Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge
Abstract
1. Introduction
2. Background and Epidemiology
3. Clinical Presentation
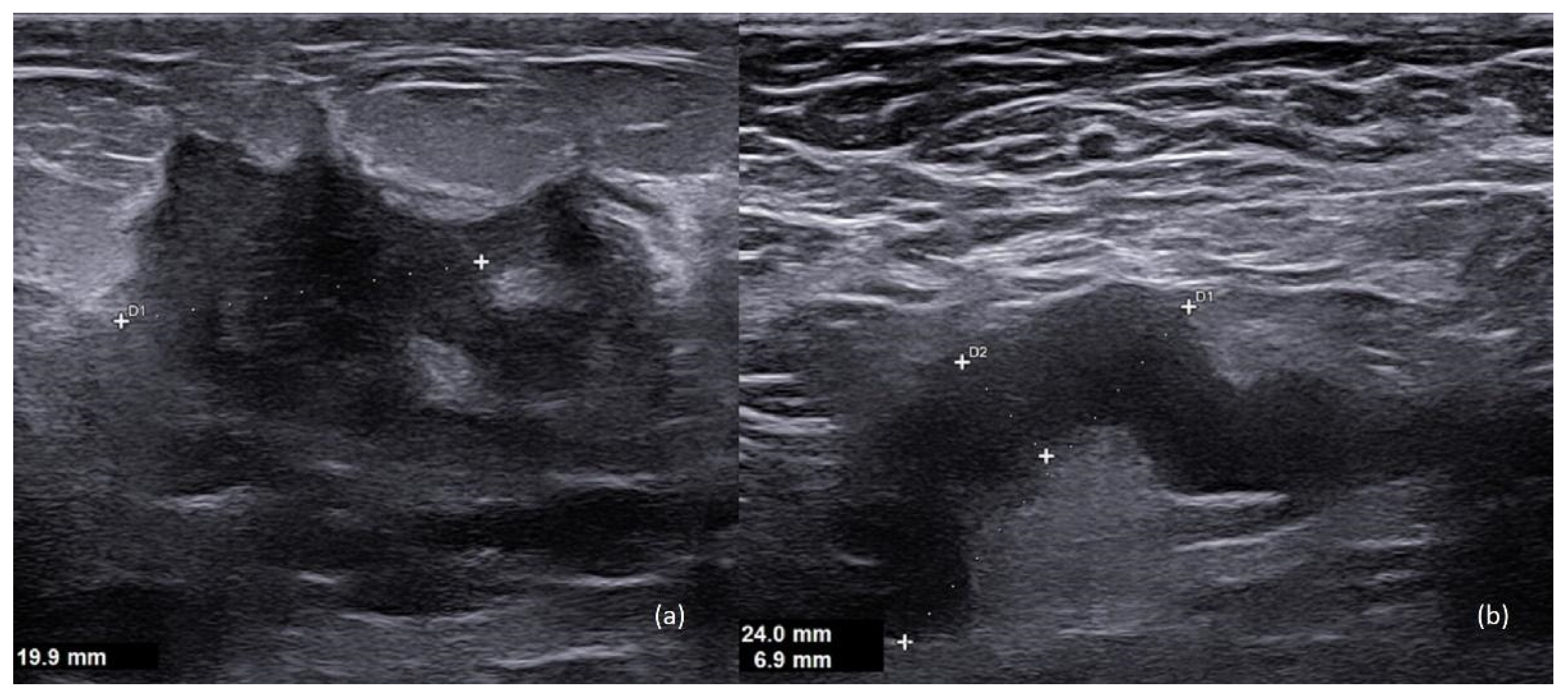
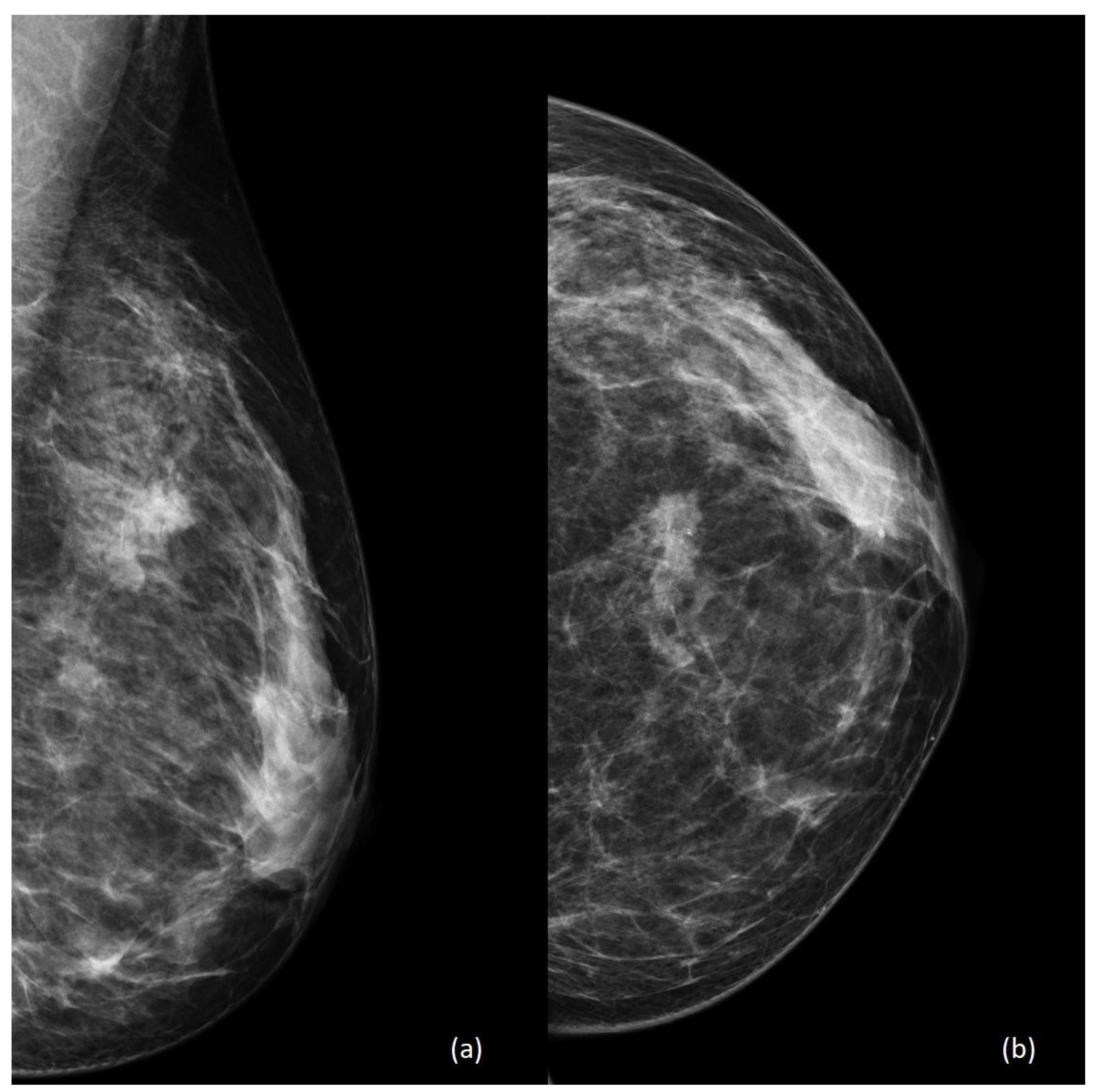
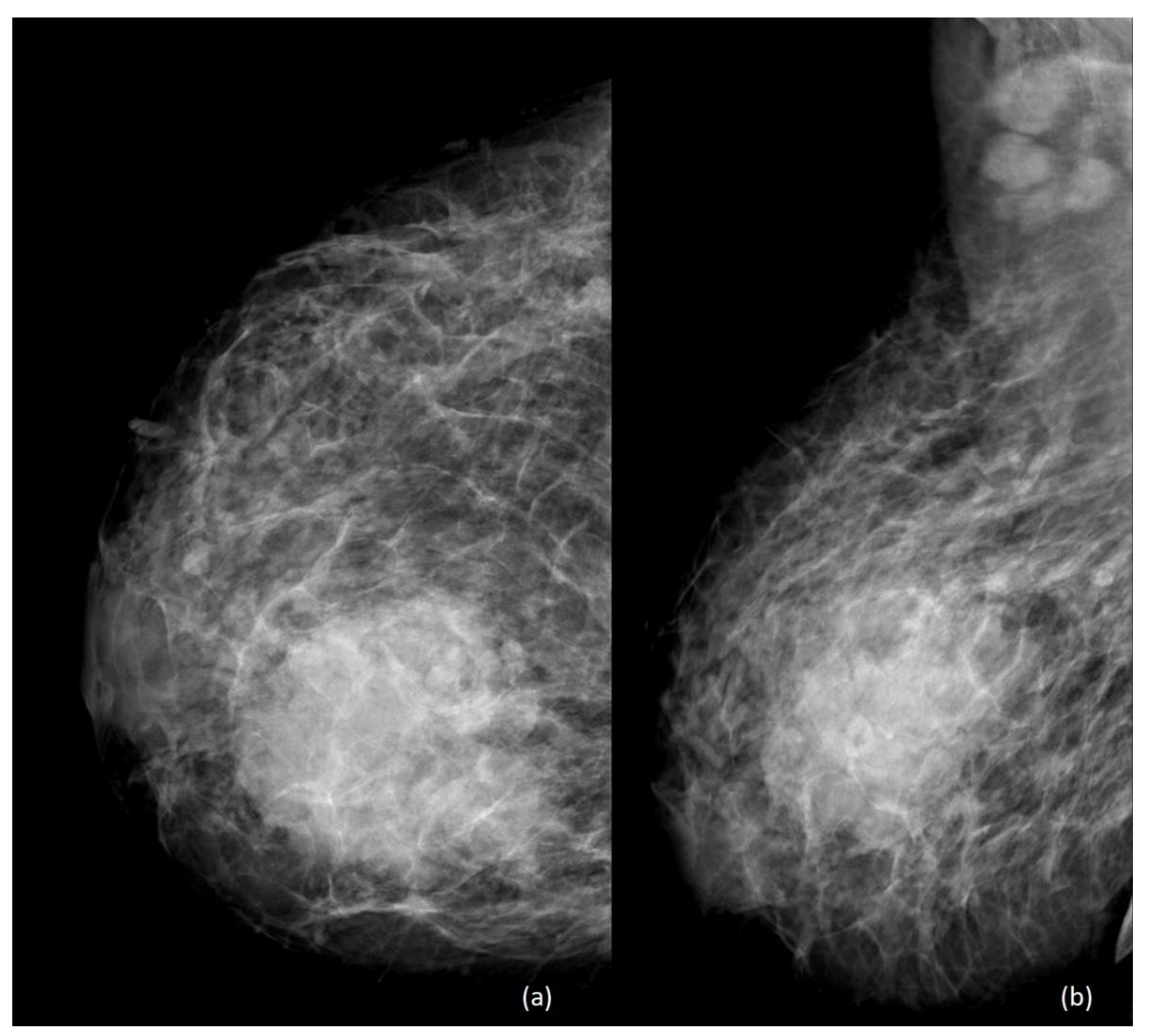
4. Imaging
5. Histology
