Evaluating Cardiac Lateralization by MRI to Simplify Estimation of Cardiopulmonary Impairment in Pectus Excavatum
Abstract
:1. Introduction
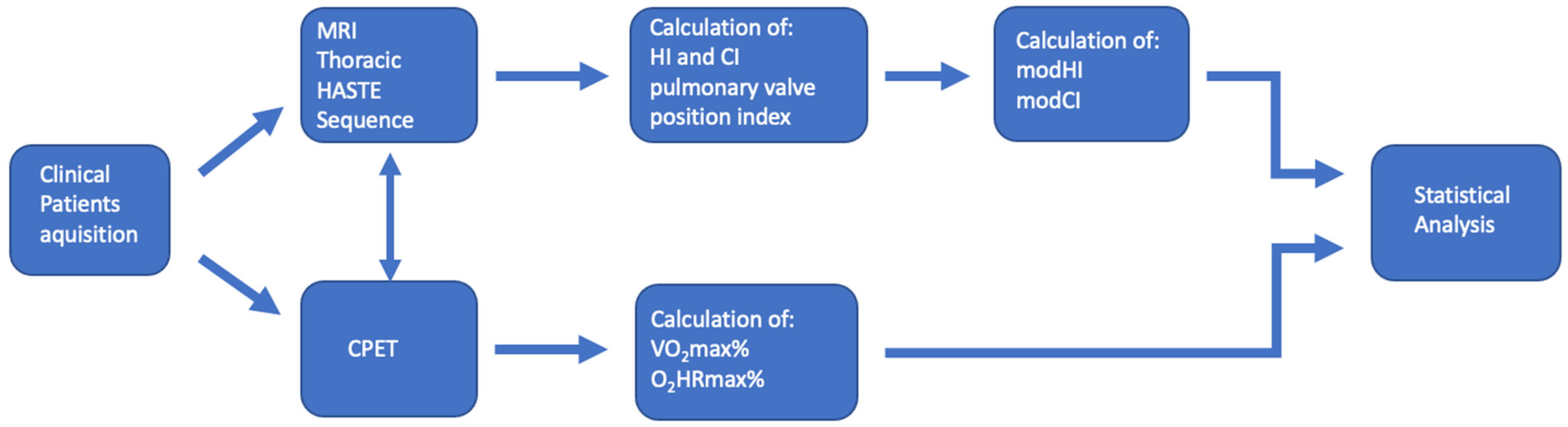
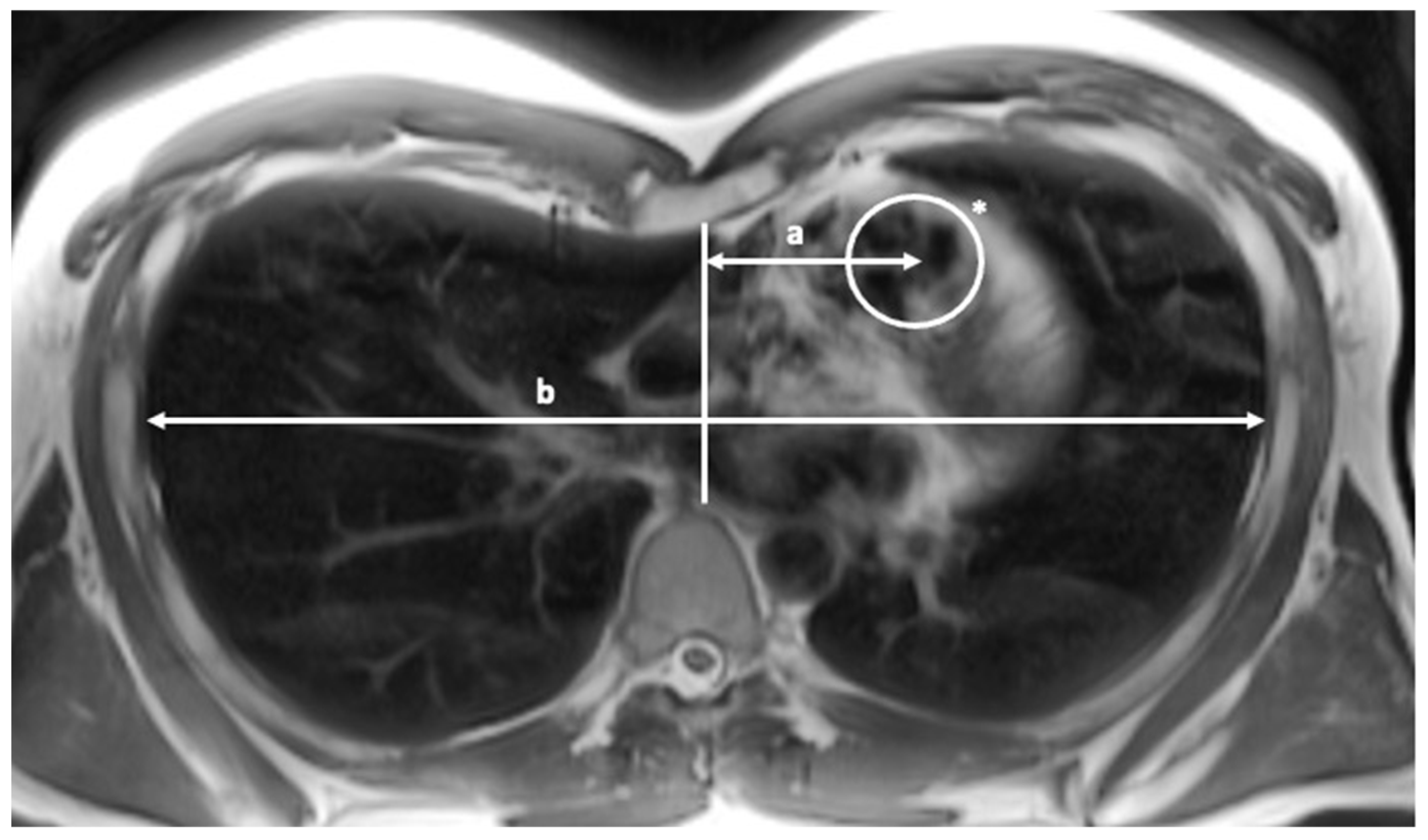
2. Materials and Methods
2.1. Patients
2.2. CPET Procedure
2.3. Statistics
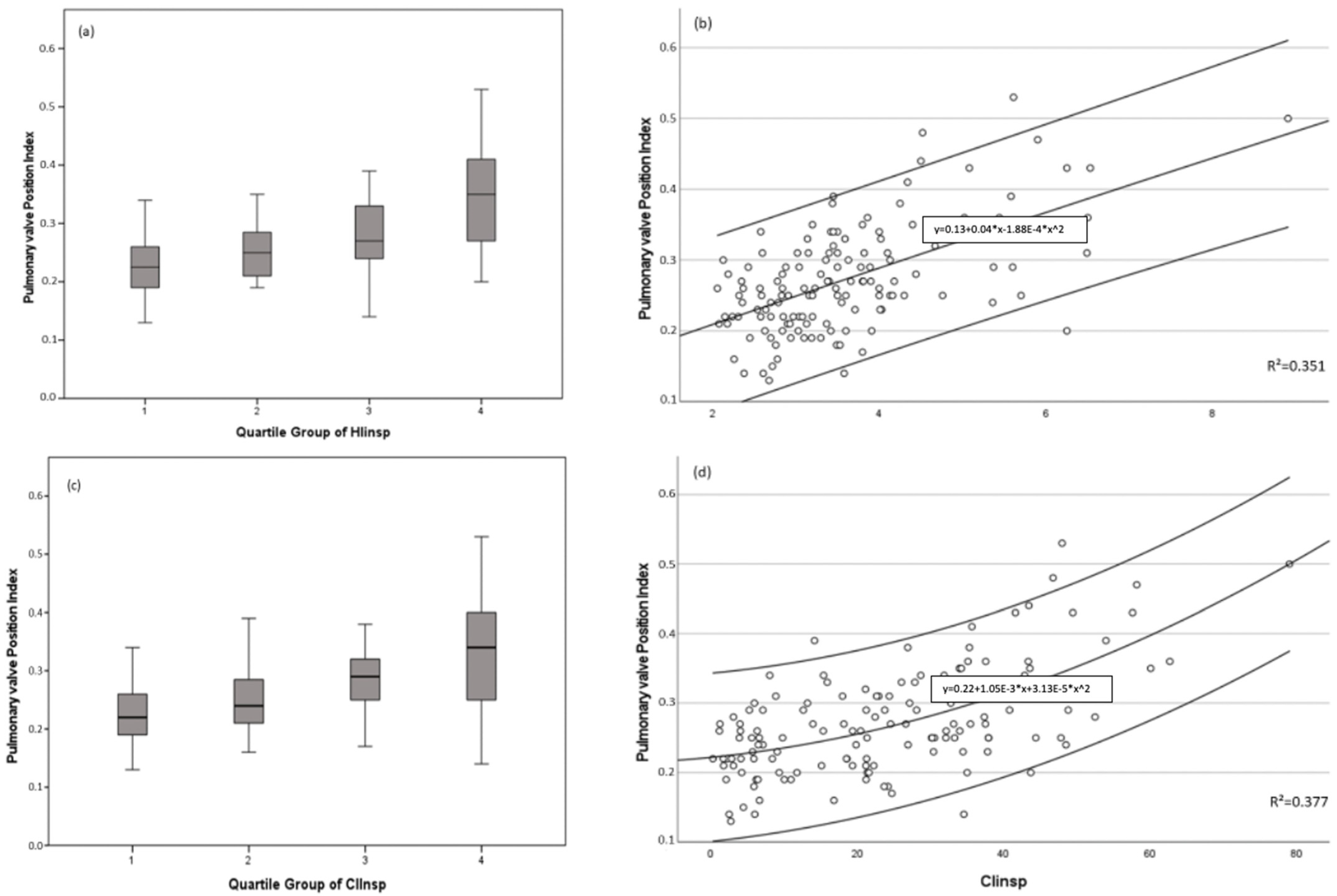
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Correction Index |
| CIInsp | End-inspiratory Correction Index |
| CPET | Cardiopulmonary exercise testing |
| HI | Haller Index |
| HIinsp | End-inspiratory Haller Index |
| MRI | Magnetic resonance imaging |
| mod CI | Modified Correction Index |
| mod HI | Modified Haller Index |
| NPV | Negative predictive value |
| O2-pulsemax% | Percent of predicted oxygen pulse (ml/beat) |
| PE | Pectus excavatum |
| PPV | Positive predictive value |
| PVRatio | Pulmonary valve position index |
| ROC | Receiver operating characteristics |
| VO2max% | Percent of predicted oxygen uptake (%) |
References
- Neviere, R.; Montaigne, D.; Benhamed, L.; Catto, M.; Edme, J.L.; Matran, R.; Wurtz, A. Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. Eur. J. Cardiothorac. Surg. 2011, 40, e77–e82. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.E., Jr. Pectus excavatum: Historical background, clinical picture, preoperative evaluation and criteria for operation. Semin. Pediatr. Surg. 2008, 17, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.; Wilde, J.; Tabard-Fougere, A.; Toso, S.; Beghetti, M.; Vallee, J.P.; Corbelli, R.; Barazzone-Argiroffo, C.; Lascombes, P.; Ruchonnet-Metrailler, I. Cardiopulmonary function in adolescent patients with pectus excavatum or carinatum. BMJ Open Respir. Res. 2021, 8, e001020. [Google Scholar] [CrossRef] [PubMed]
- Wachter, T.; Del Frari, B.; Edlinger, M.; Morandi, E.M.; Mayerl, C.; Verstappen, R.; Celep, E.; Djedovic, G.; Kinzl, J.; Schwabegger, A.H.; et al. Aesthetic outcomes after surgical repair of pectus excavatum in females: Differences between patients and professional evaluators. Arch. Plast. Surg. 2020, 47, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuss, D.; Kelly, R.E., Jr. Indications and technique of Nuss procedure for pectus excavatum. Thorac. Surg. Clin. 2010, 20, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewski, D.E. Physiologic implications of pectus