Utility and Limitations of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphadenopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methods
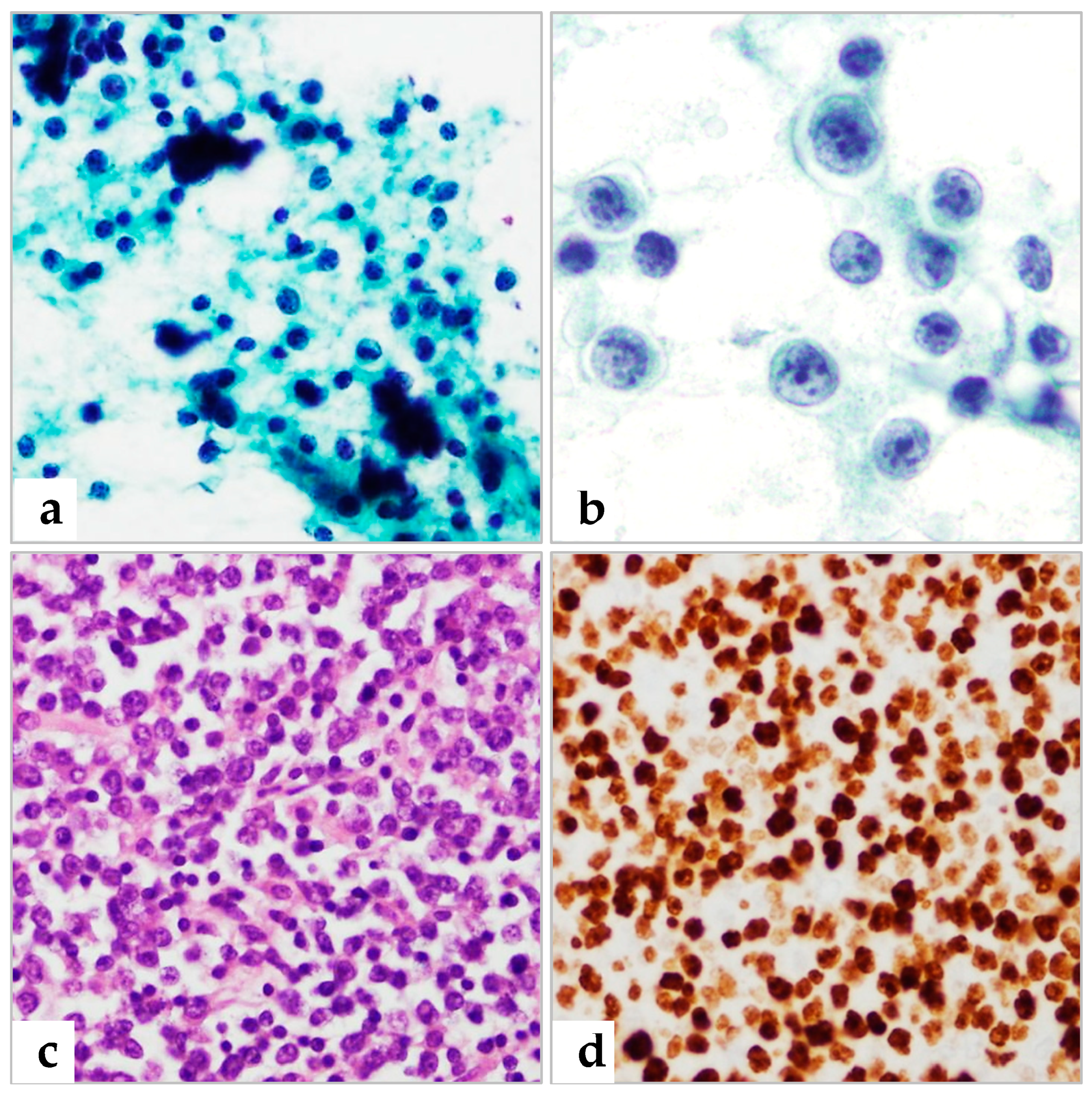
Obtaining Specimens and Ancillary Tests
2.3. Statistical Analysis
| Sensitivity | TP/(TP + FN) |
| Specificity | TN/(TN + FP) |
| Positive predictive value (PPV) | TP/(TP + FP) |
| Negative predictive values (NPV) | TN/(TN + FN) |
| Accuracy | (TP + TN)/(TP + FP + TN + FN) |
3. Results
3.1. Analysis of Lymphadenopathy Using Ultra-Sonography Guided FNAC
3.2. Comparison of Initial Cytologic and Histologic Diagnoses
3.3. Sampling Errors of FNAC
3.4. Sampling Errors of Biopsy
3.5. Cytologic Over-Diagnoses
3.6. Cytologic Under-Diagnoses
3.7. Limitations of Cytological and Histological Diagnoses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malhotra, A.S.; Lahori, M.; Nigam, A.; Khajuria, A. Profile of Lymphadenopathy: An Institutional Based Cytomorphological Study. Int. J. Appl. Basic Med. Res. 2017, 7, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Al-Abbadi, M.A.; Barroca, H.; Bode-Lesniewska, B.; Calaminici, M.; Caraway, N.P.; Chhieng, D.F.; Cozzolino, I.; Ehinger, M.; Field, A.S.; Geddie, W.R.; et al. A Proposal for the Performance, Classification, and Reporting of Lymph Node Fine-Needle Aspiration Cytopathology: The Sydney System. Acta Cytol. 2020, 64, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Rivas, H.E.T.; Zarra, K.V.; Pabón, L.A.P.; Gutierréz, M.d.l.P.G.; Ortiz, N.Z.; Fernández, M.d.M.O.; Llanos, S.N.; Álvarez, N.A.; Martín, Á.G.; Fernández, L.M.F. Ultrasound-guided fine-needle aspiration of superficial lymphadenopathy performed by Interventional pathologists: The applicability of the Sydney system from 2 years of experience and 363 cases. J. Acta Cytol. 2021, 65, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Uzun, E.; Erkilic, S. Diagnostic accuracy of Thinprep® in cervical lymph node aspiration: Assessment according to the Sydney system. J. Diagn. Cytopathol. 2022, 50, 253–262. [Google Scholar] [CrossRef]
- Baruah, A.K.; Bhuyan, G. Utility of the Sydney system for reporting of lymph node cytology in a tertiary health care set up of north-eastern India. World Cancer Res. J. 2022, 9, e2459. [Google Scholar]
- Sergi, C.; Dhiman, A.; Gray, J.A. Fine Needle Aspiration Cytology for Neck Masses in Childhood. An Illustrative Approach. Diagnostics 2018, 8, 28. [Google Scholar] [CrossRef]
- Cozzolino, I.; Vitagliano, G.; Caputo, A.; Montella, M.; Franco, R.; Ciancia, G.; Selleri, C.; Zeppa, P. CD15, CD30, and PAX5 evaluation in Hodgkin’s lymphoma on fine-needle aspiration cytology samples. Diagn. Cytopathol. 2020, 48, 211–216. [Google Scholar] [CrossRef]
- Garg, S.; Rohilla, M.; Srinivasan, R.; Bal, A.; Das, A.; Dey, P.; Gupta, N.; Gupta, P.; Rajwanshi, A. Fine-needle aspiration diagnosis of lymphoma based on cytomorphology alone: How accurate is it?-a cyto-histopathology correlative study. J. Cancer Cytopathol. 2021, 38, 164. [Google Scholar]
