Association between Urinary Creatinine Excretion and Hypothyroidism in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
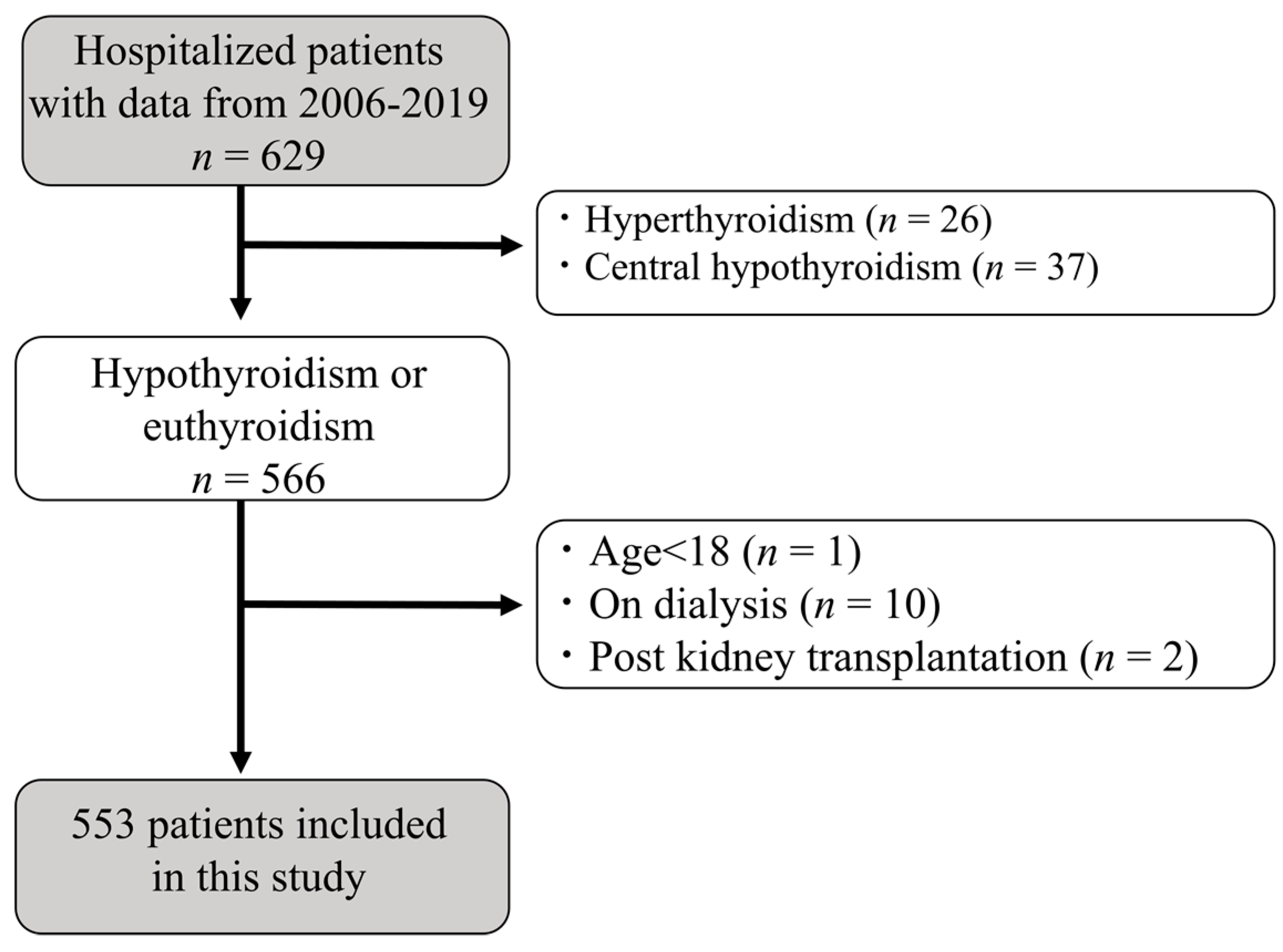
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analysis
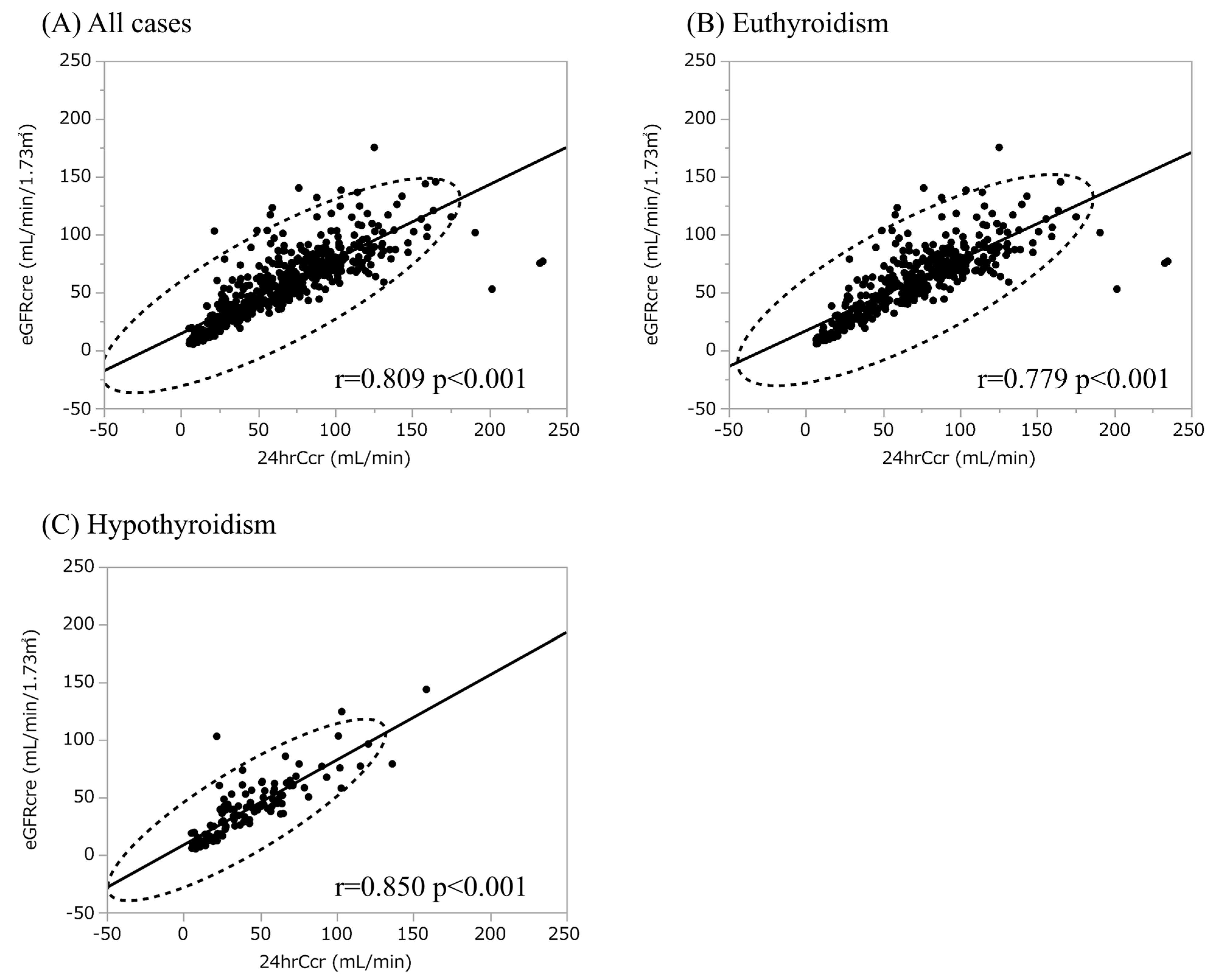
3. Results
3.1. Study Population and Clinical Characteristics
3.2. Hypothyroidism Is Not an Independent Explanatory Variable for Urinary CER
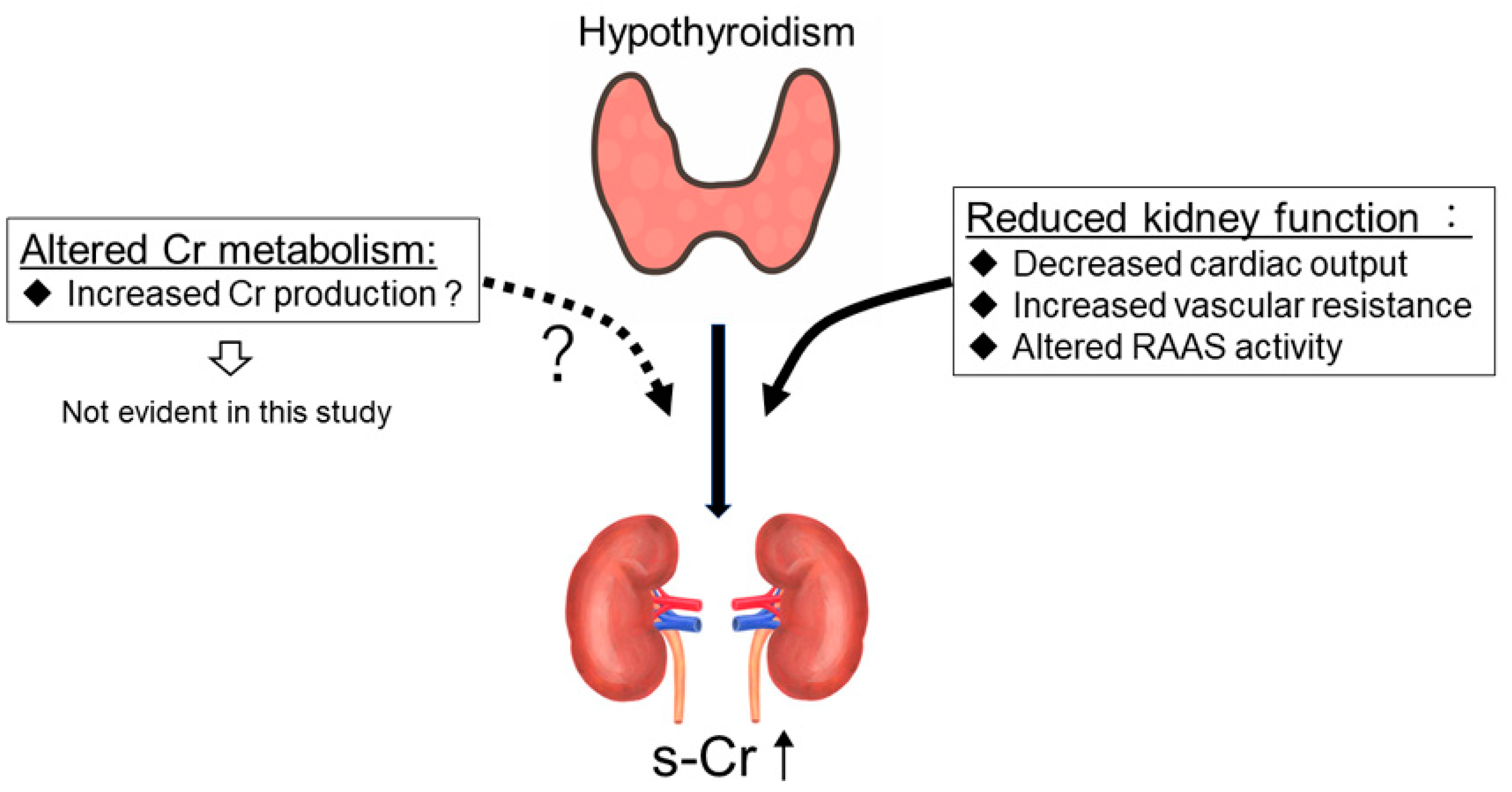
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- den Hollander, J.G.; Wulkan, R.W.; Mantel, M.J.; Berghout, A. Correlation between severity of thyroid dysfunction and renal function. Clin. Endocrinol. 2005, 62, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Berwaerts, J.; Marescau, B.; Abs, R.; Neels, H.; Mahler, C.; De Deyn, P.P. Serum creatine, creatinine, and other guanidino compounds in patients with thyroid dysfunction. Metabolism 1997, 46, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, M.; Cerantonio, A.; Pastore, I.; Liguori, R.; Greco, M.; Foti, D.; Gulletta, E.; Brunetti, A.; Fuiano, G. The correct renal function evaluation in patients with thyroid dysfunction. J. Endocrinol. Investig. 2016, 39, 495–507. [Google Scholar] [CrossRef]
- Hammond, H.K.; White, F.C.; Buxton, I.L.; Saltzstein, P.; Brunton, L.L.; Longhurst, J.C. Increased myocardial beta-receptors and adrenergic responses in hyperthyroid pigs. Am. J. Physiol. 1987, 252 (Pt 2), H283–H290. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.D.; Crawford, F.A.; Kato, S.; Spinale, F.G. The novel effects of 3,5,3’-triiodo-L-thyronine on myocyte contractile function and beta-adrenergic responsiveness in dilated cardiomyopathy. J. Thorac. Cardiovasc. Surg. 1994, 108, 672–679. [Google Scholar] [CrossRef]
- Asmah, B.J.; Wan Nazaimoon, W.M.; Norazmi, K.; Tan, T.T.; Khalid, B.A. Plasma renin and aldosterone in thyroid diseases. Horm. Metab. Res. 1997, 29, 580–583. [Google Scholar] [CrossRef]
