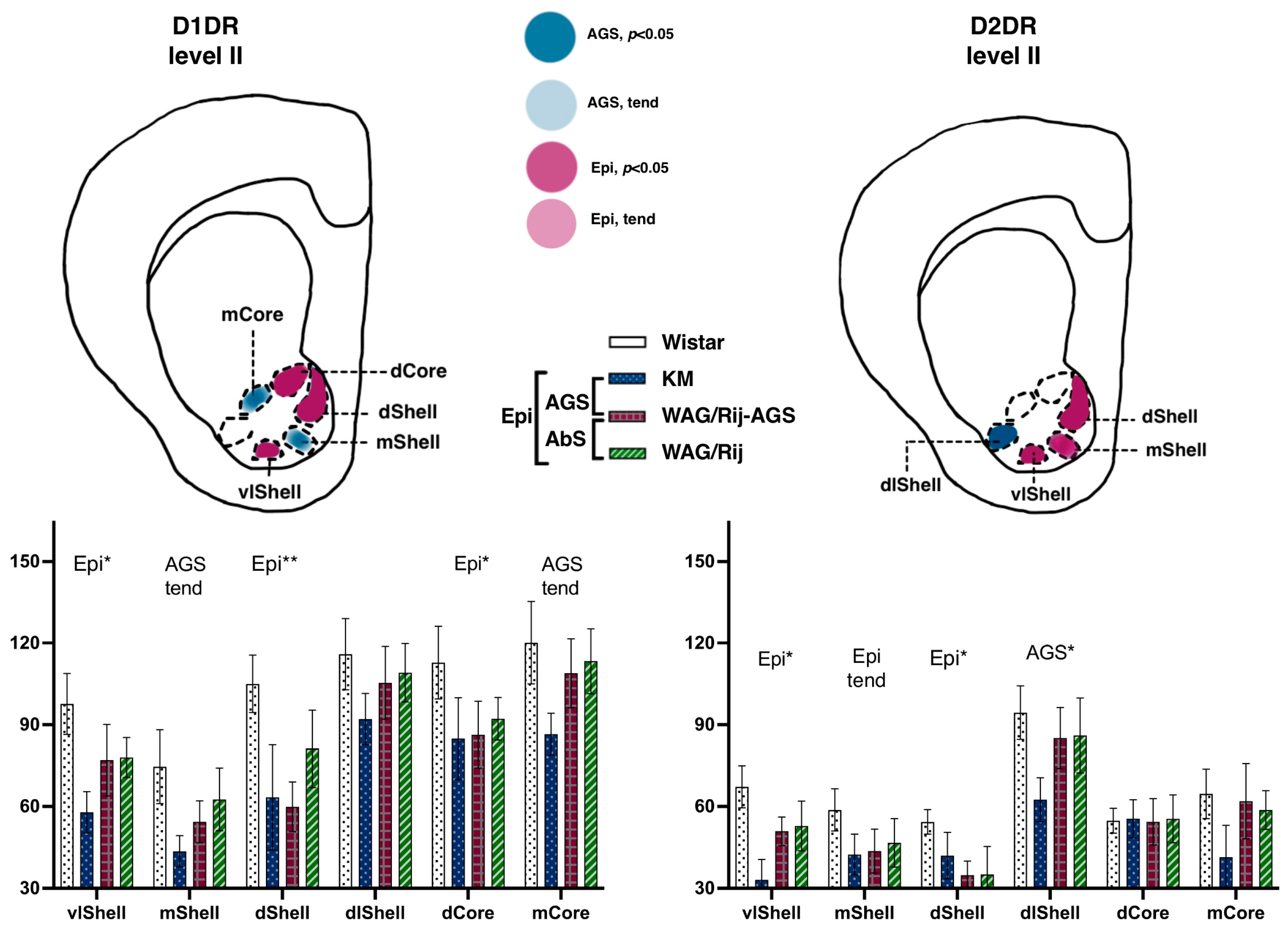
3.2.2. Ventral Striatum
Accumbal Binding Densities to D1DR
Accumbal Binding Densities to D2-like Dopamine Receptors
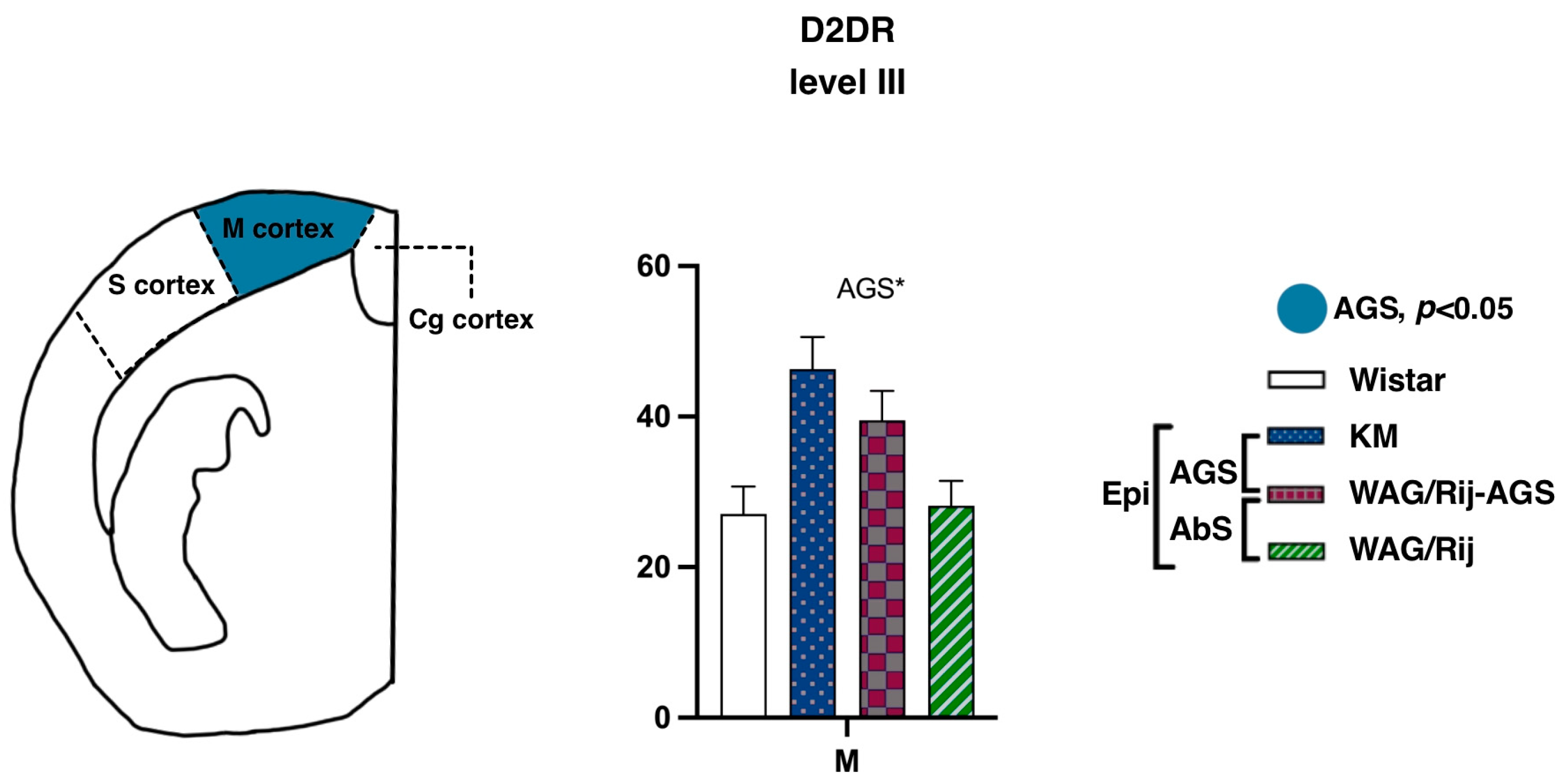
3.2.3. Motor and Somatosensory Cortex
3.3. Regional Correlations of D1DR and D2DR Binding Densities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akyuz, E.; Polat, A.K.; Eroglu, E.; Kullu, I.; Angelopoulou, E.; Paudel, Y.N. Revisiting the Role of Neurotransmitters in Epilepsy: An Updated Review. Life Sci. 2021, 265, 118826. [Google Scholar] [CrossRef]
- Tugba, E.; Medine, G.; Ozlem, A.; Deniz, K.; Filiz, O. Prolongation of absence seizures and changes in serotonergic and dopaminergic neurotransmission by nigrostriatal pathway degeneration in genetic absence epilepsy rats. Pharm. Biochem. Behav. 2022, 213, 173317. [Google Scholar] [CrossRef]
- Jones, N.C.; O’Brien, T.J. Stress, epilepsy, and psychiatric comorbidity: How can animal models inform the clinic? Epilepsy Behav. 2013, 26, 363–369. [Google Scholar] [CrossRef]
- Kanner, A. Mood disorder and epilepsy: A neurobiologic perspective of their relationship. Dialogues Clin. Neurosci. 2008, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. The holy grail of epilepsy prevention: Preclinical approaches to antiepileptogenic treatments. Neuropharmacology 2020, 167, 107605. [Google Scholar] [CrossRef] [PubMed]
- Poletaeva, I.I.; Kostyna, Z.A.; Surina, N.M.; Fedotova, I.B.; Zorina, Z.A. The Krushinsky–Molodkina genetic rat strain as a unique experimental model of seizure states. Vavilovskii Zhurnal Genet. I Sel. Vavilov J. Genet. Breed. 2017, 21, 427–434. [Google Scholar] [CrossRef]
- Poletaeva, I.I.; Surina, N.M.; Kostina, Z.A.; Perepelkina, O.V.; Fedotova, I.B. The Influence of Ionizing Radiation on Proneness to Audiogenic Seizure and Behavior in Krushinsky–Molodkina Rats. Biophysics 2020, 65, 660–665. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Zobeiri, M. Progress and outlooks in a genetic absence epilepsy model (WAG/Rij). Curr. Med. Chem. 2014, 21, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Citraro, R.; Constanti, A.; Leo, A.; Lüttjohann, A.; van Luijtelaar, G.; De Sarro, G. Upholding WAG/Rij rats as a model of absence epileptogenesis: Hidden mechanisms and a new theory on seizure development. Neurosci. Biobehav. Rev. 2016, 71, 388–408. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Zobeiri, M.; Lüttjohann, A.; Depaulis, A. Experimental treatment options in absence epilepsy. Curr. Pharm. Des. 2017, 23, 5577–5592. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, G.D. Audiogenic seizures in rats of different genetic strains. Zh Vyss. Nervn Dejat 1998, 48, 143–152. (In Russian) [Google Scholar]