6. Local and Systemic Treatments
6.1. Surgery
6.2. Radiotherapy
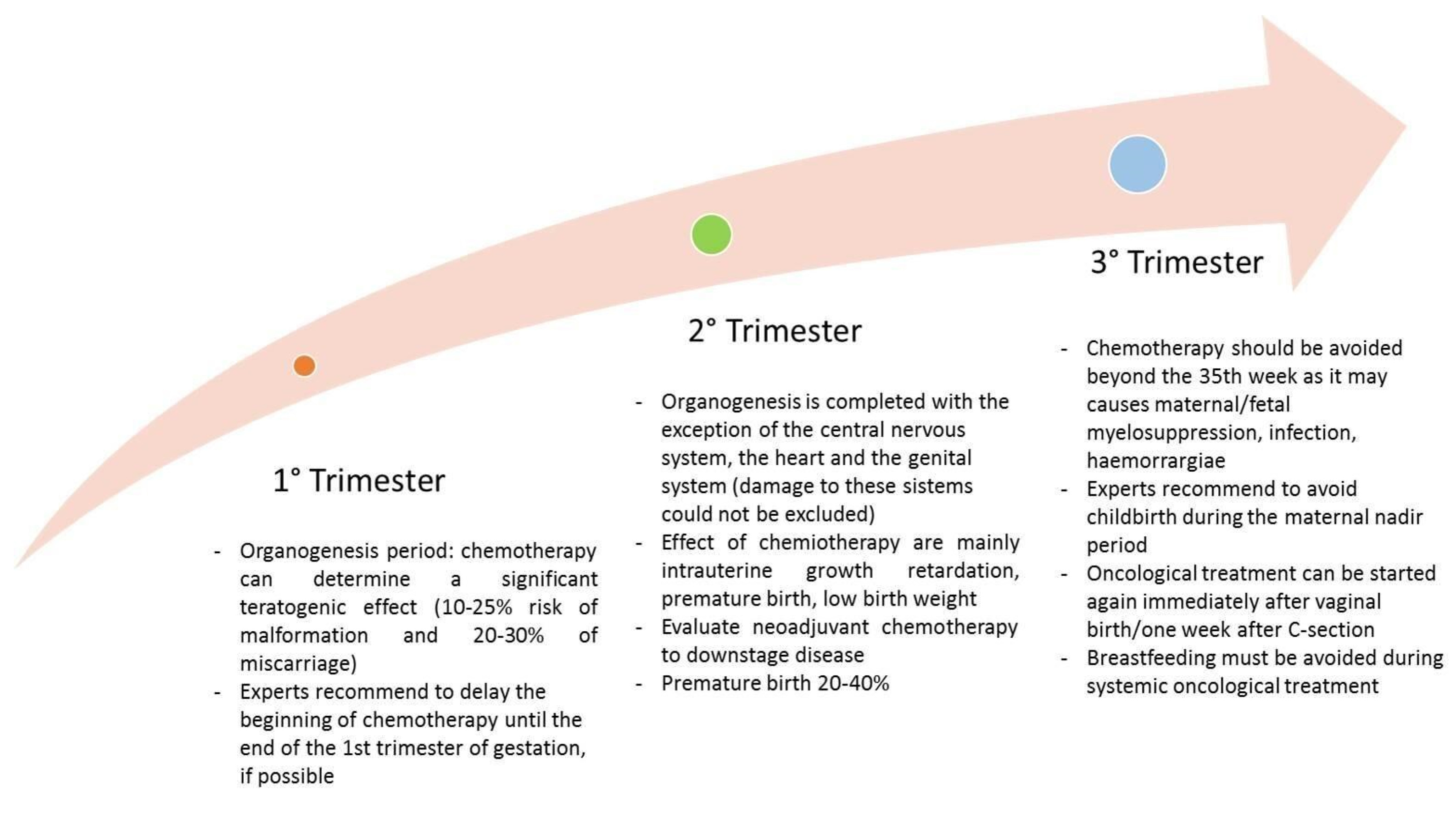
6.3. Chemotherapy
6.3.1. Maternal Factors
6.3.2. Timing
6.3.3. Drug Factors and Fetal Effects
6.4. Hormone Therapy
6.5. Target Therapy
- Healthy babies when the drug was administered only during the first trimester;
- Higher prevalence of adverse birth events (57%) or stillbirths when administered during the second and third trimester;
- Oligohydramnios/anhydramnios as the most reported event (73.3% of patients) during the second and third trimester.