excavatum. J. Thorac. Cardiovasc. Surg. 2017, 153, 218–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.J.; Jaroszewski, D.E.; Kumar, P.N.; Ewais, M.M.; Appleton, C.P.; Mookadam, F.; Gotway, M.B.; Naqvi, T.Z. Surgical repair of pectus excavatum relieves right heart chamber compression and improves cardiac output in adult patients—An intraoperative transesophageal echocardiographic study. Am. J. Surg. 2015, 210, P1118–P1125. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, R.; Badano, L.; Lestuzzi, C.; Nicolosi, G.L.; Zanuttini, D. Relation of right ventricular morphology and function in pectus excavatum to the severity of the chest wall deformity. Am. J. Cardiol. 1995, 76, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Zens, T.J.; Casar Berazaluce, A.M.; Jenkins, T.M.; Hardie, W.; Alsaied, T.; Tretter, J.T.; Moore, R.; Foster, K.; Fleck, R.J.; Hanke, R.E.; et al. The Severity of Pectus Excavatum Defect Is Associated With Impaired Cardiopulmonary Function. Ann. Thorac. Surg. 2022, 114, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.S.; Finn, J.P.; Fenchel, M.; Moghadam, A.N.; Krishnam, M.; Abrazado, M.; Ton, A.; Habibi, R.; Fonkalsrud, E.W.; Cooper, C.B. Cardiovascular magnetic resonance in patients with pectus excavatum compared with normal controls. J. Cardiovasc. Magn. Reson. 2010, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Abu-Tair, T.; Turial, S.; Hess, M.; Wiethoff, C.M.; Staatz, G.; Lollert, A.; Kampmann, C. Impact of Pectus Excavatum on Cardiopulmonary Function. Ann. Thorac. Surg. 2018, 105, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Casar Berazaluce, A.M.; Jenkins, T.M.; Garrison, A.P.; Hardie, W.D.; Foster, K.E.; Alsaied, T.; Tretter, J.; Moore, R.A.; Fleck, R.J.; Garcia, V.F.; et al. The chest wall gender divide: Females have better cardiopulmonary function and exercise tolerance despite worse deformity in pectus excavatum. Pediatr. Surg. Int. 2020, 36, 1281–1286. [Google Scholar] [CrossRef]
- Wooler, G.H.; Mashhour, Y.A.; Garcia, J.B.; Holden, M.P.; Ionescu, M.I. Pectus excavatum. Thorax 1969, 24, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.E.; Park, J.B.; Kang, C.H.; Park, S.; Kim, E.H.; Lee, J.H.; Kim, H.S.; Kim, J.T. Cardiopulmonary resuscitation in pediatric pectus excavatum patients-Where is the heart? Paediatr. Anesth. 