Multi-Techniques for Analyzing X-ray Images for Early Detection and Differentiation of Pneumonia and Tuberculosis Based on Hybrid Features
Abstract
:1. Introduction
- Enhancement of chest X-rays using overlapping of average and Laplacian filters;
- Reducing the high dimensionality deep features produced using the VGG16 and ResNet18;
- Application of a hybrid technology between deep learning models and the support vector machine (SVM) for early differentiation between pneumonia and tuberculosis;
- Fusing of the deep features of the VGG16 and ResNet18 before and after reducing the high dimensions to obtain more representative features of pneumonia and tuberculosis, which are then diagnosed by ANN;
- Early differentiation between pneumonia and tuberculosis by ANN through integrating the features of the VGG16 and ResNet18 separately with the hand-crafted features.
2. Related Work
3. Materials and Methods
3.1. Description of the Data Set
3.2. Enhancement of X-rays
3.3. Hybrid Models CNN with SVM
3.3.1. Deep Feature Extraction
3.3.2. SVM Classifier
3.4. Integrating the Deep Features of the Two CNN Models
3.5. Integrating CNN Features with Hand-Crafted Features
4. Experimental Results of the Systems
4.1. Split of the Data Set
4.2. Evaluation Metrics
4.3. Data Set Balancing and Data Augmentation
4.4. Result of Hybrid Models CNN and SVM
4.5. Result of Integrating the Deep Features of the Two CNN
4.6. Result of Integrating CNN Features with Hand-Crafted Features
4.6.1. Error Histogram
4.6.2. Best Validation Performance
4.6.3. Gradient and Validation Checks (GVC)
4.6.4. Confusion Matrix
5. Discussion and Comparison of the Achievement of the Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subramaniam, S.; Raju, N.; Ganesan, A.; Rajavel, N.; Chenniappan, M.; Prakash, C.; Dixit, S. Artificial Intelligence Technologies for Forecasting Air Pollution and Human Health: A Narrative Review. Sustainability 2022, 14, 9951. [Google Scholar] [CrossRef]
- Nemours KidsHealth. Lungs and Respiratory System (for Teens). Available online: https://kidshealth.org/en/teens/lungs.html (accessed on 15 July 2022).
- Dodia, S.; Annappa, B.; Mahesh, P.A. Recent advancements in deep learning based lung cancer detection: A systematic review. Eng. Appl. Artif. Intell. 2022, 116, 105490. [Google Scholar] [CrossRef]
- Kaklauskas, A.; Abraham, A.; Milevicius, V. Diurnal emotions, valence and the coronavirus lockdown analysis in public spaces. Eng. Appl. Artif. Intell. 2021, 98, 104122. [Google Scholar] [CrossRef]
- Kaya, F.; Ernest, J.P.; LoMauro, K.; Gengenbacher, M.; Madani, A.; Aragaw, W.W.; Zimmerman, M.D.; Sarathy, J.P. A Rabbit Model to Study Antibiotic Penetration at the Site of Infection for Nontuberculous Mycobacterial Lung Disease: Macrolide Case Study. Antimicrob. Agents Chemother. 2022, 66, e0221221. [Google Scholar] [CrossRef]
- Evora, L.; Seixas, J.; Kritski, A.L. Neural network models for supporting drug and multidrug resistant tuberculosis screening diagnosis. Neurocomputing 2017, 265, 116–126. [Google Scholar] [CrossRef]
- El Zowalaty, M.E.; Ashour, H.M. Paving the way to a new class of efficient and safe tuberculosis vaccines: The role of c-di-AMP in Mycobacterium tuberculosis immunity and virulence. Mol. Ther. Nucleic Acids 2022, 30, 13–14. [Google Scholar] [CrossRef]
- NHS. Tuberculosis (TB). Available online: https://www.nhs.uk/conditions/tuberculosis-tb/ (accessed on 15 July 2022).
