Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice
Abstract
1. Introduction
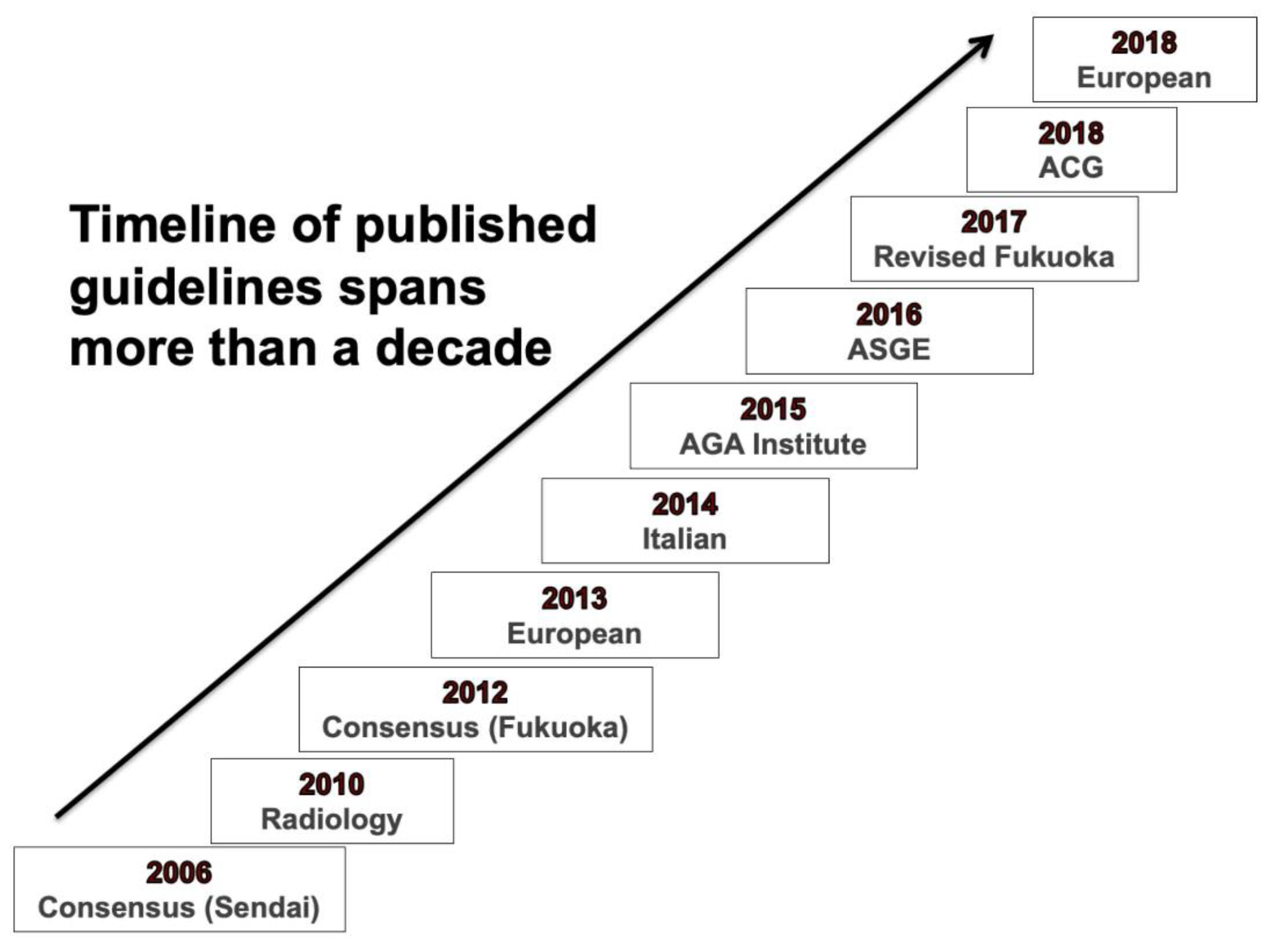
2. Comparative Description of Recommendations from Major guidelines
2.1. Cyst Types and Patient Characteristics
2.2. Preferred Imaging Modality
2.3. Indications for EUS and Cyst Fluid Analysis
2.4. Indications for Surgery
2.5. Conduct of Surveillance
3. Comparative and Validation Studies
4. Emerging Diagnostic Modalities Not Included in Guidelines
4.1. Biomarkers
4.2. Endoscopic Technology and Advanced Imaging
4.3. F-18 Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) Imaging
4.4. Machine Learning-Based Strategies
5. Practice Patterns and Awareness of the Guidelines among Clinicians
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| PCLs | Pancreatic cystic lesions; |
| PC | Pancreatic cancer; |
| MD-IPMNs | Main duct intraductal papillary mucinous neoplasms; |
| BD-IPMNs | Branched duct intraductal papillary mucinous neoplasms; |
| MCN | Mucinous cystic neoplasm; |
| SCN | serous cystic neoplasm; |
| AGA | American Gastroenterological Association; |
| ACG | American College of Gastroenterology; |
| ASGE | American Society of Gastrointestinal Endoscopy; |
| ACR | American College of Radiology; |
| MRI | Magnetic resonance imaging; |
| MRCP | Magnetic resonance cholangiopancreatography; |
| CT | Computed tomography; |
| EUS | Endoscopic ultrasound; |
| CH-EUS | Contrast harmonic enhanced endoscopic ultrasound; |
| EUS-FNA | Endoscopic ultrasound guided fine-needle aspiration; |
| CEA | Carcinoembryonic antigen; |
| PD | Pancreatic duct; |
| MPD | Main pancreatic duct; |
| CA 19-9 | Carbohydrate antigen 19-9; |
| CLE | Confocal laser endomicroscopy; |
| F-18 FDG PET | F-18 fluorodeoxyglucose positron emission tomography. |
References
- Laffan, T.A.; Horton, K.M.; Klein, A.P.; Berlanstein, B.; Siegelman, S.S.; Kawamoto, S.; Johnson, P.T.; Fishman, E.K.; Hruban, R.H. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am. J. Roentgenol. 2008, 191, 802–807. [Google Scholar] [CrossRef] [PubMed]
- De Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; van Eijck, C.H.; van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin. Gastroenterol. Hepatol. 2016, 14, 585–593.e3. [Google Scholar] [CrossRef]
- Kromrey, M.L.; Bulow, R.; Hubner, J.; Paperlein, C.; Lerch, M.M.; Ittermann, T.; Volzke, H.; Mayerle, J.; Kuhn, J.P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Acher, A.W.; Krishna, S.G.; Cloyd, J.M.; Pawlik, T.M. Comparison of Society Guidelines for the Management and Surveillance of Pancreatic Cysts: A Review. JAMA Surg. 2022, 157, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Berland, L.L.; Silverman, S.G.; Gore, R.M.; Mayo-Smith, W.W.; Megibow, A.J.; Yee, J.; Brink, J.A.; Baker, M.E.; Federle, M.P.; Foley, W.D.; et al. Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee. J. Am. Coll. Radiol. 2010, 7, 754–773. [Google Scholar] [CrossRef] [PubMed]