- Midzyanovskaya, I.S.; Kuznetsova, G.D.; Vinogradova, L.V.; Shatskova, A.B.; Coenen, A.M.L.; van Luijtelaar, G. Mixed forms of epilepsy in a subpopulation of WAG/Rij rats. Epilepsy Behav. 2004, 5, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Gurbanova, A.A.; Aker, R.; Berkman, K.; Onat, F.Y.; van Rijn, C.M.; van Luijtelaar, G. Effect of systemic and intracortical administration of phenytoin in two genetic models of absence epilepsy. Br. J. Pharmacol. 2006, 148, 1076–1082. [Google Scholar] [CrossRef]
- Meeren, H.; van Luijtelaar, G.; da Silva, F.L.; Coenen, A. Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Arch. Neurol. 2005, 62, 371–376. [Google Scholar] [CrossRef]
- Lüttjohann, A.; van Luijtelaar, G. Dynamics of networks during absence seizure’s on-and offset in rodents and man. Front. Physiol. 2015, 6, 16. [Google Scholar] [CrossRef]
- Kuznetsova, G.D.; Petrova, E.V.; Coenen, A.M.L.; Van Luijtelaar, E.L.J.M. Generalized absence epilepsy and catalepsy in rats. Physiol. Behav. 1996, 60, 1165–1169. [Google Scholar] [CrossRef]
- Deransart, C.; Riban, V.; Lê, B.-T.; Marescaux, C.; Depaulis, A. Dopamine in the striatum modulates seizures in a genetic model of absence epilepsy in the rat. Neuroscience 2000, 100, 335–344. [Google Scholar] [CrossRef]
- D’Amore, V.; von Randow, C.; Nicoletti, F.; Ngomba, R.T.; van Luijtelaar, G. Anti-absence activity of mGlu1 and mGlu5 receptor enhancers and their interaction with a GABA reuptake inhibitor: Effect of local infusions in the somatosensory cortex and thalamus. Epilepsia 2015, 56, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Poletaeva, I.I.; Fedotova, I.B.; Sourina, N.M.; Kostina, Z.A. Audiogenic Seizures—Biological Phenomenon and Experimental Model of Human Epilepsies. In Clinical and Genetic Aspects of Epilepsy; IntechOpen: London, UK, 2011; Available online: https://www.intechopen.com/chapters/19737 (accessed on 20 December 2022). [CrossRef]
- Fedotova, I.B.; Surina, N.M.; Malikova, L.A.; Raevskiĭ, K.S.; Poletaeva, I.I. The investigation of cataleptic muscle tonus changes in rats after audiogenic seizures. Zh. Vyssh. Nerv. Deiat. 2008, 58, 585620627. [Google Scholar]