- Khimova, E.; Gonzalo, X.; Popova, Y.; Eliseev, P.; Andrey, M.; Nikolayevskyy, V.; Broda, A.; Drobniewski, F. Urine biomarkers of pulmonary tuberculosis. Expert Rev. Respir. Med. 2022, 16, 615–621. [Google Scholar] [CrossRef]
- Sultan, A.S.; Elgharib, M.A.; Tavares, T.; Jessri, M.; Basile, J.R. The use of artificial intelligence, machine learning and deep learning in oncologic histopathology. J. Oral Pathol. Med. 2020, 49, 849–856. [Google Scholar] [CrossRef]
- Starshinova, A.A.; Kudryavtsev, I.; Malkova, A.; Zinchenko, U.; Karev, V.; Kudlay, D.; Glushkova, A.; Starshinova, A.Y.; Dominguez, J.; Villar-Hernández, R.; et al. Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection. Int. J. Mol. Sci. 2022, 23, 2235. [Google Scholar] [CrossRef]
- Chang, K.; Balachandar, N.; Lam, C.; Yi, D.; Brown, J.; Beers, A.; Kalpathy-Cramer, J. Distributed deep learning networks among institutions for medical imaging. J. Am. Med. Inform. Assoc. 2018, 25, 945–954. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lin, J.; Zhao, Y.; Sun, X. Deregulated Expression of miR-483-3p Serves as a Diagnostic Biomarker in Severe Pneumonia Children with Respiratory Failure and Its Predictive Value for the Clinical Outcome of Patients. Mol. Biotechnol. 2022, 64, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Farahani, A.; Khatibi, T.; Sarmadian, H.; Boskabadi, A. Proposing a two-step decision support system for differential diagnosis of tuberculosis from pneumonia. Sustain. Oper. Comput. 2022, 3, 303–316. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Shu, Y.; Zhu, J. Magnetic resonance imaging images under deep learning in the identification of tuberculosis and pneumonia. J. Healthc. Eng. 2021, 2021, 6772624. [Google Scholar] [CrossRef] [PubMed]
- Bharati, S.; Podder, P.; Rubaiyat Hossain Mondal, M. Hybrid deep learning for detecting lung diseases from X-ray images. Inform. Med. Unlocked 2020, 20, 100391. [Google Scholar] [CrossRef] [PubMed]
- Sathitratanacheewin, S.; Sunanta, P.; Pongpirul, K. Deep learning for automated classification of tuberculosis-related chest X-ray: Dataset distribution shift limits diagnostic performance generalizability. Heliyon 2020, 6, e04614. [Google Scholar] [CrossRef] [PubMed]
- Gite, S.; Mishra, A.; Kotecha, K. Enhanced lung image segmentation using deep learning. Neural Comput. Appl. 2022, 1–15. [Google Scholar] [CrossRef]
- Ayaz, M.; Shaukat, F.; Raja, G. Ensemble learning based automatic detection of tuberculosis in chest X-ray images using hybrid feature descriptors. Phys. Eng. Sci. Med. 2021, 44, 183–194. [Google Scholar] [CrossRef]
- Acharya, A.; Satapathy, R. A deep learning based approach towards the automatic diagnosis of pneumonia from chest radio-graphs. Biomed. Pharmacol. J. 2020, 13, 449–455. [Google Scholar] [CrossRef]
- Jamil, S.; Abbas, M.S.; Zia, M.F.; Rahman, M.U. A deep convolutional neural network based framework for pneumonia detection. In Proceedings of the 2021 International Conference on Digital Futures and Transformative Technologies (ICoDT2), Islamabad, Pakistan, 20–21 May 2021; pp. 1–5. [Google Scholar]