- Jin, M.; Wakely, P.E., Jr. Lymph node cytopathology: Essential ancillary studies as applied to lymphoproliferative neoplasms. J. Cancer Cytopathol. 2018, 126, 615–626. [Google Scholar] [CrossRef]
- Das, D.K. Contribution of Immunocytochemistry to the Diagnosis of Usual and Unusual Lymphoma Cases. J. Cytol. 2018, 35, 163–169. [Google Scholar] [CrossRef]
- Caputo, A.; Ciliberti, V.; Zeppa, P.; D’Antonio, A. Cytological Diagnosis of Aggressive Small-Cell Lymphomas. Acta Cytol. 2022, 66, 269–278. [Google Scholar] [CrossRef]
- Ingersoll, K.F.; Zhao, Y.; Harrison, G.P.; Li, Y.; Yang, L.-H.; Wang, E. Limited Tissue Biopsies and Hematolymphoid Neoplasms: Success Stories and Cautionary Tales. J. Am. J. Clin. Pathol. 2019, 152, 782–798. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Faquin, W.C. Cytologic evaluation of cervical lymph node metastases from cancers of unknown primary origin. Semin. Diagn. Pathol. 2015, 32, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hafez, N.H.; Tahoun, N.S. Reliability of fine needle aspiration cytology (FNAC) as a diagnostic tool in cases of cervical lymphadenopathy. J. Egypt. Natl. Cancer Inst. 2011, 23, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Malviya, A. Categorisation of lymph node aspirates using the proposed Sydney system with assessment of risk of malignancy and diagnostic accuracy. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2022, 33, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Makarenko, V.V.; DeLelys, M.E.; Hasserjian, R.P.; Ly, A. Lymph node FNA cytology: Diagnostic performance and clinical implications of proposed diagnostic categories. Cancer Cytopathol. 2022, 130, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Chantziantoniou, N.; Donnelly, A.D.; Mukherjee, M.; Boon, M.E.; Austin, R.M. Inception and Development of the Papanicolaou Stain Method. Acta Cytol. 2017, 61, 266–280. [Google Scholar] [CrossRef]
- Kocjan, G.; Chandra, A.; Cross, P.; Denton, K.; Giles, T.; Herbert, A.; Smith, P.; Remedios, D.; Wilson, P. BSCC Code of Practice--fine needle aspiration cytology. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2009, 20, 283–296. [Google Scholar] [CrossRef] [PubMed]
- VanderLaan, P.A. Fine-needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities. Cancer Cytopathol. 2016, 124, 862–870. [Google Scholar] [CrossRef]
- Altınboğa, A.A.; Yüce, G. Fine-Needle Aspiration Cytology in the Diagnosis of Lymph Nodes: Correlation with Histopathological Diagnosis. Diagn. Value Lymph Node Cytol. 2019, 30. [Google Scholar] [CrossRef]
- Peker, İ.O.; Kulaçoğlu, S.; Eruyar, T.; Ergül, G. Fine needle aspiration cytology of head and neck masses: A cytohistopathological correlation study with emphasis on false positives and false negatives. Turk. J. Ear Nose Throat 2013, 23, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Göret, C.C.; Göret, N.E.; Özdemir, Z.T.; Özkan, E.A.; Doğan, M.; Yanık, S.; Gümrükçü, G.; Aker, F.V. Diagnostic value of fine needle aspiration biopsy in non-thyroidal head and neck lesions: A retrospective study of 866 aspiration materials. J. Int. J. Clin. Exp. Pathol. 2015, 8, 8709. [Google Scholar] [PubMed]
