Performance Evaluation of RapiSure (EDGC) COVID-19 S1 RBD IgG/Neutralizing Ab Test for the Rapid Detection of SARS-CoV-2 Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
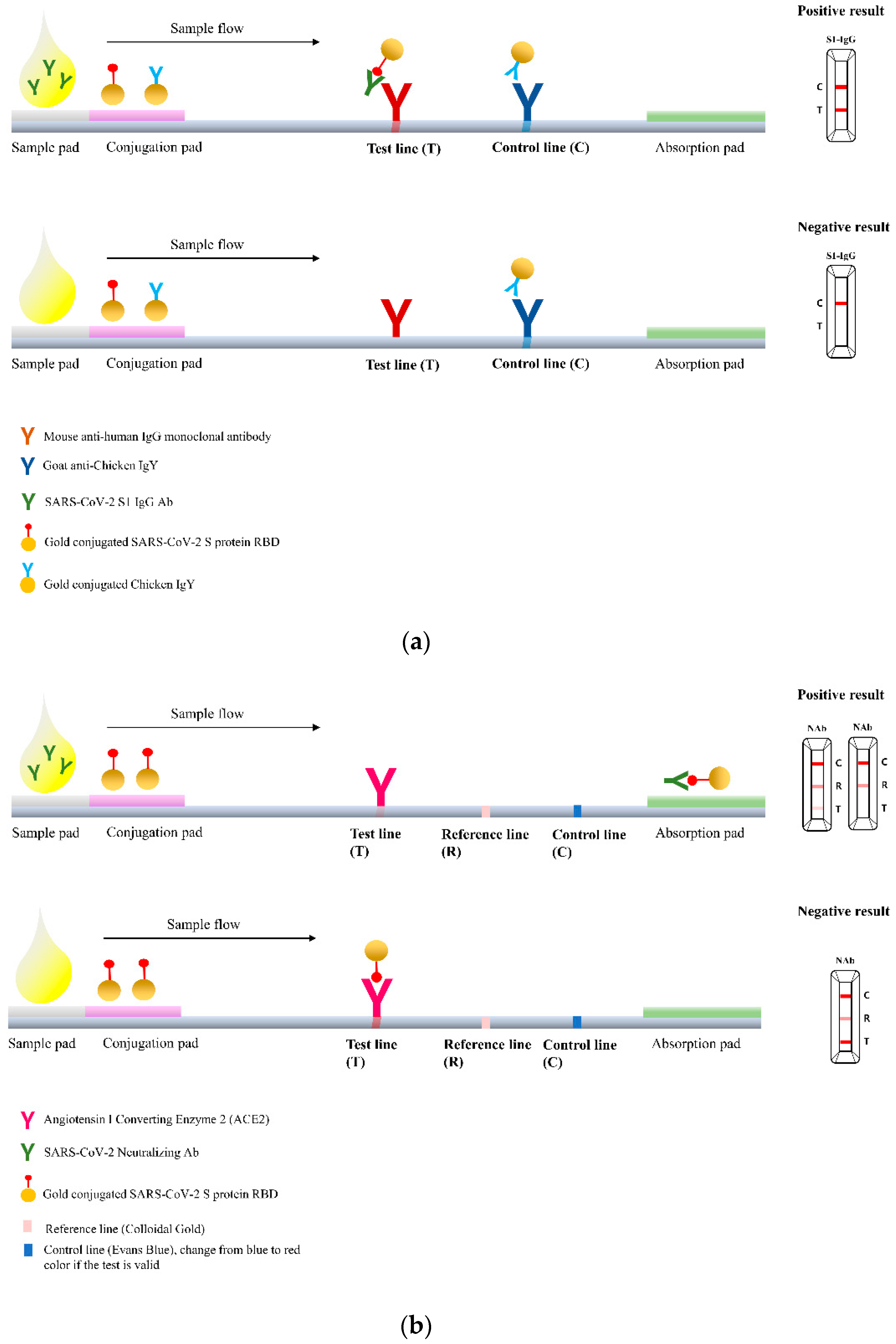
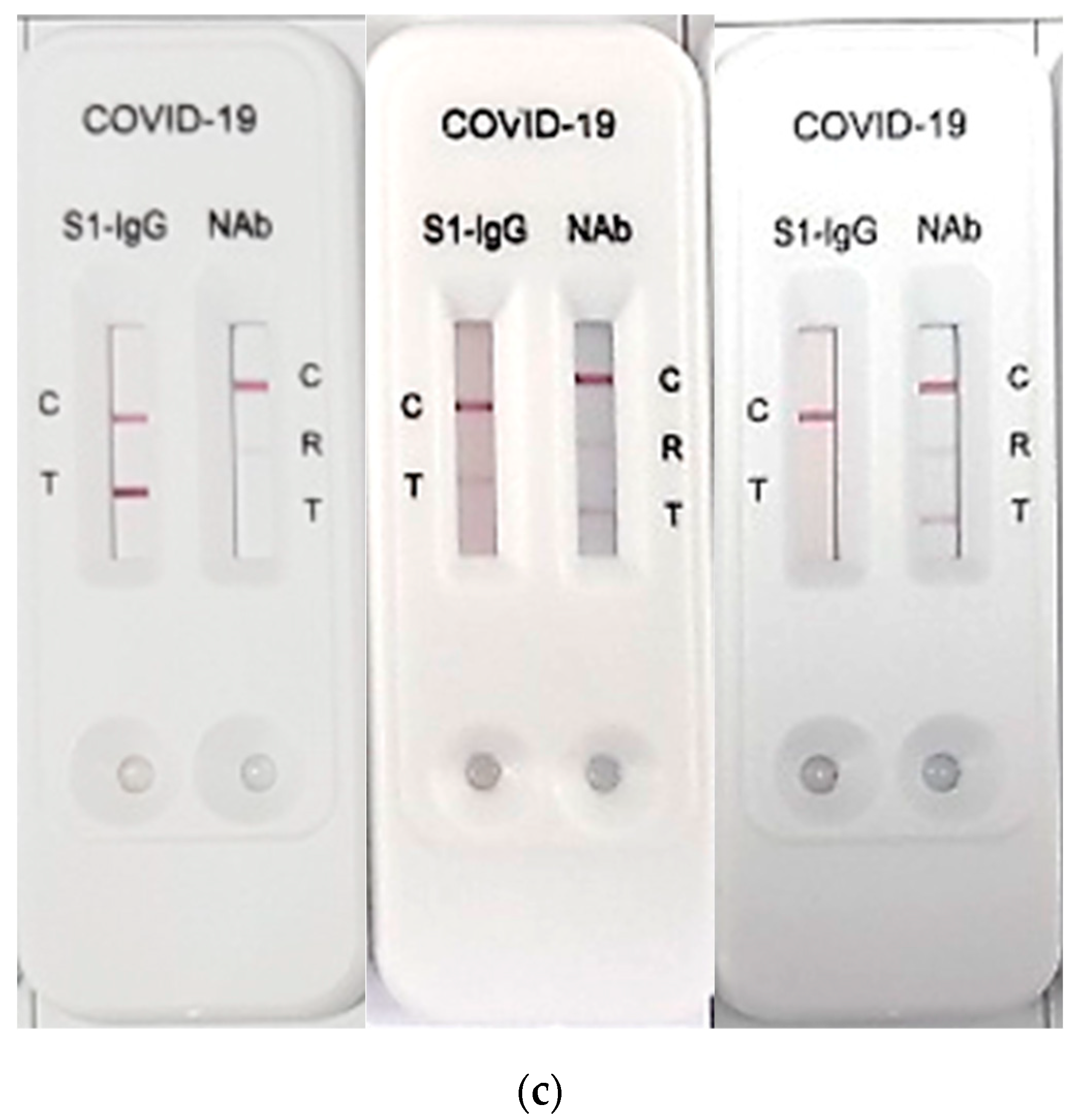
