MRI Radiomics and Predictive Models in Assessing Ischemic Stroke Outcome—A Systematic Review
Abstract
1. Introduction
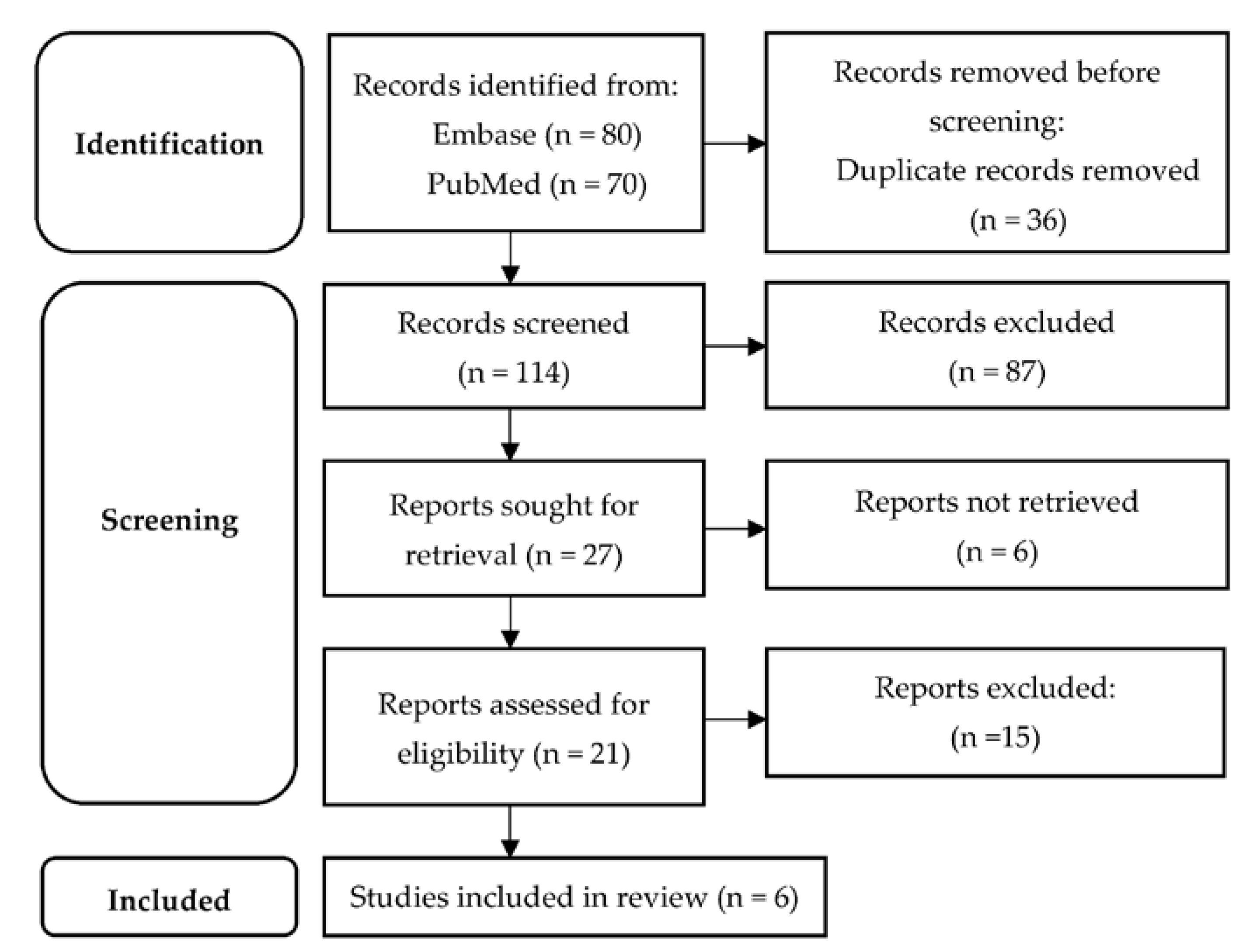
2. Materials and Methods
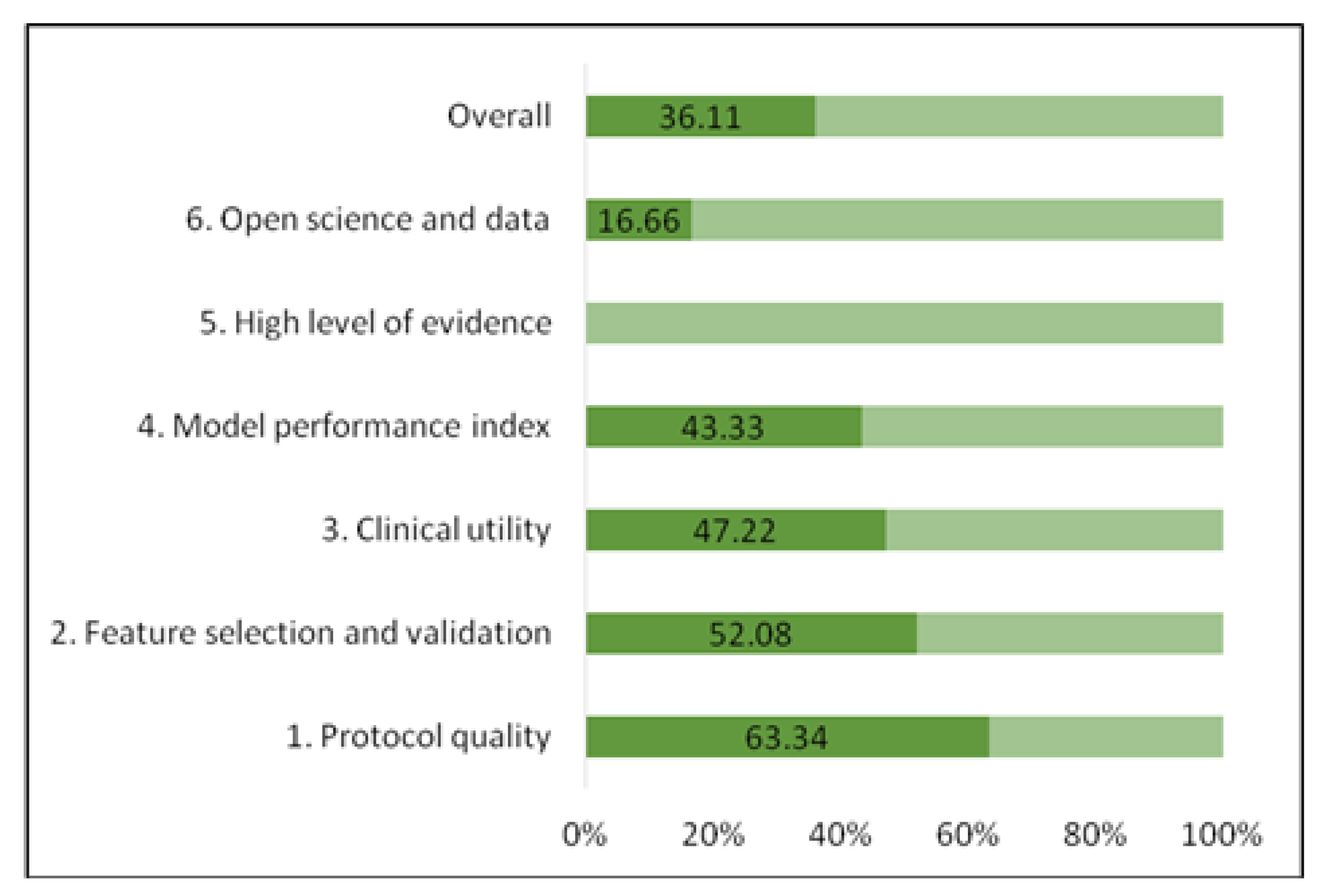
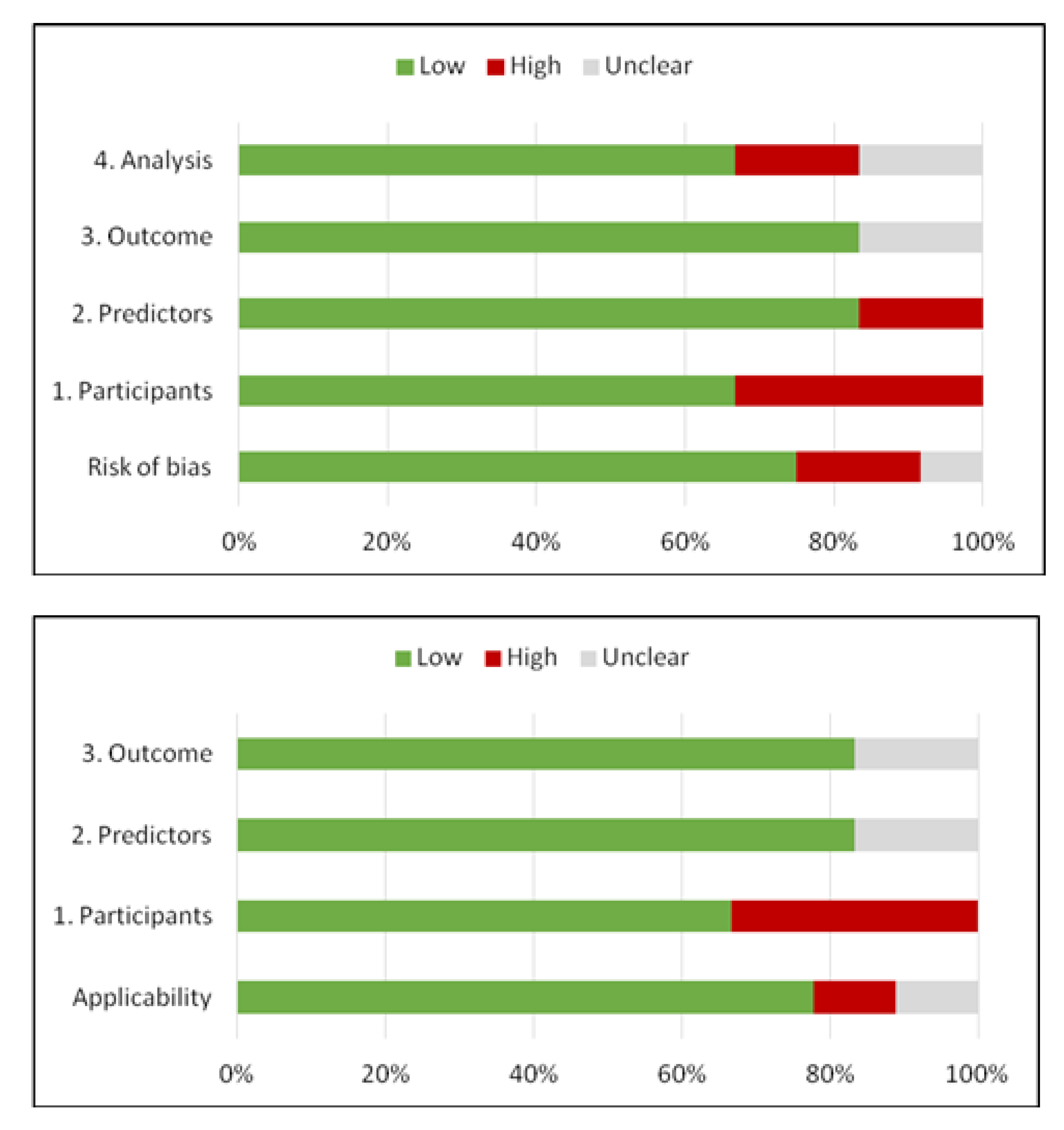
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajsic, S.; Gothe, H.; Borba, H.H.; Sroczynski, G.; Vujicic, J.; Toell, T.; Siebert, U. Economic Burden of Stroke: A Systematic Review on Post-Stroke Care. Eur. J. Health Econ. 2019, 20, 107–134. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Stroke Collaborators. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Fahey, M.; Crayton, E.; Wolfe, C.; Douiri, A. Clinical Prediction Models for Mortality and Functional Outcome Following Ischemic Stroke: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0185402. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Vinogradova, Y.; Robson, J.; May, M.; Brindle, P. Derivation and Validation of QRISK, a New Cardiovascular Disease Risk Score for the United Kingdom: Prospective Open Cohort Study. BMJ 2007, 335, 136. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Paynter, N.P.; Rifai, N.; Gaziano, J.M.; Cook, N.R. C-Reactive Protein and Parental History Improve Global Cardiovascular Risk Prediction: The Reynolds Risk Score for Men. Circulation 2008, 118, 2243–2251. [Google Scholar] [CrossRef]