- Vimal, S.; Dharwadkar, A.; Chandanwale, S.S.; Vishwanathan, V.; Kumar, H. Cytomorphological study of lymph node lesions: A study of 187 cases. J. Med. J. Dr. DY Patil Univ. 2016, 9, 43. [Google Scholar] [CrossRef]
- Suryawanshi Kishor, H.; Damle Rajshri, P.; Dravid Nandkumar, V. Spectrum of fnac in palpable head and neck lesions in a tertiary care hospital in india-a 3 years study. J. Indian J. Pathol. Oncol. 2015, 2, 7–13. [Google Scholar]
- Zhu, Y.; Song, Y.; Xu, G.; Fan, Z.; Ren, W. Causes of misdiagnoses by thyroid fine-needle aspiration cytology (FNAC): Our experience and a systematic review. J. Diagn. Pathol. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Bhide, S.P.; Katre, R.Y.; Joshi, S.R. Cytological evaluation of fine needle aspiration cytology in lymph node lesions. J. Med. Sci. Clin. Res. 2017, 5, 26869–26876. [Google Scholar] [CrossRef]
- Khdhayer, A.A.; Al-Azawi, M.J.; Al-Alash, N.; Yasseen, H.A. Fine needle aspiration cytology in the diagnosis of head and neck masses. J. Eur. Sci. J. 2016, 12, 653–659. [Google Scholar] [CrossRef]
- Caputo, A.; D’Ardia, A.; Sabbatino, F.; Picariello, C.; Ciaparrone, C.; Zeppa, P.; D’Antonio, A. Testing EGFR with Idylla on Cytological Specimens of Lung Cancer: A Review. Int. J. Mol. Sci. 2021, 22, 4852. [Google Scholar] [CrossRef]
- Acanfora, G.; Iaccarino, A.; Dello Iacovo, F.; Pisapia, P.; De Luca, C.; Giordano, C.; Bellevicine, C.; Picardi, M.; Troncone, G.; Vigliar, E. A roadmap for a comprehensive diagnostic approach to fine needle cytology of lymph node metastases. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2022, 33, 668–677. [Google Scholar] [CrossRef]
- Iacob, A.; Zazgyva, A.; Ormenisan, A.; Mezei, T.; Sin, A.; Tilinca, M. Effectiveness of fine-needle aspiration cytology in the diagnosis of lateral cervical nonthyroid tumors. Medicine (Baltimore) 2016, 95, e4448. [Google Scholar] [CrossRef]
- González, M.; de Agustín de Agustín, P. Usefulness of light microscopy in lymph node fine needle aspiration biopsy. J Acta Cytol. 2002, 46, 364–368. [Google Scholar]
- Houcine, Y.; Romdhane, E.; Blel, A.; Ksentini, M.; Aloui, R.; Lahiani, R.; Znaidi, N.; Ben Salah, M.; Rammeh, S. Evaluation of fine needle aspiration cytology in the diagnosis of cervical lymph node lymphomas. J. Craniomaxillofac. Surg. 2018, 46, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Kitamura, T.; Fujita, K.; Tate, G.; Mitsuya, T. Cytologic differential diagnosis of follicular lymphoma grades 1 and 2 from reactive follicular hyperplasia: Cytologic features of fine-needle aspiration smears with Pap stain and fluorescence in situ hybridization analysis to detect t (14; 18)(q32; q21) chromosomal translocation. J. Diagn. Cytopathol. 2006, 34, 11–17. [Google Scholar]