2020, 30, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Lollert, A.; Emrich, T.; Eichstadt, J.; Kampmann, C.; Abu-Tair, T.; Turial, S.; Duber, C.; Kreitner, K.F.; Staatz, G. Differences in myocardial strain between pectus excavatum patients and healthy subjects assessed by cardiac MRI: A pilot study. Eur. Radiol. 2018, 28, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Deviggiano, A.; Carrascosa, P.; Vallejos, J.; Bellia-Munzon, G.; Vina, N.; Rodriguez-Granillo, G.A.; Martinez-Ferro, M. Relationship between cardiac MR compression classification and CT chest wall indexes in patients with pectus excavatum. J. Pediatr. Surg. 2018, 53, 2294–2298. [Google Scholar] [CrossRef]
- Bellia-Munzon, G.; Sanjurjo, D.; Toselli, L.; Vallee, M.; Elmo, G.; Martinez-Ferro, M. Novel index to estimate the cephalocaudal extent of the excavation in pectus excavatum: The titanic index. J. Pediatr. Surg. 2022, in press. [Google Scholar] [CrossRef]
- Fagelman, K.M.; Methratta, S.; Cilley, R.E.; Wilson, M.Z.; Hollenbeak, C.S. The Depression Index: An objective measure of the severity of pectus excavatum based on vertebral diameter, a morphometric correlate to patient size. J. Pediatr. Surg. 2015, 50, 1130–1133. [Google Scholar] [CrossRef]
- Capunay, C.; Martinez-Ferro, M.; Carrascosa, P.; Bellia-Munzon, G.; Deviggiano, A.; Nazar, M.; Martinez, J.L.; Rodriguez-Granillo, G.A. Sternal torsion in pectus excavatum is related to cardiac compression and chest malformation indexes. J. Pediatr. Surg. 2020, 55, 619–624. [Google Scholar] [CrossRef]
- Martinez-Ferro, M. Chest Wall Deformities and Corrective Procedures; Springer: Berlin/Heidelberg, Germany, 2016; pp. 35–60. [Google Scholar]
- St Peter, S.D.; Juang, D.; Garey, C.L.; Laituri, C.A.; Ostlie, D.J.; Sharp, R.J.; Snyder, C.L. A novel measure for pectus excavatum: The correction index. J. Pediatr. Surg. 2011, 46, 2270–2273. [Google Scholar] [CrossRef]
- Sujka, J.A.; St Peter, S.D. Quantification of pectus excavatum: Anatomic indices. Semin. Pediatr. Surg. 2018, 27, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, G.A.; Raggio, I.M.; Deviggiano, A.; Bellia-Munzon, G.; Capunay, C.; Nazar, M.; Martinez, J.L.; Carrascosa, P.; Martinez-Ferro, M. Impact of pectus excavatum on cardiac morphology and function according to the site of maximum compression: Effect of physical exertion and respiratory cycle. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, K.Y.; Park, H.J.; Kim, H.Y.; Kang, E.Y.; Oh, Y.W.; Seo, B.K.; Je, B.K.; Choi, E.J. Development of New Cardiac Deformity Indexes for Pectus Excavatum on Computed Tomography: Feasibility for Pre- and Post-Operative Evaluation. Yonsei Med. J. 2009, 50, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, M.; Ugander, M.; Heiberg, E.; Arheden, H. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H636–H644. [Google Scholar] [CrossRef]
- Sheehan, F.; Redington, A. The right ventricle: Anatomy, physiology and clinical imaging. Heart 2008, 94, 1510–1515. [Google Scholar] [CrossRef]
- Maagaard, M.; Heiberg, J. Improved cardiac function and exercise capacity following correction of pectus excavatum: A review of current literature. Ann. Cardiothorac. Surg. 2016, 5, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Oleksak, F.; Spakova, B.; Durdikova, A.; Durdik, P.; Kralova, T.; Igaz, M.; Molnar, M.; Gura, M.; Murgas, D. Correlation of anthropometric index and cardiopulmonary exercise testing in children with pectus excavatum. Respir. Physiol. Neurobiol. 2022, 296, 103790. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Lombardo, M.; Anza, C.; Ambrosio, G. Reduced Myocardial Strain Parameters in Subjects With Pectus Excavatum: Impaired Myocardial Function or Methodological Limitations Due to Chest Deformity? Semin. Thorac. Cardiovasc. Surg. 2021, 33, 251–262. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Braga, M.; Villa, M.C.; Migliori, C.; Lombardo, M. Does chest wall conformation influence myocardial strain parameters in infants with pectus excavatum? J. Clin. Ultrasound 2021, 49, 918–928. [Google Scholar] [CrossRef]
- Kroidl, R.; Schwarz, S.; Lehnigk, B.; Fritsch, J. Kursbuch Spiroergometrie; Thieme: New York, NY, USA, 2014; Volume 3. [Google Scholar]
- Athira, S.B.; Pal, P.; Nair, P.P.; Nanda, N.; Aghoram, R. Cardiovascular autonomic function and baroreflex sensitivity in drug-resistant temporal lobe epilepsy. Epilepsy Behav. 2023, 138, 109013. [Google Scholar] [CrossRef] [PubMed]