6.6. Immunotherapy
6.7. Lactation during Systemic Therapy
7. Conclusions
Funding
Conflicts of Interest
References
- Allouch, S.; Gupta, I.; Malik, S.; Al Farsi, H.F.; Vranic, S.; Al Moustafa, A.E. During Pregnancy: A Marked Propensity to Triple-Negative Phenotype. Front. Oncol. 2020, 10, 580345. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.A. Coexistence of pregnancy and malignancy. Oncologist 2002, 7, 279–287. [Google Scholar] [CrossRef]
- Keyser, E.A.; Staat, B.C.; Fausett, M.B.; Shields, A.D. Pregnancy-associated breast cancer. Rev. Obstet. Gynecol. 2012, 5, 94–99. [Google Scholar] [PubMed]
- Beadle, B.M.; Woodward, W.A.; Middleton, L.P.; Tereffe, W.; Strom, E.A.; Litton, J.K.; Meric-Bernstam, F.; Theriault, R.L.; Buchholz, T.A.; Perkins, G.H. The impact of pregnancy on breast cancer outcomes in women. Cancer 2009, 115, 1174–1184. [Google Scholar] [CrossRef]
- Andersson, T.M.; Johansson, A.L.V.; Hsieh, C.C.; Cnattingius, S.; Lambe, M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet. Gynecol. 2009, 114, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.B.; Brandão, E.C.; Soares, A.N.; Lucena, C.E.; Antunes, C.M. Prognosis for patients diagnosed with pregnancy-associated breast cancer: A paired case-control study. Sao Paulo Med. J. 2010, 128, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.H.; Danielsen, B.; Allen, M.E.; Cress, R. Cancer associated with obstetric delivery: Results of linkage with the California cancer registry. Am. J. Obstet. Gynecol. 2003, 189, 1128–1135. [Google Scholar] [CrossRef]
- Soto-Trujillo, D.; Santos Aragón, L.N.; Kimura, Y. Pregnancy-Associated Breast Cancer: What Radiologists Must Know. Cureus 2020, 12, e10343. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Huang, W.; Shao, B.; Yan, Y.; Liang, X.; Ran, R.; Song, G.; Di, L.; Jiang, H.; et al. Clinicopathological features and prognosis of patients with pregnancy-associated breast cancer: A matched case control study. Asia Pac. J. Clin. Oncol. 2021, 17, 396–402. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Jiang, Z.; Zhao, J.; Mao, Y.; Liu, J.; Zhang, J. Clinicopathological characteristics, diagnosis, and prognosis of pregnancy-associated breast cancer. Thorac. Cancer 2019, 10, 1060–1068. [Google Scholar] [CrossRef]
- Johansson, A.L.V.; Andersson, T.M.; Hsieh, C.C.; Jirström, K.; Cnattingius, S.; Fredriksson, I.; Dickman, P.W.; Lambe, M. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int. J. Cancer 2018, 142, 1343–1354. [Google Scholar] [CrossRef]
- Middleton, L.P.; Amin, M.; Gwyn, K.; Theriault, R.; Sahin, A. Breast carcinoma in pregnant women: Assessment of clinicopathologic and immunohistochemical features. Cancer 2003, 98, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Jahanbin, B.; Soleimani, V. Histology of Pregnancy-Associated Breast Cancer. AEMB 2020, 1252, 81–86. [Google Scholar] [CrossRef]
- Ayyappan, A.P.; Kulkarni, S.; Crystal, P. Pregnancy-associated breast cancer: Spectrum of imaging appearances. Br. J. Radiol. 2010, 83, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lee, M.C. Clinical Presentation, Diagnosis and Prognosis of Pregnancy-Associated Breast Cancer. Adv. Exp. Med. Biol. 2020, 1252, 87–93. [Google Scholar] [CrossRef]
- Wanders, J.O.; Holland, K.; Veldhuis, W.B.; Mann, R.M.; Pijnappel, R.M.; Peeters, P.H.; van Gils, C.H.; Karssemeijer, N. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res. Treat. 2017, 162, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nissan, N.; Bauer, E.; Moss Massasa, E.E.; Sklair-Levy, M. Breast MRI during pregnancy and lactation: Clinical challenges and technical advances. Insights Imaging 2022, 13, 71. [Google Scholar] [CrossRef]
- Expert Panel on Breast Imaging; Di Florio-Alexander, R.M.; Slanetz, P.J.; Moy, L.; Baron, P.; Didwania, A.D.; Heller, S.L.; Holbrook, A.I.; Lewin, A.A.; Lourenco, A.P.; et al. ACR Appropriateness Criteria® Breast Imaging of Pregnant and Lactating Women. JACR 2018, 15, S263–S275. [Google Scholar] [CrossRef]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef]