- Italian Association of Hospital Gastroenterologists and Endoscopists; Italian Association for the Study of the Pancreas. Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig. Liver Dis. 2014, 46, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines, C.; American Gastroenterology, A. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822; quize 812–813. [Google Scholar] [CrossRef]
- Committee, A.S.o.P.; Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest. Endosc. 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S.; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernandez-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Verbeke, C.; Salvia, R.; Kloppel, G.; Werner, J.; McKay, C.; Friess, H.; Manfredi, R.; Van Cutsem, E.; Lohr, M.; et al. European experts consensus statement on cystic tumours of the pancreas. Dig. Liver Dis. 2013, 45, 703–711. [Google Scholar] [CrossRef]
- Lennon, A.M.; Canto, M.I. Pancreatic Cysts—Part 2: Should We Be Less Cyst Centric? Pancreas 2017, 46, 745–750. [Google Scholar] [CrossRef]
- Scheiman, J.M. Pancreatic Cysts—Part 1: Using the American Gastroenterological Association Guidelines for the Management of Pancreatic Cysts-A Practical Approach. Pancreas 2017, 46, 742–744. [Google Scholar] [CrossRef]
- Hasan, A.; Visrodia, K.; Farrell, J.J.; Gonda, T.A. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J. Gastroenterol. 2019, 25, 4405–4413. [Google Scholar] [CrossRef]
- Buerlein, R.C.D.; Shami, V.M. Management of pancreatic cysts and guidelines: What the gastroenterologist needs to know. Ther. Adv. Gastrointest. Endosc. 2021, 14, 1–21. [Google Scholar] [CrossRef]
- Singhi, A.D.; Zeh, H.J.; Brand, R.E.; Nikiforova, M.N.; Chennat, J.S.; Fasanella, K.E.; Khalid, A.; Papachristou, G.I.; Slivka, A.; Hogg, M.; et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: A clinicopathologic study of 225 patients with supporting molecular data. Gastrointest. Endosc. 2016, 83, 1107–1117.e2. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.; de Pretis, N.; Aryal-Khanal, A.; Ahmed, N.; Sah, R.; Enders, F.; Larson, J.J.; Levy, M.J.; Takahashi, N.; Topazian, M.; et al. Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut 2017, 66, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Bassi, C.; Salvia, R.; Malleo, G.; Marchegiani, G.; Rebours, V.; Levy, P.; Partelli, S.; Suleiman, S.L.; Banks, P.A.; et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: A mid-term follow-up analysis. Gut 2017, 66, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Lekkerkerker, S.J.; Besselink, M.G.; Busch, O.R.; Verheij, J.; Engelbrecht, M.R.; Rauws, E.A.; Fockens, P.; van Hooft, J.E. Comparing 3 guidelines on the management of surgically removed pancreatic cysts with regard to pathological outcome. Gastrointest. Endosc. 2017, 85, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Li, Z.; Miao, H. Accuracy of Fukuoka and American Gastroenterological Association Guidelines for Predicting Advanced Neoplasia in Pancreatic Cyst Neoplasm: A Meta-Analysis. Ann. Surg. Oncol. 2019, 26, 4522–4536. [Google Scholar] [CrossRef]
- McCarty, T.R.; Garg, R.; Rustagi, T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 698–712.e6. [Google Scholar] [CrossRef]