- Kukker, A.; Sharma, R. Modified fuzzy Q learning based classifier for Pneumonia and tuberculosis. IRBM 2021, 42, 369–377. [Google Scholar] [CrossRef]
- Duong, L.T.; Le, N.H.; Tran, T.B.; Ngo, V.M.; Nguyen, P.T. Detection of tuberculosis from chest X-ray images: Boosting the performance with vision transformer and transfer learning. Expert Syst. Appl. 2021, 184, 115519. [Google Scholar] [CrossRef]
- Win, K.Y.; Maneerat, N.; Hamamoto, K.; Sreng, S. Hybrid Learning of Hand-Crafted and Deep-Activated Features Using Particle Swarm Optimization and Optimized Support Vector Machine for Tuberculosis Screening. Appl. Sci. 2020, 10, 5749. [Google Scholar] [CrossRef]
- Hrizi, O.; Gasmi, K.; Ben Ltaifa, I.; Alshammari, H.; Karamti, H.; Krichen, M.; Mahmood, M.A. Tuberculosis disease diagnosis based on an optimized machine learning model. J. Healthc. Eng. 2022, 2022, 8950243. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Usman, M.; Ahmed, Z. An efficient deep learning-based framework for tuberculosis detection using chest X-ray images. Tuberculosis 2022, 136, 102234. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Roychoudhury, R.; Malakar, S.; Sarkar, R. An optimized fuzzy ensemble of convolutional neural networks for detecting tuberculosis from Chest X-ray images. Appl. Soft Comput. 2022, 114, 108094. [Google Scholar] [CrossRef]
- Yi, P.H.; Kim, T.K.; Lin, C.T. Comparison of radiologist versus natural language processing-based image annotations for deep learning system for tuberculosis screening on chest radiographs. Clin. Imaging 2022, 87, 34–37. [Google Scholar] [CrossRef]
- Tulo, S.K.; Ramu, P.; Swaminathan, R. Evaluation of Diagnostic Value of Mediastinum for Differentiation of Drug Sensitive, Multi and Extensively Drug Resistant Tuberculosis Using Chest X-rays. IRBM 2022, 43, 658–669. [Google Scholar] [CrossRef]
- Liu, J.; Qi, J.; Chen, W.; Nian, Y. Multi-branch fusion auxiliary learning for the detection of pneumonia from chest X-ray images. Comput. Biol. Med. 2022, 147, 105732. [Google Scholar] [CrossRef]
- Alshmrani, G.M.M.; Ni, Q.; Jiang, R.; Pervaiz, H.; Elshennawy, N.M. A deep learning architecture for multi-class lung diseases classification using chest X-ray (CXR) images. Alex. Eng. J. 2022, 64, 923–935. [Google Scholar] [CrossRef]
- Bhandari, M.; Shahi, T.B.; Siku, B.; Neupane, A. Explanatory classification of CXR images into COVID-19, Pneumonia and Tuberculosis using deep learning and XAI. Comput. Biol. Med. 2022, 150, 106156. [Google Scholar] [CrossRef]
- Sourab, S.Y.; Kabir, M.A. A comparison of hybrid deep learning models for pneumonia diagnosis from chest radiograms. Sens. Int. 2022, 3, 100167. [Google Scholar] [CrossRef]
- Kaggle. Chest X-ray Images (Pneumonia). Available online: https://www.kaggle.com/datasets/paultimothymooney/chest-xray-pneumonia (accessed on 16 July 2022).
- Kaggle. Pulmonary Chest X-ray Abnormalities. Available online: https://www.kaggle.com/datasets/kmader/pulmonary-chest-xray-abnormalities/code?select=Montgomery (accessed on 16 July 2022).
- Kaggle. Tuberculosis (TB) Chest X-ray Database. Available online: https://www.kaggle.com/datasets/tawsifurrahman/tuberculosis-tb-chest-xray-dataset (accessed on 16 July 2022).