- Sindoni, A.; Rodolico, C.; Pappalardo, M.A.; Portaro, S.; Benvenga, S. Hypothyroid myopathy: A peculiar clinical presentation of thyroid failure. Review of the literature. Rev. Endocr. Metab. Disord. 2016, 17, 499–519. [Google Scholar] [CrossRef]
- Montenegro, J.; Gonzalez, O.; Saracho, R.; Aguirre, R.; Gonzalez, O.; Martinez, I. Changes in renal function in primary hypothyroidism. Am. J. Kidney Dis. 1996, 27, 195–198. [Google Scholar] [CrossRef]
- Wilkins, L.; Fleischmann, W. Effects of Thyroid on Creatine Metabolism with a Discussion of the Mechanism of Storage and Excretion of Creatine Bodies. J. Clin. Investig. 1946, 25, 360–377. [Google Scholar] [CrossRef]
- Nørrelund, H.; Hove, K.Y.; Brems-Dalgaard, E.; Jurik, A.G.; Nielsen, L.P.; Nielsen, S.; Jørgensen, J.O.; Weeke, J.; Møller, N. Muscle mass and function in thyrotoxic patients before and during medical treatment. Clin. Endocrinol. 1999, 51, 693–699. [Google Scholar] [CrossRef]
- Klein, I.; Mantell, P.; Parker, M.; Levey, G.S. Resolution of abnormal muscle enzyme studies in hypothyroidism. Am. J. Med. Sci. 1980, 279, 159–162. [Google Scholar] [CrossRef]
- Ix, J.H.; de Boer, I.H.; Wassel, C.L.; Criqui, M.H.; Shlipak, M.G.; Whooley, M.A. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 2010, 121, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Jayagopal, V.; Keevil, B.G.; Atkin, S.L.; Jennings, P.E.; Kilpatrick, E.S. Paradoxical changes in cystatin C and serum creatinine in patients with hypo- and hyperthyroidism. Clin. Chem. 2003, 49, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Ghirlanda-Keller, C.; Zwimpfer, C.; Zoidis, E. Triiodothyronine stimulates cystatin C production in bone cells. Biochem. Biophys. Res. Commun. 2012, 419, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Xie, J.; Fan, H.; Sun, X.; Shi, B. Association Between Serum Cystatin C and Thyroid Diseases: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 766516. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Foti, D.P.; Aversa, A.; Fuiano, G.; Brunetti, A.; Simeoni, M. Cystatin C, a Controversial Biomarker in Hypothyroid Patients under Levothyroxine Therapy: THYRenal, a Pilot Cohort Observational Study. J. Clin. Med. 2020, 9, 2958. [Google Scholar] [CrossRef]