- Singhi, A.D.; McGrath, K.; Brand, R.E.; Khalid, A.; Zeh, H.J.; Chennat, J.S.; Fasanella, K.E.; Papachristou, G.I.; Slivka, A.; Bartlett, D.L.; et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018, 67, 2131–2141. [Google Scholar] [CrossRef]
- Haeberle, L.; Schramm, M.; Goering, W.; Frohn, L.; Driescher, C.; Hartwig, W.; Preissinger-Heinzel, H.K.; Beyna, T.; Neuhaus, H.; Fuchs, K.; et al. Molecular analysis of cyst fluids improves the diagnostic accuracy of pre-operative assessment of pancreatic cystic lesions. Sci. Rep. 2021, 11, 2901. [Google Scholar] [CrossRef]
- McCarty, T.R.; Paleti, S.; Rustagi, T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 93, 1019–1033.e5. [Google Scholar] [CrossRef]
- Schmitz, D.; Kazdal, D.; Allgauer, M.; Trunk, M.; Vornhusen, S.; Nahm, A.M.; Doll, M.; Weingartner, S.; Endris, V.; Penzel, R.; et al. KRAS/GNAS-testing by highly sensitive deep targeted next generation sequencing improves the endoscopic ultrasound-guided workup of suspected mucinous neoplasms of the pancreas. Genes Chromosomes Cancer 2021, 60, 489–497. [Google Scholar] [CrossRef]
- Basar, O.; Yuksel, O.; Yang, D.J.; Samarasena, J.; Forcione, D.; DiMaio, C.J.; Wagh, M.S.; Chang, K.; Casey, B.; Fernandez-Del Castillo, C.; et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest. Endosc. 2018, 88, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mittal, C.; Obuch, J.C.; Hammad, H.; Edmundowicz, S.A.; Wani, S.; Shah, R.J.; Brauer, B.C.; Attwell, A.R.; Kaplan, J.B.; Wagh, M.S. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video). Gastrointest. Endosc. 2018, 87, 1263–1269. [Google Scholar] [CrossRef]
- Yang, D.; Trindade, A.J.; Yachimski, P.; Benias, P.; Nieto, J.; Manvar, A.; Ho, S.; Esnakula, A.; Gamboa, A.; Sethi, A.; et al. Histologic Analysis of Endoscopic Ultrasound-Guided Through the Needle Microforceps Biopsies Accurately Identifies Mucinous Pancreas Cysts. Clin. Gastroenterol. Hepatol. 2019, 17, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Samarasena, J.B.; Jamil, L.H.; Chang, K.J.; Lee, D.; Ona, M.A.; Lo, S.K.; Gaddam, S.; Liu, Q.; Draganov, P.V. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: A multicenter study. Endosc. Int. Open 2018, 6, E1423–E1430. [Google Scholar] [CrossRef]
- Krishna, S.G.; Hart, P.A.; DeWitt, J.M.; DiMaio, C.J.; Kongkam, P.; Napoleon, B.; Othman, M.O.; Yew Tan, D.M.; Strobel, S.G.; Stanich, P.P.; et al. EUS-guided confocal laser endomicroscopy: Prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest. Endosc. 2020, 91, 551–563.e5. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.G.; Hart, P.A.; Malli, A.; Kruger, A.J.; McCarthy, S.T.; El-Dika, S.; Walker, J.P.; Dillhoff, M.E.; Manilchuk, A.; Schmidt, C.R.; et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin. Gastroenterol. Hepatol. 2020, 18, 432–440.e6. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Napoleon, B.; Lennon, A.M.; El-Dika, S.; Pereira, S.P.; Tan, D.; Pannala, R.; Girotra, M.; Kongkam, P.; Bertani, H.; et al. Accuracy and agreement of a large panel of endosonographers for endomicroscopy-guided virtual biopsy of pancreatic cystic lesions. Pancreatology 2022, 22, 994–1002. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Bertagna, F.; Gheza, F.; Grazioli, L.; Calanducci, D.; Giubbini, R.; Portolani, N.; Giulini, S.M. Searching for indicators of malignancy in pancreatic intraductal papillary mucinous neoplasms: The value of 18FDG-PET confirmed. Ann. Surg. Oncol. 2012, 19, 3574–3580. [Google Scholar] [CrossRef]