2.2. RapiSure COVID-19 S1 RBD IgG/Neutralizing Ab Test
2.3. Performance Comparison of the RapiSure Test with the PRNT and the STANDARD Q COVID-19 IgM/IgG Plus Test
2.4. Statistical Analysis
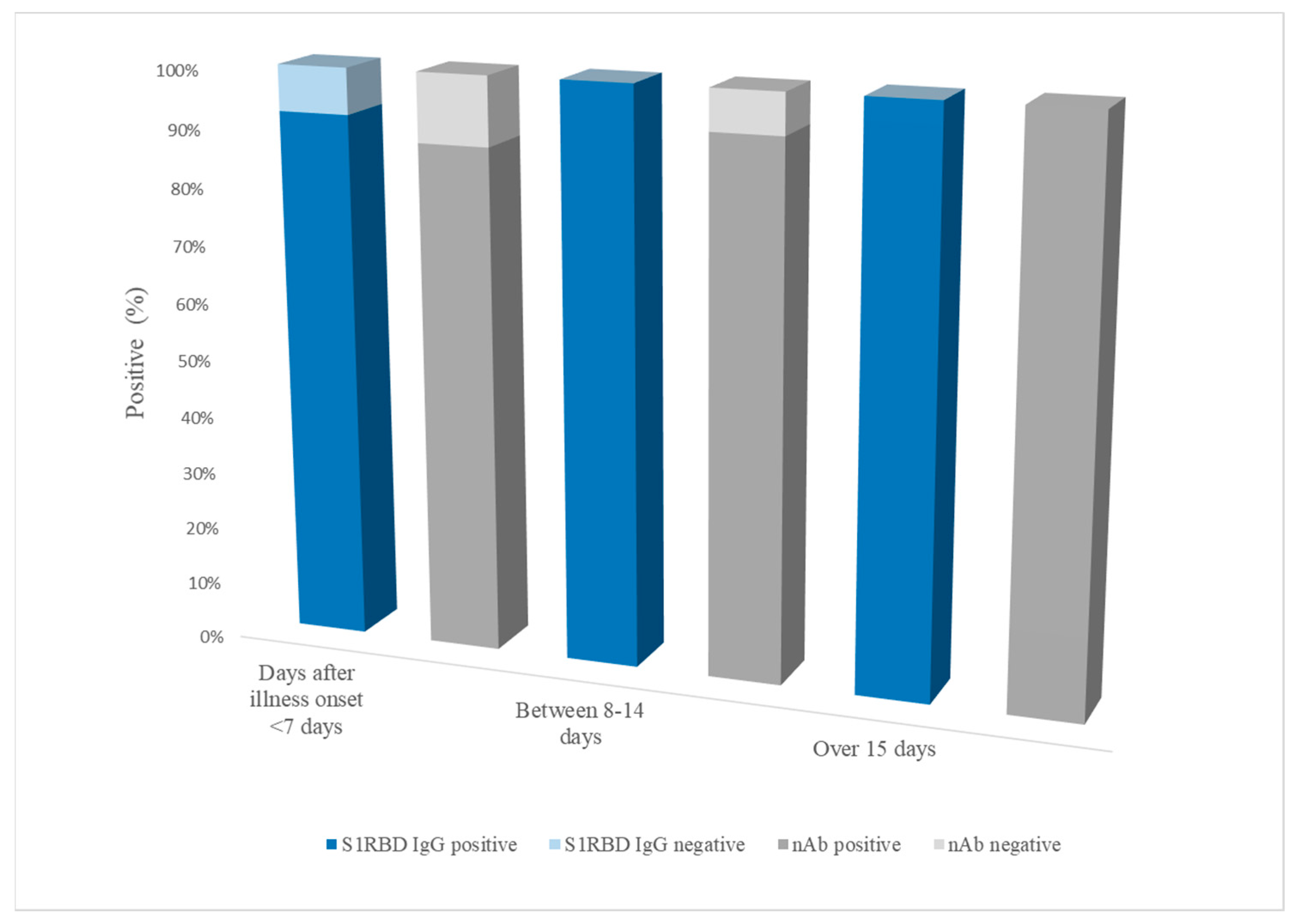
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Statement on the Twelfth Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/news/item/12-07-2022-statement-on-the-twelfth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 29 September 2022).