- Kieturakis, A.J.; Wahab, R.A.; Vijapura, C.; Mahoney, M.C. Current Recommendations for Breast Imaging of the Pregnant and Lactating Patient. AJR Am. J. Roentgenol. 2021, 216, 1462–1475. [Google Scholar] [CrossRef]
- Galati, F.; Moffa, G.; Pediconi, F. Breast imaging: Beyond the detection. Eur. J. Radiol. 2022, 146, 110051. [Google Scholar] [CrossRef] [PubMed]
- Galati, F.; Trimboli, R.M.; Pediconi, F. Special Issue “Advances in Breast MRI”. Diagnostics 2021, 11, 2297. [Google Scholar] [CrossRef] [PubMed]
- Galati, F.; Rizzo, V.; Trimboli, R.M.; Kripa, E.; Maroncelli, R.; Pediconi, F. MRI as a biomarker for breast cancer diagnosis and prognosis. BJR Open 2022, 4, 20220002. [Google Scholar] [CrossRef]
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging 2017, 45, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Nissan, N.; Furman-Haran, E.; Allweis, T.; Menes, T.; Golan, O.; Kent, V.; Barsuk, D.; Paluch-Shimon, S.; Haas, I.; Brodsky, M.; et al. Noncontrast Breast MRI During Pregnancy Using Diffusion Tensor Imaging: A Feasibility Study. J. Magn. Reson. Imaging 2019, 49, 508–517. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Codacci-Pisanelli, G.; Del Grande, M.; Scarfone, G.; Zugni, F.; Petralia, G. Whole body MRI for systemic staging of breast cancer in pregnant women. Breast 2017, 35, 177–181. [Google Scholar] [CrossRef]
- Han, S.N.; Amant, F.; Michielsen, K.; De Keyzer, F.; Fieuws, S.; Van Calsteren, K.; Dresen, R.C.; Gziri, M.M.; Vandecaveye, V. Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: A pilot study. Eur. Radiol. 2018, 28, 1862–1874. [Google Scholar] [CrossRef]
- Alex, A.; Bhandary, E.; McGuire, K.P. Anatomy and Physiology of the Breast during Pregnancy and Lactation. Adv. Exp. Med. Biol. 2020, 1252, 3–7. [Google Scholar] [CrossRef]
- Marzocca, F.; Moffa, G.; Landi, V.N.; Panzironi, G.; Kirchin, M.A.; Pediconi, F.; Galati, F. Gadoteridol-enhanced MRI of the breast: Can contrast agent injection rate impact background parenchymal enhancement? Acta Radiol. 2022, 63, 1173–1179. [Google Scholar] [CrossRef]
- Nissan, N.; Allweis, T.; Menes, T.; Brodsky, A.; Paluch-Shimon, S.; Haas, I.; Golan, O.; Miller, Y.; Barlev, H.; Carmon, E.; et al. Breast MRI during lactation: Effects on tumor conspicuity using dynamic contrast-enhanced (DCE) in comparison with diffusion tensor imaging (DTI) parametric maps. Eur. Radiol. 2020, 30, 767–777. [Google Scholar] [CrossRef]
- Avendano, D.; Marino, M.A.; Leithner, D.; Thakur, S.; Bernard-Davila, B.; Martinez, D.F.; Helbich, T.H.; Morris, E.A.; Jochelson, M.S.; Baltzer, P.A.T.; et al. Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res. 2019, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Moffa, G.; Kripa, E.; Caramanico, C.; Pediconi, F.; Galati, F. Preoperative Staging in Breast Cancer: Intraindividual Comparison of Unenhanced MRI Combined With Digital Breast Tomosynthesis and Dynamic Contrast Enhanced-MRI. Front. Oncol. 2021, 11, 661945. [Google Scholar] [CrossRef]
- Bonnier, P.; Romain, S.; Dilhuydy, J.M.; Bonichon, F.; Julien, J.P.; Charpin, C.; Lejeune, C.; Martin, P.M.; Piana, L. Influence of pregnancy on the outcome of breast cancer: A case-control study. Int. J. Cancer 1997, 72, 720–727. [Google Scholar] [CrossRef]
- Parente, J.T.; Amsel, M.; Lerner, R.; Chinea, F. Breast cancer associated with pregnancy. Obstet. Gynecol. 1988, 71, 861–864. [Google Scholar] [PubMed]
- Michieletto, S.; Saibene, T.; Evangelista, L.; Barbazza, F.; Grigoletto, R.; Rossi, G.; Ghiotto, C.; Bozza, F. Preliminary monocentric results of biological characteristics of pregnancy associated breast cancer. Breast 2014, 23, 19–25. [Google Scholar] [CrossRef]