- Deng, X.; Tang, C.; Wu, J.; Han, R.; Fang, F. Changes of nutritional status and the variations of serum indicators of patients with chronic kidney disease accompanied by hypothyroidism taking thyroid hormone replacement therapy as the therapeutic models. Saudi J. Biol. Sci. 2019, 26, 2091–2095. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Tao, X.J.; Li, Q.; Li, F.F.; Lee, K.O.; Li, D.M.; Ma, J.H. Relationship between Thyroid Function and Kidney Function in Patients with Type 2 Diabetes. Int. J. Endocrinol. 2018, 2018, 1871530. [Google Scholar] [CrossRef]
- Patil, V.P.; Shilpasree, A.S.; Patil, V.S.; Pravinchandra, K.R.; Ingleshwar, D.G.; Vani, A.C. Evaluation of renal function in subclinical hypothyroidism. J. Lab. Physicians 2018, 10, 50–55. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Kasuga, M.; Araki, E.; Oka, Y.; Hanafusa, T.; Ito, H.; Tominaga, M.; Oikawa, S.; Noda, M.; Kawamura, T.; et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J. Diabetes Investig. 2012, 3, 39–40. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology, C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: Accuracy and use for population estimates. Am. J. Kidney Dis. 2010, 56, 32–38. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Nitta, K.; Yamagata, K.; Iseki, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Hirakata, H.; Watanabe, T.; et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin. Exp. Nephrol. 2007, 11, 41–50. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312–313. [Google Scholar]
- Pan, B.; Du, X.; Zhang, H.; Hua, X.; Wan, X.; Cao, C. Relationships of Chronic Kidney Disease and Thyroid Dysfunction in Non-Dialysis Patients: A Pilot Study. Kidney Blood Press Res. 2019, 44, 170–178. [Google Scholar] [CrossRef]
- Lo, J.C.; Chertow, G.M.; Go, A.S.; Hsu, C.Y. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005, 67, 1047–1052. [Google Scholar] [CrossRef]