- Abunadi, I.; Senan, E.M. Multi-Method Diagnosis of Blood Microscopic Sample for Early Detection of Acute Lymphoblastic Leukemia Based on Deep Learning and Hybrid Techniques. Sensors 2022, 22, 1629. [Google Scholar] [CrossRef] [PubMed]
- Al-Mekhlafi, Z.G.; Senan, E.M.; Rassem, T.H.; Mohammed, B.A.; Makbol, N.M.; Alanazi, A.A.; Ghaleb, F.A. Deep Learning and Machine Learning for Early Detection of Stroke and Haemorrhage. Comput. Mater. Contin. 2022, 72, 775–796. [Google Scholar] [CrossRef]
- Mohammed, B.A.; Senan, E.M.; Rassem, T.H.; Makbol, N.M.; Alanazi, A.A.; Al-Mekhlafi, Z.G.; Almurayziq, T.S.; Ghaleb, F.A. Multi-Method Analysis of Medical Records and MRI Images for Early Diagnosis of Dementia and Alzheimer’s Disease Based on Deep Learning and Hybrid Methods. Electronics 2021, 10, 2860. [Google Scholar] [CrossRef]
- Khan, Z.A.; Hussain, T.; Ullah, F.U.M.; Gupta, S.K.; Lee, M.Y.; Baik, S.W. Randomly Initialized CNN with Densely Connected Stacked Autoencoder for Efficient Fire Detection. Eng. Appl. Artif. Intell. 2022, 116, 105403. [Google Scholar] [CrossRef]
- Fati, S.M.; Senan, E.M.; Azar, A.T. Hybrid and Deep Learning Approach for Early Diagnosis of Lower Gastrointestinal Diseases. Sensors 2022, 22, 4079. [Google Scholar] [CrossRef]
- Pintelas, E.; Pintelas, P. A 3D-CAE-CNN model for Deep Representation Learning of 3D images. Eng. Appl. Artif. Intell. 2022, 113, 104978. [Google Scholar] [CrossRef]
- Mehta, T.; Mehendale, N. Classification of X-ray images into COVID-19, pneumonia, and TB using cGAN and fine-tuned deep transfer learning models. Res. Biomed. Eng. 2021, 37, 803–813. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Senan, E.M.; Rassem, T.H.; Ali, M.A.; Shatnawi, H.S.A.; Alwazer, S.M.; Alshahrani, M. Eye Tracking-Based Diagnosis and Early Detection of Autism Spectrum Disorder Using Machine Learning and Deep Learning Techniques. Electronics 2022, 11, 530. [Google Scholar] [CrossRef]
- Senan, E.M.; Jadhav, M.E.; Kadam, A. Classification of PH2 images for early detection of skin diseases. In Proceedings of the 2021 6th International Conference for Convergence in Technology (I2CT), Maharashtra, India, 2–4 April 2021; pp. 1–7. [Google Scholar] [CrossRef]