- Sorokin, A.I.; Kudrin, V.S.; Klodt, P.M.; Tuomisto, L.; Poletaeva, I.I.; Raevskiĭ, K.S. The interstrain differences in the effects of D-amphet- amine and raclopride on dorsal striatum dopaminergic system in KM and Wistar rats (microdi- alysis study). Genetika 2004, 40, 846–849. (In Russian) [Google Scholar]
- Wicker, E.; Beck, V.C.; Kulick-Soper, C.; Kulick-Soper, C.V.; Hyder, S.K.; Campos-Rodriguez, C.; Khan, T.; N’Gouemo, P.; Forcelli, P.A. Descending projections from the substantia nigra pars reticulata differentially control seizures. Proc. Natl. Acad. Sci. USA 2019, 116, 27084–27094. [Google Scholar] [CrossRef] [PubMed]
- Birioukova, L.; Midzyanovskaya, I.; Lensu, S.; Tuomisto, L.; van Luijtelaar, G. Distribution of D1-like and D2-like dopamine receptors in the brain of genetic epileptic WAG/Rij rats. Epilepsy Res. 2005, 63, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance. Official Journal of the European Union. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 20 December 2022).
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Blunt, S.B.; Jenner, P.; Marsden, C.D. Autoradiographic study of striatal D1- and D2-like dopamine receptors in 6-OHDA- lesioned rats receiving foetal ventral mesencephalic grafts and chronic treatment with L-DOPA and carbidopa. Brain Res. 1992, 582, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Dellu-Hagedorn, F.; Fitoussi, A.; De Deurwaerdere, P.J. Correlative Analysis of Dopaminergic and Serotonergic Metabolism across the Brain to Study Monoaminergic Function and Interaction. J. Neurosci. Methods 2017, 280, 54–63. [Google Scholar] [CrossRef]
- Boyson, S.J.; McGonigle, P.; Molinoff, P.B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci. 1986, 6, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, F.; Oskamp, A.; Lang, M.; Hawlitschka, A.; Zilles, K.; Wree, A.; Bauer, A. Intrastriatal administration of botulinum neurotoxin A normalizes striatal D2R binding and reduces striatal D1R binding in male hemiparkinsonian rats. J. Neurosci. Res. 2018, 96, 75–86. [Google Scholar] [CrossRef]
- Fedotova, I.B.; Surina, N.M.; Nikolaev, G.M.; Revishchin, A.V.; Poletaeva, I.I. Rodent brain pathology, audiogenic epilepsy. Biomedicines 2021, 9, 1641. [Google Scholar] [CrossRef]