- Carrero, J.J.; Johansen, K.L.; Lindholm, B.; Stenvinkel, P.; Cuppari, L.; Avesani, C.M. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016, 90, 53–66. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, E.I.; Kaptein, E.M.; Nicoloff, J.T.; Massry, S.G. Thyroid function in patients with nephrotic syndrome and normal renal function. Am. J. Nephrol. 1982, 2, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A. Creatine and Creatinine; Longmans, Green and Company Limited: London, UK, 1928; Volume 24. [Google Scholar]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R. Creatinine excretion in renal failure. Proc. Soc. Exp. Biol. Med. 1954, 85, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Diago, C.A.A.; Senaris, J.A.A. Should we pay more attention to low creatinine levels? Endocrinol. Diabetes Nutr. (Engl. Ed.) 2020, 67, 486–492. [Google Scholar] [PubMed]
- Burger, M. Die Bedeutung des Kreatininkoefizienten fur die quantitative bewertund der muskulatur als korpergewichtskomponente. Z. Ges. Exp. Med. 1919, 9, 361–399. [Google Scholar]
- Rule, A.D.; Bailey, K.R.; Schwartz, G.L.; Khosla, S.; Lieske, J.C.; Melton, L.J., 3rd. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009, 75, 1071–1078. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; McManus, C.; Smith, J.; Stevens, V.; Nixon, D.W. Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. Am. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef]
- Baghi, M.A.; Sirajudeen, J.; Naushad, V.A.; Alarbi, K.S.; Benshaban, N. Severe hypothyroidism-induced rhabdomyolysis: A case report. Clin. Case Rep. 2021, 9, e05107. [Google Scholar] [CrossRef]
- Tynkevich, E.; Flamant, M.; Haymann, J.P.; Metzger, M.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Houillier, P.; Froissart, M.; Stengel, B.; et al. Decrease in urinary creatinine excretion in early stage chronic kidney disease. PLoS ONE 2014, 9, e111949. [Google Scholar] [CrossRef]