- Ma, J.; Xia, D.; Wang, Y.; Niu, X.; Jiang, S.; Liu, Z.; Guo, H. A comprehensive comparison among metaheuristics (MHs) for geohazard modeling using machine learning: Insights from a case study of landslide displacement prediction. Eng. Appl. Artif. Intell. 2022, 114, 105150. [Google Scholar] [CrossRef]
- Liang, P.; Wang, W.; Yuan, X.; Liu, S.; Zhang, L.; Cheng, Y. Intelligent fault diagnosis of rolling bearing based on wavelet transform and improved ResNet under noisy labels and environment. Eng. Appl. Artif. Intell. 2022, 115, 105269. [Google Scholar] [CrossRef]
- Gayathri, J.L.; Abraham, B.; Sujarani, M.S.; Nair, M.S. A computer-aided diagnosis system for the classification of COVID-19 and non-COVID-19 pneumonia on chest X-ray images by integrating CNN with sparse autoencoder and feed forward neural network. Comput. Biol. Med. 2022, 141, 105134. [Google Scholar] [CrossRef]
- Steiniger, Y.; Kraus, D.; Meisen, T. Survey on deep learning based computer vision for sonar imagery. Eng. Appl. Artif. Intell. 2022, 114, 105157. [Google Scholar] [CrossRef]
- Alshudukhi, J.; Aljaloud, S.; Alharbi, T.S.; Abebaw, S. Convolutional Neural Network Architectures to Solve a Problem of Tuberculosis Classification Using X-ray Images of the Lungs. J. Nanomater. 2022, 2022, 2509830. [Google Scholar] [CrossRef]
- Fati, S.M.; Senan, E.M.; ElHakim, N. Deep and Hybrid Learning Technique for Early Detection of Tuberculosis Based on X-ray Images Using Feature Fusion. Appl. Sci. 2022, 12, 7092. [Google Scholar] [CrossRef]
- Senan, E.M.; Jadhav, M.E. Techniques for the Detection of Skin Lesions in PH 2 Dermoscopy Images Using Local Binary Pattern (LBP). In International Conference on Recent Trends in Image Processing and Pattern Recognition; Springer: Singapore, 2020; pp. 14–25. [Google Scholar] [CrossRef]
- Mohan, R.; Kadry, S.; Rajinikanth, V.; Majumdar, A.; Thinnukool, O. Automatic Detection of Tuberculosis Using VGG19 with Seagull-Algorithm. Life 2022, 12, 1848. [Google Scholar] [CrossRef]
- Senan, E.M.; Jadhav, M.E. Diagnosis of dermoscopy images for the detection of skin lesions using SVM and KNN. In Proceedings of the Third International Conference on Sustainable Computing, Jaipur, India, 19–20 March 2022; pp. 125–134. [Google Scholar] [CrossRef]