- Marikakis, N.; Yiangou, C.; Agrawal, A. Hormone receptor expression in pregnancy-associated breast cancer: A systematic review of the literature. Eur. J. Surg. Oncol. 2018, 44, 913. [Google Scholar] [CrossRef]
- Doger, E.; Calışkan, E.; Mallmann, P. Pregnancy associated breast cancer and pregnancy after breast cancer treatment. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 247–255. [Google Scholar] [CrossRef]
- McDaniel, S.M.; Rumer, K.K.; Biroc, S.L.; Metz, R.P.; Singh, M.; Porter, W.; Schedin, P. Remodeling of the Mammary Microenvironment after Lactation Promotes Breast Tumor Cell Metastasis. Am. J. Pathol. 2006, 168, 608–620. [Google Scholar] [CrossRef]
- Korakiti, A.M.; Moutafi, M.; Zografos, E.; Dimopoulos, M.A.; Zagouri, F. The Genomic Profile of Pregnancy-Associated Breast Cancer: A Systematic Review. Front. Oncol. 2020, 10, 1773. [Google Scholar] [CrossRef]
- Azim, H.A.; Partridge, A.H. Biology of breast cancer in young women. Breast Cancer Res. 2014, 16, 427. [Google Scholar] [CrossRef]
- Pai, V.P.; Marshall, A.M.; Hernandez, L.L.; Buckley, A.R.; Horseman, N.D. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009, 11, R81. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Joyce, J.A. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle 2007, 6, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Shousha, S. Breast carcinoma presenting during or shortly after pregnancy and lactation. Arch. Pathol. Lab. Med. 2000, 124, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Genin, A.-S.; Lesieur, B.; Gligorov, J.; Antoine, M.; Selleret, L.; Rouzier, R. Pregnancy-associated breast cancers: Do they differ from other breast cancers in young women? Breast 2012, 21, 550–555. [Google Scholar] [CrossRef]
- Bae, S.Y.; Jung, S.P.; Jung, E.S.; Park, S.M.; Lee, S.K.; Yu, J.H.; Lee, J.E.; Kim, S.W.; Nam, S.J. Clinical Characteristics and Prognosis of Pregnancy-Associated Breast Cancer: Poor Survival of Luminal B Subtype. Oncology 2018, 95, 163–169. [Google Scholar] [CrossRef]
- Negro, A.; Brar, B.K.; Lee, K.F. Essential roles of Her2/erbB2 in cardiac development and function. Recent Progress Horm. Res. 2004, 59, 1–12. [Google Scholar] [CrossRef]
- Madaras, L.; Kovács, K.A.; Szász, A.M.; Kenessey, I.; Tőkés, A.M.; Székely, B.; Baranyák, Z.; Kiss, O.; Dank, M.; Kulka, J. Clinicopathological features and prognosis of pregnancy associated breast cancer—A matched case control study. Pathol. Oncol. Res. 2014, 20, 581–590. [Google Scholar] [CrossRef]
- Haber, D.A.; Buckler, A.J.; Glaser, T.; Call, K.M.; Pelletier, J.; Sohn, R.L.; Douglass, E.C.; Housman, D.E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor. Cell 1990, 61, 1257–1269. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Deng, C.X.; Man, Y.g. Aberrant p63 and WT-1 expression in myoepithelial cells of pregnancy-associated breast cancer: Implications for tumor aggressiveness and invasiveness. Int. J. Biol. Sci. 2009, 5, 82–96. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Chuang, S.C.; Lin, C.H.; Lu, Y.S.; Hsiung, C.A. Association of pregnancy and mortality in women diagnosed with breast cancer: A Nationwide Population Based Study in Taiwan. Int. J. Cancer 2018, 143, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rodríguez, J.L.; Vrba, L.; Futscher, B.W.; Hu, C.; Komenaka, I.K.; Meza-Montenegro, M.M.; Gutierrez-Millan, L.E.; Daneri-Navarro, A.; Thompson, P.A.; Martinez, M.E. Differentially Expressed MicroRNAs in Postpartum Breast Cancer in Hispanic Women. PLoS ONE 2015, 10, e0124340. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.A.; Gómez-Macias, G.; Valera, V.A.; Sobel, M.; Merino, M.J. MiR-21 Expression in Pregnancy-Associated Breast Cancer: A Possible Marker of Poor Prognosis. J. Cancer 2011, 2, 67–75. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Nejdlova, M.; Johnson, T. Anaesthesia for Non-Obstetric Procedures during Pregnancy. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 203–206. [Google Scholar] [CrossRef]