- Roques, F.; Michel, P.; Goldstone, A.R.; Nashef, S.A.M. The Logistic EuroSCORE. Eur. Heart J. 2003, 24, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Gioulekas, F.; Papavasileiou, V.; Strbian, D.; Michel, P. ASTRAL, DRAGON and SEDAN Scores Predict Stroke Outcome More Accurately than Physicians. Eur. J. Neurol. 2016, 23, 1651–1657. [Google Scholar] [CrossRef]
- Papavasileiou, V.; Milionis, H.; Michel, P.; Makaritsis, K.; Vemmou, A.; Koroboki, E.; Manios, E.; Vemmos, K.; Ntaios, G. ASTRAL Score Predicts 5-Year Dependence and Mortality in Acute Ischemic Stroke. Stroke 2013, 44, 1616–1620. [Google Scholar] [CrossRef]
- Wang, A.; Pednekar, N.; Lehrer, R.; Todo, A.; Sahni, R.; Marks, S.; Stiefel, M.F. DRAGON Score Predicts Functional Outcomes in Acute Ischemic Stroke Patients Receiving Both Intravenous Tissue Plasminogen Activator and Endovascular Therapy. Surg. Neurol. Int. 2017, 8, 149. [Google Scholar] [CrossRef]
- Brüning, T.; Al-Khaled, M. The SEDAN Score and the Risk of Intracerebral Hemorrhage in Monocenter-Study (S25.003). Neurology 2014, 82 (Supple. 10), S25.003. [Google Scholar]
- Chen, Q.; Xia, T.; Zhang, M.; Xia, N.; Liu, J.; Yang, Y. Radiomics in Stroke Neuroimaging: Techniques, Applications, and Challenges. Aging. Dis. 2021, 12, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Franceschi, E.; Vollmuth, P.; Dhermain, F.; Weller, M.; Preusser, M.; Smits, M.; Galldiks, N. Radiomics in Neuro-Oncological Clinical Trials. Lancet Digit. Health 2022, 4, e841–e849. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Fernandes, P.T.; Avelar, W.M.; Santos, S.L.M.; Castellano, G.; Li, L.M. Texture Analysis of Computed Tomography Images of Acute Ischemic Stroke Patients. Braz. J. Med. Biol. Res. 2009, 42, 1076–1079. [Google Scholar] [CrossRef]
- Sikiö, M.; Kölhi, P.; Ryymin, P.; Eskola, H.J.; Dastidar, P. MRI Texture Analysis and Diffusion Tensor Imaging in Chronic Right Hemisphere Ischemic Stroke. J. Neuroimaging 2015, 25, 614–619. [Google Scholar] [CrossRef]
- Ortiz-Ramón, R.; Valdés Hernández, M.D.C.; González-Castro, V.; Makin, S.; Armitage, P.A.; Aribisala, B.S.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M.; Moratal, D. Identification of the Presence of Ischaemic Stroke Lesions by Means of Texture Analysis on Brain Magnetic Resonance Images. Comput. Med. Imaging Graph. 2019, 74, 12–24. [Google Scholar] [CrossRef]
- Kassner, A.; Liu, F.; Thornhill, R.E.; Tomlinson, G.; Mikulis, D.J. Prediction of Hemorrhagic Transformation in Acute Ischemic Stroke Using Texture Analysis of Postcontrast T1-Weighted MR Images. J. Magn. Reson. Imaging 2009, 30, 933–941. [Google Scholar] [CrossRef]