- Kraay, A.N.; Nelson, K.N.; Zhao, C.Y.; Demory, D.; Weitz, J.S.; Lopman, B.A. Modeling serological testing to inform relaxation of social distancing for COVID-19 control. Nat. Commun. 2021, 12, 7063. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Ferreira, C.E.; Gómez-Dantés, H.; Junqueira Bellei, N.C.; López, E.; Nogales Crespo, K.A.; O’Ryan, M.; Villegas, J. The Role of Serology Testing in the Context of Immunization Policies for COVID-19 in Latin American Countries. Viruses 2021, 13, 2391. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, S.A.; Leung, N.H.; Bull, M.B.; Yan, L.-m.; Li, A.P.; Poon, L.L.; Cowling, B.J. The hurdles from bench to bedside in the realization and implementation of a universal influenza vaccine. Front. Immunol. 2018, 9, 1479. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Crawford, K.H.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020, 58, e02107–e02120. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Prévost, J.; Gasser, R.; Beaudoin-Bussières, G.; Richard, J.; Duerr, R.; Laumaea, A.; Anand, S.P.; Goyette, G.; Benlarbi, M.; Ding, S. Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Rep. Med. 2020, 1, 100126. [Google Scholar] [CrossRef]
- Perera, R.A.; Mok, C.K.; Tsang, O.T.; Lv, H.; Ko, R.L.; Wu, N.C.; Yuan, M.; Leung, W.S.; Chan, J.M.; Chik, T.S. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 2020, 25, 2000421. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xia, H.; Zou, J.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Cutler, M.; Cooper, D.; Muik, A. BNT162b2-elicited neutralization of B. 1.617 and other SARS-CoV-2 variants. Nature 2021, 596, 273–275. [Google Scholar] [CrossRef]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P.; Girardin, R.C.; Rathod, B. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.-A. Humoral responses and serological assays in SARS-CoV-2 infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.J.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Guidelines for COVID-19 Antibody Testing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html (accessed on 6 February 2023).
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Bae, J.-Y.; Bae, S.; Hwang, S.; Kwon, K.T.; Chang, H.-H.; Lee, W.K.; Cui, C.; Lee, G.E.; Kim, S.-W. Neutralizing antibody responses to SARS-CoV-2 in Korean patients who have recovered from COVID-19. Yonsei Med. J. 2021, 62, 584. [Google Scholar] [CrossRef]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Lin, Y.-C.; Abe, K.T.; Mubareka, S.; Shigayeva, A.; Zhong, Z.; Girardin, R.C.; DuPuis, A. Evaluating humoral immunity against SARS-CoV-2: Validation of a plaque-reduction neutralization test and a multilaboratory comparison of conventional and surrogate neutralization assays. Microbiol. Spectr. 2021, 9, e00821–e00886. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research Chapman and Hall, 1st ed.; Chapman and Hall/CRC: London, UK; New York, NY, USA, 1991. [Google Scholar]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E. SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef]
- Kubina, R.; Dziedzic, A. Molecular and serological tests for COVID-19. A comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics 2020, 10, 434. [Google Scholar] [CrossRef]