- Kauhanen, S.; Rinta-Kiikka, I.; Kemppainen, J.; Gronroos, J.; Kajander, S.; Seppanen, M.; Alanen, K.; Gullichsen, R.; Nuutila, P.; Ovaska, J. Accuracy of 18F-FDG PET/CT, Multidetector CT, and MR Imaging in the Diagnosis of Pancreatic Cysts: A Prospective Single-Center Study. J. Nucl. Med. 2015, 56, 1163–1168. [Google Scholar] [CrossRef]
- Ohta, K.; Tanada, M.; Sugawara, Y.; Teramoto, N.; Iguchi, H. Usefulness of positron emission tomography (PET)/contrast-enhanced computed tomography (CE-CT) in discriminating between malignant and benign intraductal papillary mucinous neoplasms (IPMNs). Pancreatology 2017, 17, 911–919. [Google Scholar] [CrossRef]
- Hayashi, M.; Mikata, R.; Horikoshi, T.; Senoo, J.; Kusakabe, Y.; Ohyama, H.; Yasui, S.; Uchida, Y.; Uchiyama, K.; Kishimoto, T.; et al. Diffusion-Weighted Magnetic Resonance Imaging and 18-Fluorodeoxglucose Positron Emission Tomography With Computed Tomography for Evaluating Malignancy of Branch Duct and Mixed Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas 2019, 48, e43–e45. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Shim, S.R.; Jeong, S.Y.; Kim, S.J. Comparison of Preoperative Imaging Modalities for the Assessment of Malignant Potential of Pancreatic Cystic Lesions: A Network Meta-analysis. Clin. Nucl. Med. 2022, 47, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Masica, D.L.; Dal Molin, M.; Douville, C.; Thoburn, C.J.; Afsari, B.; Li, L.; Cohen, J.D.; Thompson, E.; Allen, P.J.; et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Marchegiani, G.; Salvia, R.; Verona, E.B.M.o.I. Guidelines on Pancreatic Cystic Neoplasms: Major Inconsistencies With Available Evidence and Clinical Practice- Results From an International Survey. Gastroenterology 2021, 160, 2234–2238. [Google Scholar] [CrossRef]
- Westerveld, D.; Goddard, A.; Harris, N.; Khullar, V.; Forde, J.; Draganov, P.V.; Forsmark, C.E.; Yang, D. Survey Study on the Practice Patterns of the Evaluation and Management of Incidental Pancreatic Cysts. Dig. Dis. Sci. 2019, 64, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.S.; Gatsonis, C.; Zeh, H.J.; Carlos, R.C.; O’Dwyer, P.J.; Team, E.A. Comparing the clinical impact of pancreatic cyst surveillance programs: A trial of the ECOG-ACRIN cancer research group (EA2185). Contemp. Clin. Trials 2020, 97, 106144. [Google Scholar] [CrossRef] [PubMed]
| AGA (2015) | Fukuoka (2017) | ACR (2017) | ACG (2018) | European (2018) | |
|---|---|---|---|---|---|