- Wilson, F.P.; Xie, D.; Anderson, A.H.; Leonard, M.B.; Reese, P.P.; Delafontaine, P.; Horwitz, E.; Kallem, R.; Navaneethan, S.; Ojo, A.; et al. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2014, 9, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, M.J.; Lee, H.S.; Oh, H.J.; Ko, K.I.; Kim, C.H.; Doh, F.M.; Koo, H.M.; Kim, H.R.; Han, J.H.; et al. Thyroid hormone replacement therapy attenuates the decline of renal function in chronic kidney disease patients with subclinical hypothyroidism. Thyroid 2013, 23, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Mario, F.D.; Pofi, R.; Gigante, A.; Rivoli, L.; Rosato, E.; Isidori, A.M.; Cianci, R.; Barbano, B. Hypothyroidism and Nephrotic Syndrome: Why, When and How to Treat. Curr. Vasc. Pharmacol. 2017, 15, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Soveri, I.; Berg, U.B.; Bjork, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Back, S.E.; Group, S.G.R. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]

| Clinical Parameters | All Participants (n = 553) | Low CER (n = 184) | Middle CER (n = 184) | High CER (n = 185) | p for Trend |
|---|---|---|---|---|---|
| Sex (Male), n (%) | 285 (52) | 61 (33) | 83 (45) | 141 (76) | <0.001 ** |
| Age (yr) | 60 ± 15 | 65 ± 15 | 61 ± 14 | 55 ± 14 | <0.001 ** |
| BMI (kg/m2) | 24.3 ± 4.8 | 22.3 ± 3.9 | 24.6 ± 4.3 | 26.1 ± 5.4 | <0.001 ** |
| TSH (µU/mL) | 4.88 ± 15.97 | 5.54 ± 13.99 | 4.29 ± 11.33 | 4.81 ± 21.03 | 0.004 ** |
| FT4 (ng/dL) | 1.19 ± 0.21 | 1.17 ± 0.22 | 1.17 ± 0.19 | 1.22 ± 0.20 | 0.052 |
| FT3 (pg/mL) | 2.49 ± 0.60 | 2.27 ± 0.67 | 2.50 ± 0.49 | 2.72 ± 0.53 | <0.001 ** |
| Hypothyroidism n (%) | 121 (22) | 48 (26) | 44 (24) | 29 (16) | 0.016 * |
| s-Cr (mg/dL) | 1.44 ± 1.27 | 1.62 ± 1.41 | 1.44 ± 1.37 | 1.26 ± 0.97 | 0.826 |
| eGFRcre (mL/min/1.73 m2) | 55.7 ± 30.5 | 51.5 ± 35.8 | 55.2 ± 27.7 | 60.4 ± 26.9 | <0.001 ** |
| 24hrCcr (mL/min) | 64.6 ± 38.4 | 45.3 ± 31.1 | 64.9 ± 32.8 | 83.7 ± 40.7 | <0.001 ** |
| Urinary output (mL/day) | 1665 ± 710 | 1447 ± 617 | 1674 ± 674 | 1873 ± 769 | <0.001 ** |
| Urinary CER (g/day) | 1.01 ± 0.38 | 0.64 ± 0.14 | 0.97 ± 0.07 | 1.42 ± 0.31 | <0.001 ** |