- Makoah, N.A.; Tipih, T.; Litabe, M.M.; Brink, M.; Sempa, J.B.; Goedhals, D.; Burt, F.J. A systematic review and meta-analysis of the sensitivity of antibody tests for the laboratory confirmation of COVID-19. Future Virol. 2022, 17, 119–139. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Xue, J.-H.; Wang, Y.-J.; Li, W.; Li, Q.-L.; Xu, Q.-Y.; Niu, J.-J.; Liu, L.-L. Anti-Receptor-Binding Domain Immunoglobulin G Antibody as a Predictor of Seropositivity for Anti-SARS-CoV-2 Neutralizing AntibodyAnti-RBD IgG predicts neutralizing Ab. Arch. Pathol. Lab. Med. 2022, 146, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.; Pozzetto, B.; Trabaud, M.-A.; Escuret, V.; Rabilloud, M.; Langlois-Jacques, C.; Paul, A.; Guibert, N.; D’Aubarède-Frieh, C.; Massardier-Pilonchery, A. Evaluation of high-throughput SARS-CoV-2 serological assays in a longitudinal cohort of patients with mild COVID-19: Clinical sensitivity, specificity, and association with virus neutralization test. Clin. Chem. 2021, 67, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, A.; Pieri, M.; Sarubbi, S.; Pelagalli, M.; Calugi, G.; Tomassetti, F.; Bernardini, S.; Nuccetelli, M. Evaluation of serological anti-SARS-CoV-2 chemiluminescent immunoassays correlated to live virus neutralization test, for the detection of anti-RBD antibodies as a relevant alternative in COVID-19 large-scale neutralizing activity monitoring. Clin. Immunol. 2022, 234, 108918. [Google Scholar] [CrossRef]
- Khateeb, J.; Li, Y.; Zhang, H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care 2021, 25, 244. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.; Malik, A.A.; De la Cruz, E.; Jorge, A. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 28, 481–485. [Google Scholar] [CrossRef]
- Takheaw, N.; Liwsrisakun, C.; Chaiwong, W.; Laopajon, W.; Pata, S.; Inchai, J.; Duangjit, P.; Pothirat, C.; Bumroongkit, C.; Deesomchok, A. Correlation Analysis of Anti-SARS-CoV-2 RBD IgG and Neutralizing Antibody against SARS-CoV-2 Omicron Variants after Vaccination. Diagnostics 2022, 12, 1315. [Google Scholar] [CrossRef]
- Hachmann, N.; Miller, J.; Collier, A.-r.; Ventura, J.; Yu, J.; Rowe, M.; Bondzie, E.; Powers, O.; Surve, N.; Hall, K. Neutralization Escape by the SARS-CoV-2 Omicron Variants BA. 2.12. 1, BA. 4, and BA. 5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Rössler, A.; Knabl, L.; Raschbichler, L.-M.; Peer, E.; von Laer, D.; Borena, W.; Kimpel, J. Reduced sensitivity of antibody tests after omicron infection. Lancet Microbe 2022, 4, e10–e11. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Kawasuji, H.; Morinaga, Y.; Tani, H.; Kimura, M.; Yamada, H.; Yoshida, Y.; Takegoshi, Y.; Kaneda, M.; Murai, Y.; Kimoto, K. Delayed neutralizing antibody response in the acute phase correlates with severe progression of COVID-19. Sci. Rep. 2021, 11, 16535. [Google Scholar] [CrossRef]
- Fang, F.C.; Naccache, S.N.; Greninger, A.L. The laboratory diagnosis of coronavirus disease 2019—Frequently asked questions. Clin. Infect. Dis. 2020, 71, 2996–3001. [Google Scholar] [CrossRef]
- Cheng, M.P.; Yansouni, C.P.; Basta, N.E.; Desjardins, M.; Kanjilal, S.; Paquette, K.; Caya, C.; Semret, M.; Quach, C.; Libman, M. Serodiagnostics for Severe Acute Respiratory Syndrome–Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 173, 450–460. [Google Scholar] [CrossRef]
- Kim, D.; Ali, S.T.; Kim, S.; Jo, J.; Lim, J.-S.; Lee, S.; Ryu, S. Estimation of serial interval and reproduction number to quantify the transmissibility of SARS-CoV-2 omicron variant in South Korea. Viruses 2022, 14, 533. [Google Scholar] [CrossRef]
- Shim, H.W.; Shin, J.h.; Shin, S.C.; Lee, H.J.; So, K.S.; Lee, S.Y.; Jun, J.W.; Seo, J.K.; Lee, H.S.; Lee, S.Y. Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test. Diagnostics 2022, 12, 1924. [Google Scholar] [CrossRef]
- WHO. Final WHO SARS-CoV-2 Serology Test Kit Evaluation Results. Available online: https://www.who.int/publications/m/item/final-who-sars-cov-2-serology-test-kit-evaluation-results (accessed on 6 February 2023).