| Target patient population | Asymptomatic PCLs Do not apply to solid pseudopapillary neoplasms, cystic neuroendocrine tumors, and MD-IPMN without branch duct involvement | MD-IPMNs and BD-IPMNs | Incidentally discovered asymptomatic PCLs | All newly diagnosed PCLs without a strong family history of pancreatic cancer or genetic variants known to predispose to pancreatic cancer. Included all cyst types: Neoplastic pancreatic cysts (MD-IPMNs, BD-IPMNs, MCNs, solid pseudopapillary neoplasms, cystic NETs, serous cyst adenomas) and non-neoplastic (pseudocyst) | All PCLs PCLs are classified into 4 distinct categories
|
| Choice of imaging modality | MRI | Pancreas protocol CT or MRI-MRCP | Contrast-enhanced MRI or pancreas-protocol CT | MRI-MRCP | MRI-MRCP |
| Indications of EUS and FNA | ≥2 high-risk features: Size ≥ 3 cm, dilated MPD, or presence of a solid component | Any of the following present “worrisome features”: Clinical pancreatitis secondary to cyst, cyst ≥ 3 cm, enhancing mural nodule < 5 mm, thickened or enhancing cyst walls, MPD 5–9 mm, abrupt change in the caliber of the PD with distal pancreatic atrophy, lymphadenopathy, increased serum CA 19-9, cyst growth rate ≥ 5 mm/2 years | Presence of any of the “worrisome features” or “high-risk stigmata” with the exception of cyst ≥ 3 cm without any additional “worrisome feature” or “high-risk stigmata” that alternatively can be followed. Worrisome features: cyst ≥ 3 cm, thickened/enhancing cyst wall, nonenhancing mural nodule, MPD ≥ 7 mm. High-risk stigmata: obstructive jaundice, enhancing solid component, MPD | Any of the following present: Obstructive jaundice or acute pancreatitis secondary to the cyst, presence of mural nodule or solid component, MPD > 5 mm, change in the caliber of PD with upstream atrophy, cyst size ≥ 3 cm, increase in cyst size ≥ 3 mm/year | PCLs with clinical or radiological features of concern for malignancy |
| Cyst fluid analysis | Positive cytology—highest specificity in diagnosing malignancy | Cytological and molecular analysis—considered investigational—should be performed only in expert centers | Recommend cyst fluid aspiration (cyst fluid CEA and cytology) to differentiate mucinous and non-mucinous PCLs | Cyst fluid CEA may be considered to differentiate IPMN and MCN from other cyst types. Recommended to send cyst fluid cytology Molecular markers may be considered in cases with unclear diagnosis | Cyst fluid CEA combined with cytology or KRAS/GNAS mutation analysis recommended for better diagnostic performance |
| Indications of surgery | 2 criteria should be met: solid component and a dilated duct and/or concerning features on EUS and FNA | Presence of “High-risk stigmata”: Obstructive jaundice with cystic lesion in the head of the pancreas, enhancing mural nodule ≥ 5 mm, MPD ≥ 10 mm, MD-IPMN, cytology suspicious or positive for malignancy | Presence of any of the “worrisome features” or “high-risk stigmata” with the exception of cyst ≥ 3 cm without any additional “worrisome feature” or “high-risk stigmata” that alternatively can be followed. | Recommend ‘multidisciplinary referral’ for following: Cytology showing high-grade dysplasia or malignancy, mural nodule, concerning features on EUS, all MD-IPMNs, solid pseudopapillary neoplasm | Absolute indications: Obstructive jaundice, presence of an enhancing mural nodule (≥5 mm) or a solid component, positive cytology or MPD ≥ 10 mm Relative indications: MPD dilatation between 5 and 9.9 mm, cystic growth rate ≥ 5 mm/year, increased level of serum CA 19.9 (>37 U/mL), symptoms (new-onset diabetes or acute pancreatitis), enhancing mural nodules (<5 mm), and/or a cyst diameter ≥ 40 mm |