- Danilin, L.K.; Spindler, M.; Soros, P.; Bantel, C. Heart rate and heart rate variability in patients with chronic inflammatory joint disease: The role of pain duration and the insular cortex. BMC Musculoskelet. Disord. 2022, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, E.E.; Mikheev, A.; Brinkmann, I.M.; Gilani, N.; Babb, J.S.; Basukala, D.; Benkert, T.; Veraart, J.; Chandarana, H. Cardiac Phase and Flow Compensation Effects on REnal Flow and Microstructure AnisotroPy MRI in Healthy Human Kidney. J. Magn. Reson. Imaging 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Birkemeier, K.L.; Podberesky, D.J.; Salisbury, S.; Serai, S. Breathe in … breathe out … stop breathing: Does phase of respiration affect the Haller index in patients with pectus excavatum? AJR Am. J. Roentgenol. 2011, 197, W934–W939. [Google Scholar] [CrossRef] [PubMed]
- Lollert, A.; Funk, J.; Tietze, N.; Turial, S.; Laudemann, K.; Duber, C.; Staatz, G. Morphologic assessment of thoracic deformities for the preoperative evaluation of pectus excavatum by magnetic resonance imaging. Eur. Radiol. 2015, 25, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef]
- Mirmozaffari, M.; Shadkam, E.; Khalili, S.M.; Yazdani, M. Developing a Novel Integrated Generalised Data Envelopment Analysis (DEA) to Evaluate Hospitals Providing Stroke Care Services. Bioengineering 2021, 8, 207. [Google Scholar] [CrossRef]
- Mirmozaffari, M.; Yazdani, R.; Shadkam, E.; Khalili, S.; Mahjoob, M.; Boskabadi, A. An integrated artificial intelligence model for efficiency assessment in pharmaceutical companies during the COVID-19 pandemic. Sustain. Oper. Comput. 2022, 3, 156–167. [Google Scholar] [CrossRef]
- Mirmozaffari, M.; Yazdani, R.; Shadkam, E.; Khalili, S.M.; Tavassoli, L.S.; Boskabadi, A. A Novel Hybrid Parametric and Non-Parametric Optimisation Model for Average Technical Efficiency Assessment in Public Hospitals during and Post-COVID-19 Pandemic. Bioengineering 2021, 9, 7. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Holland, S.K.; Jacola, L.M.; Lindsell, C.; Privitera, M.D.; Szaflarski, M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008, 12, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.M. Neural correlates of heart-focused interoception: A functional magnetic resonance imaging meta-analysis. Philos. Trans. R. Soc. B 2016, 371, 20160018. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.; Asrani, P.; Lee, S.; Frush, D.; Han, B.K.; Chelliah, A.; Farooqi, K.M. Cardiovascular computed tomography in pediatric congenital heart disease: A state of the art review. J. Cardiovasc. Comput. Tomogr. 2022, 16, 467–482. [Google Scholar] [CrossRef] [PubMed]