- Cozzolino, I.; Giudice, V.; Mignogna, C.; Selleri, C.; Caputo, A.; Zeppa, P. Lymph node fine-needle cytology in the era of personalised medicine. Is there a role? Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2019, 30, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, P.; Cozzolino, I.; Caraway, N.P.; Al-Abbadi, M.A.; Barroca, H.; Bode-Lesniewska, B.; Calaminici, M.; Chhieng, D.F.; Ehinger, M.; Geddie, W.R. Announcement: The international system for reporting lymph node cytopathology. J. Acta Cytol. 2020, 64, 299–305. [Google Scholar] [CrossRef]
- Jiménez-Heffernan, J.A.; Vicandi, B.; López-Ferrer, P.; Hardisson, D.; Viguer, J.M. Value of fine needle aspiration cytology in the initial diagnosis of Hodgkin’s disease. J. Acta Cytol. 2001, 45, 300–306. [Google Scholar] [CrossRef]
- Chhieng, D.C.; Cangiarella, J.F.; Symmans, W.F.; Cohen, J.M. Fine-needle aspiration cytology of Hodgkin disease: A study of 89 cases with emphasis on false-negative cases. Cancer 2001, 93, 52–59. [Google Scholar] [CrossRef]
- Al Kadah, B.; Popov, H.H.; Schick, B.; Knobber, D. Cervical lymphadenopathy: Study of 251 patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 745–752. [Google Scholar] [CrossRef]
- Petrone, G.; Rossi, E.D.; Gallus, R.; Petrelli, L.; Marrone, S.; Rizzo, D.; Piras, A.; Garofalo, G.; Rindi, G.; Galli, J.; et al. Utility of ultrasound-guided fine needle aspiration cytology in assessing malignancy in head and neck pathology. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2021, 32, 407–415. [Google Scholar] [CrossRef]
- Eryilmaz, O.T.; Ucak, R.; Ozagari, A.A.; Kabukcuoglu, F. Diagnostic value of lymph node fine-needle aspiration cytology. Cytojournal 2021, 18, 8. [Google Scholar] [CrossRef]
- Vigliar, E.; Acanfora, G.; Iaccarino, A.; Mascolo, M.; Russo, D.; Scalia, G.; Della Pepa, R.; Bellevicine, C.; Picardi, M.; Troncone, G. A Novel Approach to Classification and Reporting of Lymph Node Fine-Needle Cytology: Application of the Proposed Sydney System. Diagnostics 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, N.; Kumar, P.; Bhardwaj, S.; Srinivasan, R.; Dey, P.; Rohilla, M.; Bal, A.; Das, A.; Rajwanshi, A. Assessment of risk of malignancy by application of the proposed Sydney system for classification and reporting lymph node cytopathology. Cancer Cytopathol. 2021, 129, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Shyu, S.; Rajgariah, A.; Saoud, C.; Rogers, N.; Ali, S.Z. Image-guided lymph node fine-needle aspiration: The Johns Hopkins Hospital experience. J. Am. Soc. Cytopathol. 2021, 10, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Rammeh, S.; Romdhane, E.; Sassi, A.; Belhajkacem, L.; Blel, A.; Ksentini, M.; Lahiani, R.; Farah, F.; Salah, M.B.; Ferjaoui, M. Accuracy of fine-needle aspiration cytology of head and neck masses. Diagn. Cytopathol. 2019, 47, 394–399. [Google Scholar] [CrossRef]
- Schwock, J.; Geddie, W.R. Diagnosis of B-cell non-hodgkin lymphomas with small-/intermediate-sized cells in cytopathology. Pathol. Res. Int. 2012, 2012, 164934. [Google Scholar] [CrossRef]