| Urinary protein (g/day) | 2.1 ± 6.7 | 1.7 ± 3.9 | 2.5 ± 9.0 | 2.3 ± 6.3 | 0.562 |
| Albumin (g/dL) | 3.6 ± 0.8 | 3.4 ± 0.8 | 3.6 ± 0.8 | 3.8 ± 0.8 | <0.001 ** |
| Hemoglobin (g/dL) | 12.3 ± 2.3 | 11.6 ± 2.3 | 12.2 ± 2.1 | 13.2 ± 2.4 | <0.001 ** |
| Total cholesterol (mg/dL) | 196 ± 62 | 192 ± 63 | 196 ± 62 | 200 ± 60 | 0.134 |
| HbA1c (%) | 7.1 ± 2.0 | 6.9 ± 2.0 | 7.2 ± 2.1 | 7.1 ± 2.1 | 0.370 |
| Nephrotic syndrome n (%) | 52 (9) | 19 (10) | 20 (11) | 13 (7) | 0.277 |
| ACE-i/ARB intake n (%) | 128 (23) | 40 (22) | 42 (23) | 46 (25) | 0.477 |
| Levothyroxine intake n (%) | 55 (10) | 23 (13) | 18 (10) | 14 (8) | 0.114 |
| Glucocorticoid intake n (%) | 26 (5) | 13 (7) | 5 (3) | 8 (4) | 0.215 |
| Clinical Parameters | All Participants (n = 553) | Hypothyroidism (n = 121) | Euthyroidism (n = 432) | p-Value |
|---|---|---|---|---|
| Sex (Male), n (%) | 285 (52) | 66 (55) | 219 (51) | 0.454 |
| Age (yr) | 60 ± 15 | 63 ± 16 | 59 ± 15 | 0.224 |
| BMI (kg/m2) | 24.3 ± 4.8 | 24.8 ± 6.0 | 24.2 ± 4.8 | 0.208 |
| TSH (µIU/mL) | 4.88 ± 15.97 | 15.74 ± 31.91 | 1.84 ± 15.97 | <0.001 ** |
| FT4 (ng/dL) | 1.19 ± 0.21 | 1.03 ± 0.26 | 1.23 ± 0.16 | <0.001 ** |
| FT3 (pg/mL) | 2.49 ± 0.60 | 2.25 ± 0.73 | 2.56 ± 0.53 | <0.001 ** |
| s-Cr (mg/dL) | 1.44 ± 1.27 | 2.14 ± 1.67 | 1.24 ± 1.06 | <0.001 ** |
| Cystatin C (mg/L) | 1.66 ± 1.06 | 2.21 ± 1.17 | 1.51 ± 0.98 | <0.001 ** |
| eGFRcre (mL/min/1.73 m2) | 55.7 ± 30.5 | 38.9 ± 26.0 | 60.5 ± 30.1 | <0.001 ** |
| 24hrCcr (mL/min) | 64.6 ± 38.4 | 41.4 ± 29.9 | 71.2 ± 38.0 | <0.001 ** |
| Urinary output (mL/day) | 1665 ± 710 | 1579 ± 745 | 1689 ± 699 | 0.134 |
| Urinary CER (g/day) | 1.01 ± 0.38 | 0.91 ± 0.35 | 1.04 ± 0.38 | 0.001 ** |
| Urinary protein (g/day) | 2.1 ± 6.7 | 3.5 ± 5.7 | 1.8 ± 7.0 | <0.001 ** |
| Albumin (g/dL) | 3.6 ± 0.8 | 3.2 ± 1.0 | 3.7 ± 0.7 | <0.001 ** |
| Hemoglobin (g/dL) | 12.3 ± 2.3 | 12.0 ± 2.4 | 12.5 ± 2.3 | 0.042 * |
| Total cholesterol (mg/dL) | 196 ± 62 | 204 ± 72 | 194 ± 59 | 0.110 |
| HbA1c (%) | 7.1 ± 2.0 | 6.5 ± 1.6 | 7.2 ± 2.1 | <0.001 ** |