- Senan, E.M.; Abunadi, I.; Jadhav, M.E.; Fati, S.M. Score and Correlation Coefficient-Based Feature Selection for Predicting Heart Failure Diagnosis by Using Machine Learning Algorithms. Comput. Math. Methods Med. 2021, 2021, 8500314. [Google Scholar] [CrossRef]
- Senan, E.M.; Jadhav, M.E.; Rassem, T.H.; Aljaloud, A.S.; Mohammed, B.A.; Al-Mekhlafi, Z.G. Early Diagnosis of Brain Tumour MRI Images Using Hybrid Techniques between Deep and Machine Learning. Comput. Math. Methods Med. 2022, 2022, 8330833. [Google Scholar] [CrossRef]















| Phase | 80:20 | Testing Phases 20% | |
|---|---|---|---|
| Classes | Training (80%) | Validation (20%) | |
| Pneumonia | 2734 | 684 | 855 |
| Tuberculosis | 663 | 166 | 207 |
| Normal | 1013 | 253 | 317 |
| Phase | Training Phase | ||
|---|---|---|---|
| Classes | Pneumonia | Tuberculosis | Normal |
| Before augmentation | 2734 | 663 | 1013 |
| After augmentation | 5468 | 5304 | 5065 |
| Techniques | Type of Class | Accuracy % | Sensitivity % | Specificity % | Precision % | AUC % |
|---|---|---|---|---|---|---|
| SVM with features of VGG16 | Normal | 98.1 | 98.12 | 98.87 | 96 | 97.39 |
| Pneumonia | 98.6 | 99.24 | 98.14 | 98.9 | 98.61 | |
| Tuberculosis | 91.8 | 94.36 | 99.28 | 93.6 | 95.49 | |
| average ratio | 97.50 | 97.24 | 98.76 | 96.17 | 97.16 | |
| SVM with features of ResNet18 | Normal | 98.10 | 97.84 | 99.41 | 96.00 | 97.30 |
| Pneumonia | 98.10 | 97.54 | 97.35 | 98.10 | 97.89 | |
| Tuberculosis | 88.40 | 88.37 | 98.94 | 91.50 | 94.53 | |
| average ratio | 96.70 | 94.58 | 98.57 | 95.20 | 96.57 |
| Techniques | Type of Class | Accuracy % | Sensitivity % | Specificity % | Precision % | AUC % |
|---|---|---|---|---|---|---|
| ANN based on fusion CNN before PCA | Normal | 98.7 | 99.29 | 99.37 | 97.2 | 98.34 |
| Pneumonia | 99.3 | 98.86 | 99.1 | 99.6 | 98.97 | |
| Tuberculosis | 95.2 | 95.28 | 99.26 | 96.1 | 97.76 | |
| average ratio | 98.50 | 97.81 | 99.24 | 97.63 | 98.36 | |
| ANN based on fusion CNN after PCA | Normal | 96.50 | 96.58 | 99.30 | 98.10 | 98.82 |
| Pneumonia | 99.40 | 98.67 | 96.74 | 98.40 | 99.25 | |
| Tuberculosis | 92.80 | 93.41 | 99.16 | 94.60 | 96.71 | |
| average ratio | 97.80 | 96.22 | 98.40 | 97.03 | 98.26 |
| Techniques | Type of Class | Accuracy % | Sensitivity % | Specificity % | Precision % | AUC % |
|---|---|---|---|---|---|---|
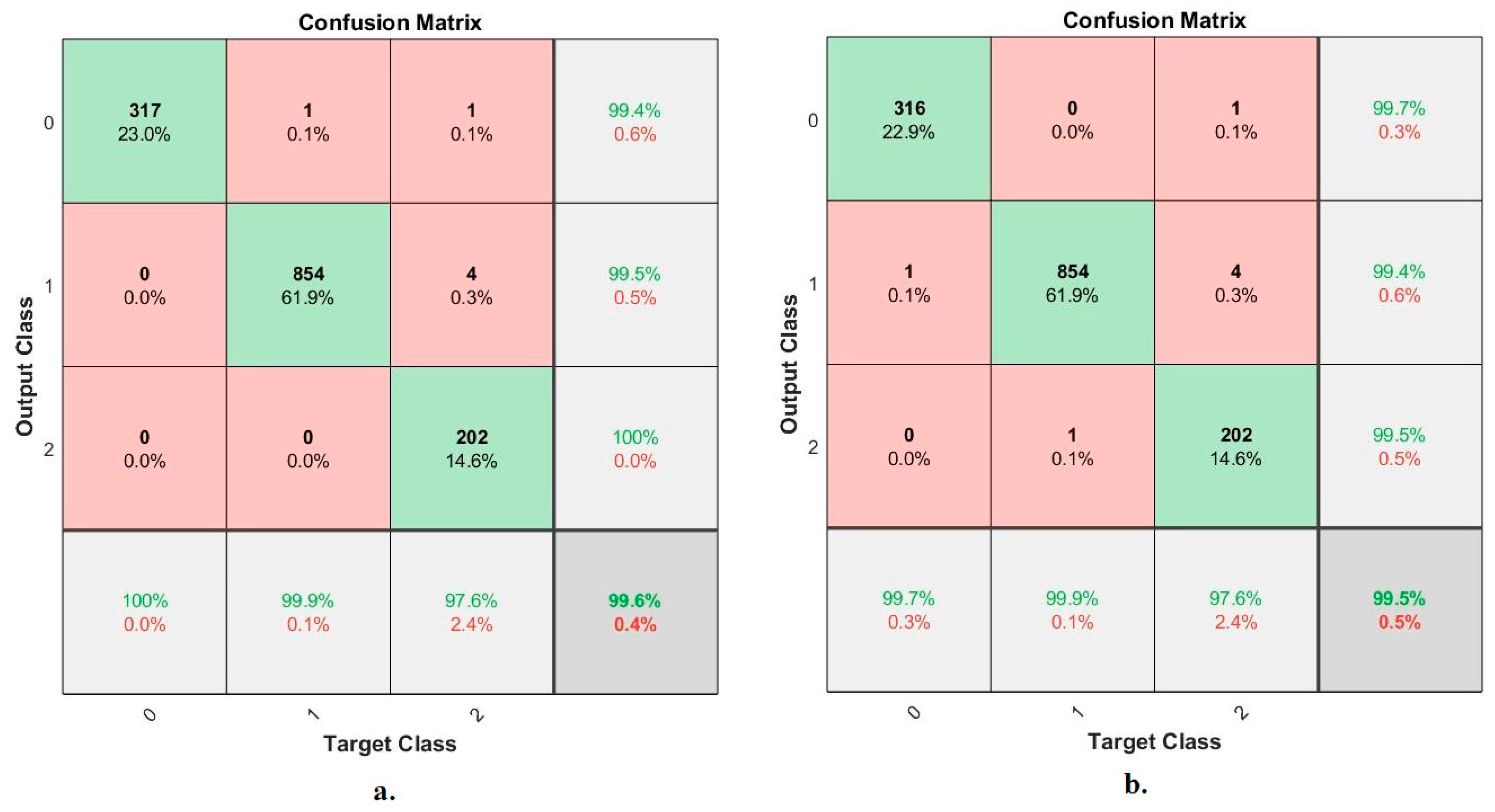