- Mehdi, G.; Singh, A.K.; Hasan, M.; Ansari, H.A.; Rehman, S.; Mirza, S.; Sherwani, R.K. Cytological evaluation of enlarged lymph nodes in metastatic disease: A hospital-based assessment. J. Clin. Cancer Investig. J. 2015, 4, 152–157. [Google Scholar]



| Characteristics | No. of Patients (%) | |
|---|---|---|
| Gender | Male | 247 (57) |
| Female | 185 (43) | |
| Age (mean age 58.2 years) | <30 years | 34 (8) |
| (range 8–89 years) | 30–50 years | 96 (22) |
| >50 years | 302 (70) | |
| Lymph node size (mean size 1.9 cm) | <2.0 cm | 265 (61) |
| (range 0.2–5.0 cm) | 2.0–3.0 cm | 120 (28) |
| >3.0 cm | 47 (11) | |
| Lesion location | Cervical | 257 (59) |
| Supraclavicular | 59 (14) | |
| Mediastinal | 86 (20) | |
| Axillary | 22 (5) | |
| Inguinal | 8 (20) |
| Cytologic Diagnosis (n = 432) | Histologic Diagnosis (n = 432) | ||||
|---|---|---|---|---|---|
| TIFD (39 ‡) | Benign (155) | Metastasis (199) | NHL (35) | HL (4) | |
| Inadequate (15 †) | 3 | 7 | 5 † | 0 | 0 |
| Benign (155) | 24 | 119 | 7 † | 5 ** | 0 |
| Atypical (39) | 5 | 16 | 12 | 6 | 0 |
| Suspicious (7) | 0 | 3 ‡,* | 3 | 1 | 0 |
| Metastasis (192) | 7 ‡ | 10 ‡,* | 172 | 3 | 0 |
| NHL (20) | 0 | 0 | 0 | 20 | 0 |
| HL (4) | 0 | 0 | 0 | 0 | 4 |
| Primary Site | No. of Cases (n = 172) | Histologic Diagnoses | No. of Cases (n = 172) | Gender (M:F) | Age (Range) |
|---|---|---|---|---|---|
| Lung | 58 | Adenocarcinoma | 26 | 46:12 | 43–82 |
| Squamous cell ca. | 19 | ||||
| Small cell carcinoma | 10 | ||||
| Large cell carcinoma | 1 | ||||
| Mucoepidermoid ca. | 1 | ||||
| LCNEC | 1 | ||||
| Thyroid gland | 50 | Papillary thyroid ca. | 48 | 11:39 | 24–75 |
| Medullary ca. | 2 | ||||
| Breast | 18 | Ductal ca. | 17 | 0:18 | 35–65 |
| Mucinous ca. | 1 | ||||
| Oral cavity | 9 | Squamous cell ca. | 9 | 5:4 | 36–77 |
| Skin | 5 | Malignant melanoma | 4 | 1:4 | 48–75 |
| Squamous cell ca. | 1 | ||||
| Tonsil | 5 | Squamous cell ca. | 3 | 5:0 | 38–52 |
| Adenocarcinoma | 2 | ||||
| Pharynx | 4 | Squamous cell ca. | 2 | 4:0 | 48–79 |
| Adenosquamous ca. | 1 | ||||
| Undifferentiated ca. | 1 | ||||
| Esophagus | 3 | Squamous cell ca. | 3 | 3:0 | 60–68 |
| Ovary | 3 | Serous ca. | 3 | 0:3 | 53–76 |
| Bladder | 3 | Urothelial ca. | 3 | 2:1 | 60–70 |
| Salivary gland | 2 | Salivary duct ca. | 1 | 2:0 | 39–56 |
| Undifferentiated ca. | 1 | ||||
| Larynx | 2 | Squamous cell ca. | 2 | 2:0 | 65–71 |
| Stomach | 2 | Adenocarcinoma | 1 | 1:1 | 58–70 |
| Squamous cell ca. | 1 | ||||
| Rectum | 2 | Adenocarcinoma | 2 | 1:1 | 73–76 |
| Liver | 1 | Hepatocellular ca. | 1 | 0:1 | 48 |
| Kidney | 1 | Renal cell ca. | 1 | 1:0 | 64 |
| Cervix | 1 | Squamous cell ca. | 1 | 0:1 | 56 |
| Unknown origin | 3 | Squamous cell ca. | 1 | 2:1 | 48–68 |
| Carcinoma | 2 |
| Cytologic Diagnosis | No. of Cases (n = 22) | Histologic Diagnosis | No. of Cases (n = 22) |
|---|---|---|---|
| TIFD | 3 | ||
| Benign | 7 | ||