| Nephrotic syndrome n (%) | 52 (9) | 25 (21) | 27 (6) | <0.001 ** |
| ACE-i/ARB intake n (%) | 128 (23) | 40 (33) | 88 (20) | 0.003 ** |
| Levothyroxine intake n (%) | 55 (10) | 34 (28) | 21 (5) | <0.001 ** |
| Glucocorticoid intake n (%) | 26 (5) | 7 (6) | 19 (4) | 0.524 |
| Variable | B | 95% CI | β | t | p-Value |
|---|---|---|---|---|---|
| Sex (Male) | 0.138 | 0.108 to 0.167 | 0.366 | 9.22 | <0.001 ** |
| Age | −0.007 | −0.009 to −0.005 | −0.267 | −6.52 | <0.001 ** |
| BMI | 0.028 | 0.022 to 0.034 | 0.362 | 9.12 | <0.001 ** |
| TSH | −0.001 | −0.003 to 0.001 | −0.041 | −0.97 | 0.333 |
| FT4 | 0.217 | 0.064 to 0.369 | 0.118 | 2.79 | 0.006 ** |
| FT3 | 0.192 | 0.142 to 0.243 | 0.304 | 7.48 | <0.001 ** |
| Hypothyroidism | 0.064 | 0.027 to 0.103 | 0.143 | 3.39 | <0.001 ** |
| s-Cr | −0.034 | −0.058 to −0.009 | −0.113 | −2.67 | 0.008 ** |
| eGFRcre | 0.002 | 0.001 to 0.003 | 0.135 | 3.21 | 0.001 ** |
| 24hrCcr | 0.005 | 0.004 to 0.006 | 0.503 | 13.65 | <0.001 ** |
| Urinary output | 0.000 | 0.000 to 0.000 | 0.271 | 6.60 | <0.001 ** |
| Urinary protein | 0.001 | −0.003 to 0.006 | 0.026 | 0.62 | 0.536 |
| Albumin | 0.099 | 0.061 to 0.137 | 0.212 | 5.09 | <0.001 ** |
| Hemoglobin | 0.045 | 0.032 to 0.058 | 0.277 | 6.76 | <0.001 ** |
| Total cholesterol | 0.000 | −0.000 to 0.001 | 0.015 | 0.36 | 0.719 |
| HbA1c | 0.018 | 0.002 to 0.033 | 0.096 | 2.27 | 0.024 * |
| Nephrotic syndrome | −0.031 | −0.085 to 0.023 | −0.048 | −1.14 | 0.255 |
| ACE-i/ARB intake | 0.005 | 0.032 to 0.043 | 0.012 | 0.28 | 0.779 |
| Levothyroxine intake | −0.052 | −0.105 to −0.000 | −0.083 | −1.97 | 0.050 * |
| Glucocorticoid intake | 0.064 | −0.010 to 0.138 | 0.072 | 1.69 | 0.091 |
| Variable | B | 95% CI | β | t | p-Value |
|---|---|---|---|---|---|
| (A) | |||||
| Hypothyroidism | 0.025 | −0.035 to 0.085 | 0.031 | 0.83 | 0.407 |
| Sex (male) | 0.173 | 0.152 to 0.194 | 0.459 | 16.23 | <0.001 ** |
| Age | −0.003 | −0.004 to −0.001 | −0.107 | −3.60 | <0.001 ** |
| BMI | 0.027 | 0.023 to 0.031 | 0.344 | 12.49 | <0.001 ** |
| 24hrCcr | 0.005 | 0.005 to 0.006 | 0.526 | 15.95 | <0.001 ** |