- Ju, F.; Abaimov, D.A.; Surina, N.M.; Poletaeva, I.I.; Fedotova, I.B.; Kovalev, G.I. Binding of Specific Ligand by D2- and NMDA-Receptors of Striatum Cells in Two Rat Strains Predisposed and Resistant to Audiogenic Seizures. Bull. Exp. Biol. Med. 2012, 154. [Google Scholar] [CrossRef]
- Chuvakova, L.N.; Funikov, S.Y.; Rezvykh, A.P.; Davletshin, A.I.; Evgen’ev, M.B.; Litvinova, S.A.; Fedotova, I.B.; Poletaeva, I.I.; Garbuz, D.G. Transcriptome of the Krushinsky-Molodkina audiogenic rat strain and identification of possible audiogenic epilepsy-associated genes. Front. Mol. Neurosci. 2021, 14, 738930. [Google Scholar] [CrossRef]
- Kowski, A.B.; Holtkamp, M. Electrically induced limbic seizures: Preliminary findings in a rodent model. J. Exp. Neurosci. 2015, 25, 7–14. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, R.; Wang, K.; Zhang, Z.; Wang, J.; Tan, X.; Zhang, J.; Mei, Y.; Chan, Q.; Xu, J.; et al. Connectivity-based parcellation of the nucleus accumbens into core and shell portions for stereotactic target localization and alterations in each NA c subdivision in mTLE patients. Hum. Brain Mapp. 2018, 39, 1232–1245. [Google Scholar] [CrossRef]
- Zou, W.; Guo, Z.; Suo, L.; Zhu, J.; He, H.; Li, X.; Wang, K.; Chen, R. Nucleus accumbens shell modulates seizure propagation in a mouse temporal lobe epilepsy model. Front. Cell Dev. Biol. 2022, 10, 1031872. [Google Scholar] [CrossRef] [PubMed]
- Nizinska, K.; Szydlowska, K.; Vouros, A.; Kiryk, A.; Stepniak, A.; Vasilaki, E.; Lukasiuk, K. Behavioral characteristics as potential biomarkers of the development and phenotype of epilepsy in a rat model of temporal lobe epilepsy. Sci. Rep. 2021, 11, 8665. [Google Scholar] [CrossRef] [PubMed]
- Rebik, A.A.; Riga, V.D.; Smirnov, K.S.; Sysoeva, O.V.; Midzyanovskaya, I.S. Social Behavioral Deficits in Krushinsky-Molodkina Rats, an Animal Model of Audiogenic Epilepsy. J. Pers. Med. 2022, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Ospina, J.P.; Larson, A.G.; Jalilianhasanpour, R.; Williams, B.; Diez, I.; Dhand, A.; Dickerson, B.C.; Perez, D.L. Individual differences in social network size linked to nucleus accumbens and hippocampal volumes in functional neurological disorder: A pilot study. J. Affect. Disord. 2019, 258, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Strasser, L.; Downes, M.; Kung, J.; Cross, J.H.; De Haan, M. Prevalence and risk factors for autism spectrum disorder in epilepsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.Y.; Kulikov, M.A.; Midzyanovskaya, I.S.; Folomkina, A.A. Dopamine-dependent nature of depression-like behavior in WAG/Rij rats with genetic absence epilepsy. Neurosci. Behav. Physiol. 2008, 38, 119–128. [Google Scholar] [CrossRef]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef]