| VGG16, LBP, DWT and GLCM | Normal | 100 | 99.80 | 99.56 | 99.40 | 99.64 |
| Pneumonia | 99.90 | 99.60 | 99.10 | 99.50 | 99.25 | |
| Tuberculosis | 97.60 | 98.10 | 99.60 | 100 | 99.84 | |
| average ratio | 99.60 | 99.17 | 99.42 | 99.63 | 99.58 | |
| ResNet18, LBP, DWT and GLCM | Normal | 99.70 | 99.50 | 100 | 99.70 | 99.54 |
| Pneumonia | 99.90 | 99.75 | 99.10 | 99.40 | 99.76 | |
| Tuberculosis | 97.60 | 98.22 | 100 | 99.50 | 99.48 | |
| average ratio | 99.50 | 99.16 | 99.70 | 99.53 | 99.59 |
| Techniques | Classes | Pneumonia | Tuberculosis | Normal | Accuracy % | |
|---|---|---|---|---|---|---|
| Hybrid method | VGG16 + SVM | 98.6 | 91.8 | 98.1 | 97.5 | |
| ResNet18 + SVM | 98.1 | 88.4 | 98.1 | 96.7 | ||
| Incorporating features before PCA | VGG16 + ResNet18 | 98.7 | 99.3 | 95.2 | 98.5 | |
| Incorporating features after PCA | VGG16 + ResNet18 | 96.5 | 99.4 | 92.8 | 97.8 | |
| Fusion features | ANN classifier | VGG16 and LDG | 100 | 99.9 | 97.6 | 99.6 |
| ResNet18 and LDG | 99.7 | 99.9 | 97.6 | 99.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.A.; Senan, E.M.; Shatnawi, H.S.A.; Alkhraisha, Z.M.; Al-Azzam, M.M.A. Multi-Techniques for Analyzing X-ray Images for Early Detection and Differentiation of Pneumonia and Tuberculosis Based on Hybrid Features. Diagnostics 2023, 13, 814. https://doi.org/10.3390/diagnostics13040814
Ahmed IA, Senan EM, Shatnawi HSA, Alkhraisha ZM, Al-Azzam MMA. Multi-Techniques for Analyzing X-ray Images for Early Detection and Differentiation of Pneumonia and Tuberculosis Based on Hybrid Features. Diagnostics. 2023; 13(4):814. https://doi.org/10.3390/diagnostics13040814
Chicago/Turabian StyleAhmed, Ibrahim Abdulrab, Ebrahim Mohammed Senan, Hamzeh Salameh Ahmad Shatnawi, Ziad Mohammad Alkhraisha, and Mamoun Mohammad Ali Al-Azzam. 2023. "Multi-Techniques for Analyzing X-ray Images for Early Detection and Differentiation of Pneumonia and Tuberculosis Based on Hybrid Features" Diagnostics 13, no. 4: 814. https://doi.org/10.3390/diagnostics13040814
APA StyleAhmed, I. A., Senan, E. M., Shatnawi, H. S. A., Alkhraisha, Z. M., & Al-Azzam, M. M. A. (2023). Multi-Techniques for Analyzing X-ray Images for Early Detection and Differentiation of Pneumonia and Tuberculosis Based on Hybrid Features. Diagnostics, 13(4), 814. https://doi.org/10.3390/diagnostics13040814