| Biometric/Morphometric Data | Pectus Excavatum | Control | p | |
|---|---|---|---|---|
| N = 113 | N = 36 | |||
| Age (years) | x ± SD | 19.03 ± 7.8 | 19.06 ± 2.35 | 0.893 |
| Median | [16.03] | [18.5] | ||
| Range | (9.80–44.5) | (16.2–26.4) | ||
| Indexed position of pulmonary valve | x ± SD | 0.29 ± 0.08 | 0.224 ± 0.05 | <0.001 |
| Median | [0.28] | [0.22] | ||
| Range | (0.14–0.53) | (0.13–0.34) | ||
| HIins | x ± SD | 3.97 ± 1.11 | 2.57 ± 0.29 | <0.001 |
| Median | [3.63] | [2.62] | ||
| Range | (2.26–8.9) | (2.06–3.08) | ||
| modHI | x ± SD | 14.15 ± 3.91 | 12.15 ± 3.38 | 0.007 |
| Median | [13.82] | [11.35] | ||
| Range | (8.3–31.25) | (7.06–20.49) | ||
| CIins (%) | x ± SD | 30.62 ± 13.39 | 4.87 ± 2.47 | <0.001 |
| Median | [30.23] | [4.91] | ||
| Range | (6.67–78.98) | (0.34–9.85) | ||
| modCI | x ± SD | 107.07 ± 42.06 | 22.64 ± 11.94 | <0.001 |
| Median | [101.49] | [21.39] | ||
| Range | (25.11–244.27) | (1.54–47.66) | ||
| VO2max% | O2-pulsemax% | |||
|---|---|---|---|---|
| Cut-Off | modHI 13 | modCI 100 | modHI 16 | modCI 120 |
| PPV | 32% | 33% | 46% | 46% |
| NPV | 91% | 90% | 85% | 89% |
| Sensitivity | 82% | 77% | 54% | 71% |
| Specificity | 52% | 57% | 81% | 74% |
| χ2 | 7.919 | 8.068 | 10.986 | 15.862 |
| p | 0.005 | 0.005 | 0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Tair, T.; Turial, S.; Willershausen, I.; Alkassar, M.; Staatz, G.; Kampmann, C. Evaluating Cardiac Lateralization by MRI to Simplify Estimation of Cardiopulmonary Impairment in Pectus Excavatum. Diagnostics 2023, 13, 844. https://doi.org/10.3390/diagnostics13050844
Abu-Tair T, Turial S, Willershausen I, Alkassar M, Staatz G, Kampmann C. Evaluating Cardiac Lateralization by MRI to Simplify Estimation of Cardiopulmonary Impairment in Pectus Excavatum. Diagnostics. 2023; 13(5):844. https://doi.org/10.3390/diagnostics13050844
Chicago/Turabian StyleAbu-Tair, Tariq, Salmai Turial, Ines Willershausen, Muhannad Alkassar, Gundula Staatz, and Christoph Kampmann. 2023. "Evaluating Cardiac Lateralization by MRI to Simplify Estimation of Cardiopulmonary Impairment in Pectus Excavatum" Diagnostics 13, no. 5: 844. https://doi.org/10.3390/diagnostics13050844