| Albumin | 0.050 | 0.023 to 0.078 | 0.108 | 3.61 | <0.001 ** |
| Hemoglobin | −0.004 | −0.014 to 0.005 | −0.027 | −0.89 | 0.372 |
| Constant | 0.067 | <0.001 ** | |||
| (B) | |||||
| FT3 | 0.017 | −0.024 to 0.058 | 0.027 | 0.82 | 0.410 |
| Sex (male) | 0.172 | 0.151 to 0.192 | 0.455 | 16.05 | <0.001 ** |
| Age | −0.003 | −0.004 to −0.001 | −0.105 | −3.54 | <0.001 ** |
| BMI | 0.027 | 0.022 to 0.031 | 0.342 | 12.36 | <0.001 ** |
| 24hrCcr | 0.005 | 0.004 to 0.006 | 0.512 | 16.02 | <0.001 ** |
| Albumin | 0.043 | 0.013 to 0.072 | 0.091 | 2.87 | 0.004 ** |
| Hemoglobin | −0.004 | −0.014 to 0.005 | −0.028 | −0.90 | 0.368 |
| Constant | 0.049 | <0.001 ** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka-Uchiyama, N.; Tsuji, K.; Takahashi, K.; Fukushima, K.; Takeuchi, H.; Kitamura, S.; Inagaki, K.; Uchida, H.A.; Wada, J. Association between Urinary Creatinine Excretion and Hypothyroidism in Patients with Chronic Kidney Disease. Diagnostics 2023, 13, 669. https://doi.org/10.3390/diagnostics13040669
Matsuoka-Uchiyama N, Tsuji K, Takahashi K, Fukushima K, Takeuchi H, Kitamura S, Inagaki K, Uchida HA, Wada J. Association between Urinary Creatinine Excretion and Hypothyroidism in Patients with Chronic Kidney Disease. Diagnostics. 2023; 13(4):669. https://doi.org/10.3390/diagnostics13040669
Chicago/Turabian StyleMatsuoka-Uchiyama, Natsumi, Kenji Tsuji, Kensaku Takahashi, Kazuhiko Fukushima, Hidemi Takeuchi, Shinji Kitamura, Kenichi Inagaki, Haruhito A. Uchida, and Jun Wada. 2023. "Association between Urinary Creatinine Excretion and Hypothyroidism in Patients with Chronic Kidney Disease" Diagnostics 13, no. 4: 669. https://doi.org/10.3390/diagnostics13040669
APA StyleMatsuoka-Uchiyama, N., Tsuji, K., Takahashi, K., Fukushima, K., Takeuchi, H., Kitamura, S., Inagaki, K., Uchida, H. A., & Wada, J. (2023). Association between Urinary Creatinine Excretion and Hypothyroidism in Patients with Chronic Kidney Disease. Diagnostics, 13(4), 669. https://doi.org/10.3390/diagnostics13040669