- Froehlich, K.; Schmidt, A.; Heger, J.I.; Al-Kawlani, B.; Aberl, C.A.; Jeschke, U.; Loibl, S.; Markert, U.R. Breast Cancer, Placenta and Pregnancy. Eur. J. Cancer 2019, 115, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Toesca, A.; Gentilini, O.; Peccatori, F.; Azim, H.A.; Amant, F. Locoregional Treatment of Breast Cancer during Pregnancy. Gynecol. Surg. 2014, 11, 279–284. [Google Scholar] [CrossRef]
- Fernández-Delgado, J.; López-Pedraza, M.J.; Blasco, J.A.; Andradas-Aragones, E.; Sánchez-Méndez, J.I.; Sordo-Miralles, G.; Reza, M.M. Satisfaction with and Psychological Impact of Immediate and Deferred Breast Reconstruction. Ann. Oncol. 2008, 19, 1430–1434. [Google Scholar] [CrossRef]
- Lohsiriwat, V.; Peccatori, F.A.; Martella, S.; Azim, H.A.; Sarno, M.A.; Galimberti, V.; De Lorenzi, F.; Intra, M.; Sangalli, C.; Rotmensz, N.; et al. Immediate Breast Reconstruction with Expander in Pregnant Breast Cancer Patients. Breast 2013, 22, 657–660. [Google Scholar] [CrossRef]
- Gropper, A.B.; Calvillo, K.Z.; Dominici, L.; Troyan, S.; Rhei, E.; Economy, K.E.; Tung, N.M.; Schapira, L.; Meisel, J.L.; Partridge, A.H.; et al. Sentinel Lymph Node Biopsy in Pregnant Women with Breast Cancer. Ann. Surg. Oncol. 2014, 21, 2506–2511. [Google Scholar] [CrossRef]
- Masannat, Y.A.; Hanby, A.; Horgan, K.; Hardie, L.J. DNA Damaging Effects of the Dyes Used in Sentinel Node Biopsy: Possible Implications for Clinical Practice. J. Surg. Res. 2009, 154, 234–238. [Google Scholar] [CrossRef]
- Amant, F.; Deckers, S.; Van Calsteren, K.; Loibl, S.; Halaska, M.; Brepoels, L.; Beijnen, J.; Cardoso, F.; Gentilini, O.; Lagae, L.; et al. Breast Cancer in Pregnancy: Recommendations of an International Consensus Meeting. Eur. J. Cancer 2010, 46, 3158–3168. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Koukourakis, M.I.; Azria, D.; Belkacémi, Y. Optimal Timing for Adjuvant Radiation Therapy in Breast Cancer. Crit. Rev. Oncol./Hematol. 2009, 71, 102–116. [Google Scholar] [CrossRef]
- Kal, H.B.; Struikmans, H. Radiotherapy during Pregnancy: Fact and Fiction. Lancet Oncol. 2005, 6, 328–333. [Google Scholar] [CrossRef]
- Valentin, J. (Ed.) Pregnancy and Medical Radiation, 1st ed.; ICRP Publication; Pergamon Press: Oxford, UK, 2000; ISBN 978-0-08-043901-3. [Google Scholar]
- Michalet, M.; Dejean, C.; Schick, U.; Durdux, C.; Fourquet, A.; Kirova, Y. Radiotherapy and Pregnancy. Cancer/Radiothérapie 2022, 26, 417–423. [Google Scholar] [CrossRef]
- Amant, F.; Berveiller, P.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.V.; Lambertini, M.; et al. Gynecologic Cancers in Pregnancy: Guidelines Based on a Third International Consensus Meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef]
- Van Hasselt, J.G.C.; van Calsteren, K.; Heyns, L.; Han, S.; Mhallem Gziri, M.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R.; Amant, F. Optimizing Anticancer Drug Treatment in Pregnant Cancer Patients: Pharmacokinetic Analysis of Gestation-Induced Changes for Doxorubicin, Epirubicin, Docetaxel and Paclitaxel. Ann. Oncol. 2014, 25, 2059–2065. [Google Scholar] [CrossRef]
- Cardonick, E.; Iacobucci, A. Use of Chemotherapy during Human Pregnancy. Lancet Oncol. 2004, 5, 283–291. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Peng, X.; Chen, B.; Pentassuglia, L.; Lim, C.C. Mechanisms of Anthracycline Cardiac Injury: Can We Identify Strategies for Cardioprotection? Prog. Cardiovasc. Dis. 2010, 53, 105–113. [Google Scholar] [CrossRef]
- Zagouri, F.; Sergentanis, T.N.; Chrysikos, D.; Dimitrakakis, C.; Tsigginou, A.; Zografos, C.G.; Dimopoulos, M.-A.; Papadimitriou, C.A. Taxanes for Breast Cancer During Pregnancy: A Systematic Review. Clin. Breast Cancer 2013, 13, 16–23. [Google Scholar] [CrossRef]