| Surveillance interval based on cyst size 1–2 cm | MRI in 1-year | MRI or CT in 1-year | Surveillance recommendations differ based on cyst size (i.e., <1.5 cm, 1.5–2.5 cm, >2.5 cm), patient age, interval growth, and presence of risk factors. | MRI in 1-year | MRI or EUS in 6 months in conjunction with CA 19-9 and clinical evaluation |
| 2–3 cm | MRI in 1-year | EUS in 3–6 months | MRI or EUS in 6–12 months | MRI or EUS in 6 months in conjunction with CA 19-9 and clinical evaluation | |
| 3–4 cm | MRI in 1-year | MRI or EUS in 3–6 months | MRI or EUS every 6–12 months (multidisciplinarygroup referral) | MRI or EUS in 6 months in conjunction with CA 19-9 and clinical evaluation | |
| Discontinuation of surveillance | If no significant change in the cyst characteristics after 5 years of surveillance or if the patient no longer surgical candidate | No data to evaluate discontinuation of surveillance | Advocate 9- to 10-year follow-up for most patients, terminating at the age of 80 years. | Lifetime surveillance unless the patient is no longer surgical candidate | Lifetime surveillance unless the patient is no longer surgical candidate |
| Surveillance after surgery | MRI surveillance every 2 years | IPMNs—require lifetime surveillance—every 6–12 months | No comment on post-surveillance resection strategies | IPMN—every 2 years if no remnant cyst. IPMN in remnant pancreas—surveillance based on the largest IPMN MCNs without cancer—no surveillance Solid pseudopapillary neoplasm—yearly surveillance for at least 5 years SCAs, pseudocyst, other benign cysts—no surveillance | IPMN with high-grade dysplasia or MD-IPMN—every 6 months for the first 2 years, followed by yearly surveillance. IPMN with low-grade dysplasia—followed the same manner as non-resected IPMN IPMN in the remnant pancreas, no high-grade dysplasia or MD-IPMN—followed same manner as non-resected BD-IPMN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohapatra, S.; Krishna, S.G.; Pannala, R. Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice. Diagnostics 2023, 13, 749. https://doi.org/10.3390/diagnostics13040749
Mohapatra S, Krishna SG, Pannala R. Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice. Diagnostics. 2023; 13(4):749. https://doi.org/10.3390/diagnostics13040749
Chicago/Turabian StyleMohapatra, Sonmoon, Somashekar G. Krishna, and Rahul Pannala. 2023. "Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice" Diagnostics 13, no. 4: 749. https://doi.org/10.3390/diagnostics13040749
APA StyleMohapatra, S., Krishna, S. G., & Pannala, R. (2023). Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice. Diagnostics, 13(4), 749. https://doi.org/10.3390/diagnostics13040749