| RT-PCR a | Sensitivity (95% CI b) | Specificity (95% CI) | PRNT c50 and PRNT90 | Sensitivity (95% CI) | Specificity (95% CI) | Percent Agreement (95% CI) | Cohen’s Kappa (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||||||
| RapiSure | Positive | 76 | 4 | 97.4% (91.1–99.3%) | 96.7% (91.9–98.7%) | 71 | 0 | 93.4% (85.5–97.2%) | 100% (97.0–100%) | 97.5% (94.3–99.2%) | 0.95 (0.90–0.99) |
| Negative | 2 | 118 | 5 | 124 | |||||||
| STANDARD Q IgG | Sensitivity (95% CI) | Specificity (95% CI) | Percent Agreement a (95% CI) | Cohen’s Kappa (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Overall | |||||
| RapiSure S1 RBD IgG | Positive | 66 | 14 | 95.7% (88.0–98.5%) | 89.3% (82.9–93.5%) | 91.5% (86.8–94.6%) | 0.82 (0.73–0.90) | ||
| Negative | 3 | 117 | |||||||
| RT-PCR | Positive | 66 | 12 | 95.7% (87.8–99.1%) | 90.8% (84.5–95.2%) | 92.5% (87.9–95.7%) | 0.84 (0.76–0.92) | ||
| Negative | 3 | 119 | |||||||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dilution Factor | S1-IgG | nAb | S1-IgG | nAb | S1-IgG | nAb | S1-IgG | nAb | S1-IgG | nAb |
| 1:2 | + | + | + | + | + | + | + | + | + | + |
| 1:4 | + | + | + | + | + | + | + | + | + | + |
| 1:8 | + | + | + | + | + | + | + | + | + | + |
| 1:16 | + | + | + | + | + | + | + | + | + | + |
| 1:32 | + | + | + | + | + | + | + | + | + | − |
| 1:64 | + | − | + | + | + | + | + | + | − | − |
| 1:128 | − | N/T a | + | − | + | + | + | + | N/T | N/T |
| 1:256 | − | N/T | + | − | − | − | − | − | N/T | N/T |
| Categorization | Titer | Numbers of Positive/Total Samples | RapiSure nAb Results | Sensitivity (95% CI) | p-Value a | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| PRNT50 | Low titer | 1:20, 1:40, 1:80 | 13/76 | 10 | 3 | 76.9% (49.7–91.8) | 0.0090 |
| High titer | >1:80 | 63/76 | 61 | 2 | 96.8% (89.1–99.1) | ||
| PRNT90 | Low titer | 1:10, 1:20, 1:40 | 10/76 | 7 | 3 | 70.0% (39.7–89.2) | 0.0014 |
| High titer | >1:40 | 66/76 | 64 | 2 | 97.0% (89.3–99.2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.N.; Yoon, J.; Jang, W.S.; Lim, C.S. Performance Evaluation of RapiSure (EDGC) COVID-19 S1 RBD IgG/Neutralizing Ab Test for the Rapid Detection of SARS-CoV-2 Antibodies. Diagnostics 2023, 13, 643. https://doi.org/10.3390/diagnostics13040643
Kim HN, Yoon J, Jang WS, Lim CS. Performance Evaluation of RapiSure (EDGC) COVID-19 S1 RBD IgG/Neutralizing Ab Test for the Rapid Detection of SARS-CoV-2 Antibodies. Diagnostics. 2023; 13(4):643. https://doi.org/10.3390/diagnostics13040643
Chicago/Turabian StyleKim, Ha Nui, Jung Yoon, Woong Sik Jang, and Chae Seung Lim. 2023. "Performance Evaluation of RapiSure (EDGC) COVID-19 S1 RBD IgG/Neutralizing Ab Test for the Rapid Detection of SARS-CoV-2 Antibodies" Diagnostics 13, no. 4: 643. https://doi.org/10.3390/diagnostics13040643
APA StyleKim, H. N., Yoon, J., Jang, W. S., & Lim, C. S. (2023). Performance Evaluation of RapiSure (EDGC) COVID-19 S1 RBD IgG/Neutralizing Ab Test for the Rapid Detection of SARS-CoV-2 Antibodies. Diagnostics, 13(4), 643. https://doi.org/10.3390/diagnostics13040643