- Lambertini, M.; Martel, S.; Campbell, C.; Guillaume, S.; Hilbers, F.S.; Schuehly, U.; Korde, L.; Azim, H.A.; Di Cosimo, S.; Tenglin, R.C.; et al. Pregnancies during and after Trastuzumab and/or Lapatinib in Patients with Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: Analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) Trials: Pregnancies in Women With HER2+ Breast Cancer. Cancer 2019, 125, 307–316. [Google Scholar] [CrossRef]
- Buonomo, B.; Brunello, A.; Noli, S.; Miglietta, L.; Del Mastro, L.; Lambertini, M.; Peccatori, F.A. Tamoxifen Exposure during Pregnancy: A Systematic Review and Three More Cases. Breast Care 2020, 15, 148–156. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Codacci-Pisanelli, G.; Mellgren, G.; Buonomo, B.; Baldassarre, E.; Lien, E.A.; Bifulco, E.; Hustad, S.; Zachariassen, E.; Johansson, H.; et al. First-in-Human Pharmacokinetics of Tamoxifen and Its Metabolites in the Milk of a Lactating Mother: A Case Study. ESMO Open 2020, 5, e000859. [Google Scholar] [CrossRef]
- Pagani, O.; Partridge, A.; Azim, H., Jr.; Peccatori, F.; Ruggeri, M.; Sun, Z. Abstract OT3-02-01: POSITIVE: A Study Evaluating Pregnancy and Disease Outcome and Safety of Interrupting Endocrine Therapy for Young Women with Endocrine-Responsive Breast Cancer Who Desire Pregnancy (IBCSG 48-14/BIG 8-13). Cancer Res. 2017, 77, OT3-02. [Google Scholar] [CrossRef]
- Witzel, I.D.; Müller, V.; Harps, E.; Janicke, F.; deWit, M. Trastuzumab in Pregnancy Associated with Poor Fetal Outcome. Ann. Oncol. 2008, 19, 191–192. [Google Scholar] [CrossRef]
- Zagouri, F.; Sergentanis, T.N.; Chrysikos, D.; Papadimitriou, C.A.; Dimopoulos, M.-A.; Bartsch, R. Trastuzumab Administration during Pregnancy: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2013, 137, 349–357. [Google Scholar] [CrossRef]
- Yildirim, N.; Bahceci, A. Use of Pertuzumab and Trastuzumab during Pregnancy. Anti-Cancer Drugs 2018, 29, 810–813. [Google Scholar] [CrossRef]
- Bayraktar, S.; Batoo, S.; Okuno, S.; Glück, S. Immunotherapy in Breast Cancer. J. Carcinog. 2019, 18, 2. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Menzies, A.M. Immune Checkpoint Inhibitors in Challenging Populations: Immune Therapy in Difficult Populations. Cancer 2017, 123, 1904–1911. [Google Scholar] [CrossRef]
- Hepner, A.; Negrini, D.; Hase, E.A.; Exman, P.; Testa, L.; Trinconi, A.F.; Filassi, J.R.; Francisco, R.P.V.; Zugaib, M.; O’Connor, T.L.; et al. Cancer During Pregnancy: The Oncologist Overview. World J. Oncol. 2019, 10, 28–34. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Migliavacca Zucchetti, B.; Buonomo, B.; Bellettini, G.; Codacci-Pisanelli, G.; Notarangelo, M. Lactation during and after Breast Cancer. In Diseases of the Breast during Pregnancy and Lactation; Alipour, S., Omranipour, R., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1252, pp. 159–163. ISBN 978-3-030-41595-2. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galati, F.; Magri, V.; Arias-Cadena, P.A.; Moffa, G.; Rizzo, V.; Pasculli, M.; Botticelli, A.; Pediconi, F. Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge. Diagnostics 2023, 13, 604. https://doi.org/10.3390/diagnostics13040604
Galati F, Magri V, Arias-Cadena PA, Moffa G, Rizzo V, Pasculli M, Botticelli A, Pediconi F. Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge. Diagnostics. 2023; 13(4):604. https://doi.org/10.3390/diagnostics13040604
Chicago/Turabian StyleGalati, Francesca, Valentina Magri, Paula Andrea Arias-Cadena, Giuliana Moffa, Veronica Rizzo, Marcella Pasculli, Andrea Botticelli, and Federica Pediconi. 2023. "Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge" Diagnostics 13, no. 4: 604. https://doi.org/10.3390/diagnostics13040604
APA StyleGalati, F., Magri, V., Arias-Cadena, P. A., Moffa, G., Rizzo, V., Pasculli, M., Botticelli, A., & Pediconi, F. (2023). Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge. Diagnostics, 13(4), 604. https://doi.org/10.3390/diagnostics13040604