- Midzyanovskaya, I.S.; Shatskova, A.B.; MacDonald, E.; van Luijtelaar, G.; Tuomisto, L. Brain aminergic deficiency in absence epileptic rats: Dependency on seizure severity and their functional coupling at rest. J. Behav. Brain Sci. 2020, 10, 29–45. [Google Scholar] [CrossRef]
- Coenen, A.M.; van Luijtelaar, E.L. Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- Midzyanovskaya, I.S.; Birioukova, L.M.; Storvik, M.; van Luijtelaar, G.; Tuomisto, L.M. The prefrontal cortex shows widespread decrease in H3 histamine receptor binding densities in rats with genetic generalized epilepsies. Epilepsy Res. 2022, 182, 106921. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, N.M.; van Luijtelaar, E.L.J.M.; Jansen, S.J.; Cools, A.R.; Ellenbroek, B.A. Dopamine characteristics in different rat genotypes: The relation to absence epilepsy. Neurosci. Res. 2000, 38, 165–173. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyba, E.T.; Midzyanovskaya, I.S.; Birioukova, L.M.; Tuomisto, L.M.; van Luijtelaar, G.; Abbasova, K.R. Striatal Patchwork of D1-like and D2-like Receptors Binding Densities in Rats with Genetic Audiogenic and Absence Epilepsies. Diagnostics 2023, 13, 587. https://doi.org/10.3390/diagnostics13040587
Tsyba ET, Midzyanovskaya IS, Birioukova LM, Tuomisto LM, van Luijtelaar G, Abbasova KR. Striatal Patchwork of D1-like and D2-like Receptors Binding Densities in Rats with Genetic Audiogenic and Absence Epilepsies. Diagnostics. 2023; 13(4):587. https://doi.org/10.3390/diagnostics13040587
Chicago/Turabian StyleTsyba, Evgeniya T., Inna S. Midzyanovskaya, Lidia M. Birioukova, Leena M. Tuomisto, Gilles van Luijtelaar, and Kenul R. Abbasova. 2023. "Striatal Patchwork of D1-like and D2-like Receptors Binding Densities in Rats with Genetic Audiogenic and Absence Epilepsies" Diagnostics 13, no. 4: 587. https://doi.org/10.3390/diagnostics13040587
APA StyleTsyba, E. T., Midzyanovskaya, I. S., Birioukova, L. M., Tuomisto, L. M., van Luijtelaar, G., & Abbasova, K. R. (2023). Striatal Patchwork of D1-like and D2-like Receptors Binding Densities in Rats with Genetic Audiogenic and Absence Epilepsies. Diagnostics, 13(4), 587. https://doi.org/10.3390/diagnostics13040587