| Inadequate/non-diagnostic | 15 | Ductal carcinoma | 2 |
| Adenocarcinoma | 1 | ||
| Squamous cell carcinoma | 1 | ||
| Papillary thyroid carcinoma | 1 | ||
| Adenocarcinoma | 3 | ||
| Benign | 7 | Small cell carcinoma | 2 |
| Squamous cell carcinoma | 1 | ||
| Papillary thyroid carcinoma | 1 |
| Histologic Diagnosis | No. of Cases (n = 49) | Cytologic Diagnosis | No. of Cases (n = 49) |
|---|---|---|---|
| Inadequate/non-diagnostic | 3 | ||
| Benign | 24 | ||
| Atypical cells | 5 | ||
| TIFD | 39 | Adenocarcinoma | 3 |
| Small cell carcinoma | 2 | ||
| Squamous cell carcinoma | 1 | ||
| Malignant melanoma | 1 | ||
| Small cell carcinoma | 3 | ||
| Squamous cell carcinoma | 3 | ||
| Benign | 10 | Adenocarcinoma | 2 |
| Papillary thyroid carcinoma | 1 | ||
| Malignant melanoma | 1 |
| Cytologic Diagnosis | Histologic Diagnosis | FP/FN (n = 8) |
|---|---|---|
| Suggestive of malignancy | Foreign body granuloma | FP (n = 1) |
| Suspicious for lymphoma | Reactive hyperplasia | FP (n = 1) |
| Meta. papillary thyroid ca. | Reactive hyperplasia | FP (n = 1) |
| Reactive hyperplasia | Follicular lymphoma, grade 1 | FN (n = 1) |
| Reactive hyperplasia | Mantle cell lymphoma | FN (n = 1) |
| Reactive hyperplasia | EBV-positive diffuse large B-cell lymphoma | FN (n = 1) |
| Reactive hyperplasia | Diffuse large B-cell lymphoma | FN (n = 1) |
| Reactive hyperplasia | Diffuse large B-cell lymphoma | FN (n = 1) |
| Analysis | Group I | Group II |
|---|---|---|
| Malignant + Suspicious (%) | Malignant + Suspicious + Atypical (%) | |
| Sensitivity | 97.8 | 96.0 |
| Specificity | 97.5 | 94.9 |
| Positive predictive value (PPV) | 98.7 | 97.2 |
| Negative predictive values (NPV) | 96.0 | 92.9 |
| Accuracy | 97.7 | 95.6 |
| Under-diagnosis rate | 1.3 | 2.3 |
| Over-diagnosis rate | 0.8 | 1.6 |
| Sampling error rate | 5.1 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, H.J.; Lee, J.; Kim, D.Y.; Kim, J.-S.; Shin, M.-S.; Noh, I.; Koh, J.S.; Kim, E.J.; Lee, S.-S. Utility and Limitations of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphadenopathy. Diagnostics 2023, 13, 728. https://doi.org/10.3390/diagnostics13040728
Ha HJ, Lee J, Kim DY, Kim J-S, Shin M-S, Noh I, Koh JS, Kim EJ, Lee S-S. Utility and Limitations of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphadenopathy. Diagnostics. 2023; 13(4):728. https://doi.org/10.3390/diagnostics13040728
Chicago/Turabian StyleHa, Hwa Jeong, Jeeyong Lee, Da Yeon Kim, Jung-Soon Kim, Myung-Soon Shin, Insup Noh, Jae Soo Koh, Eun Ju Kim, and Seung-Sook Lee. 2023. "Utility and Limitations of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphadenopathy" Diagnostics 13, no. 4: 728. https://doi.org/10.3390/diagnostics13040728
APA StyleHa, H. J., Lee, J., Kim, D. Y., Kim, J.-S., Shin, M.-S., Noh, I., Koh, J. S., Kim, E. J., & Lee, S.-S. (2023). Utility and Limitations of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphadenopathy. Diagnostics, 13(4), 728. https://doi.org/10.3390/diagnostics13040728