- Qiu, W.; Kuang, H.; Nair, J.; Assis, Z.; Najm, M.; McDougall, C.; McDougall, B.; Chung, K.; Wilson, A.T.; Goyal, M.; et al. Radiomics-Based Intracranial Thrombus Features on CT and CTA Predict Recanalization with Intravenous Alteplase in Patients with Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2019, 40, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Betrouni, N.; Yasmina, M.; Bombois, S.; Pétrault, M.; Dondaine, T.; Lachaud, C.; Laloux, C.; Mendyk, A.-M.; Henon, H.; Bordet, R. Texture Features of Magnetic Resonance Images: An Early Marker of Post-Stroke Cognitive Impairment. Transl. Stroke Res. 2020, 11, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Midya, A.; Chakraborty, J.; Gönen, M.; Do, R.K.G.; Simpson, A.L. Influence of CT Acquisition and Reconstruction Parameters on Radiomic Feature Reproducibility. J. Med. Imaging 2018, 5, 011020. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Ronald, J.; Vernuccio, F.; Nelson, R.C.; Ramirez-Giraldo, J.C.; Solomon, J.; Patel, B.N.; Samei, E.; Marin, D. Reproducibility of CT Radiomic Features within the Same Patient: Influence of Radiation Dose and CT Reconstruction Settings. Radiology 2019, 293, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, R.; Pastor-Juan, M.D.R.; Canales-Vázquez, J.; Castro-García, M.; Villas, M.V.; Mansilla Legorburo, F.; Sabater, S. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology 2018, 288, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mackin, D.; Fave, X.; Zhang, L.; Fried, D.; Yang, J.; Taylor, B.; Rodriguez-Rivera, E.; Dodge, C.; Jones, A.K.; Court, L. Measuring Computed Tomography Scanner Variability of Radiomics Features. Investig. Radiol. 2015, 50, 757–765. [Google Scholar] [CrossRef]
- Ugga, L.; Perillo, T.; Cuocolo, R.; Stanzione, A.; Romeo, V.; Green, R.; Cantoni, V.; Brunetti, A. Meningioma MRI Radiomics and Machine Learning: Systematic Review, Quality Score Assessment, and Meta-Analysis. Neuroradiology 2021, 63, 1293–1304. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Kim, D.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Kim, J.H. A Systematic Review Reporting Quality of Radiomics Research in Neuro-Oncology: Toward Clinical Utility and Quality Improvement Using High-Dimensional Imaging Features. BMC Cancer 2020, 20, 29. [Google Scholar] [CrossRef]
- Brancato, V.; Cerrone, M.; Lavitrano, M.; Salvatore, M.; Cavaliere, C. A Systematic Review of the Current Status and Quality of Radiomics for Glioma Differential Diagnosis. Cancers 2022, 14, 2731. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. For the PROBAST Group† PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.; Ban, R.; Ren, J.-L.; Liu, Y.; Wang, W.; Dai, S.; Yuan, T. FLAIR and ADC Image-Based Radiomics Features as Predictive Biomarkers of Unfavorable Outcome in Patients With Acute Ischemic Stroke. Front. Neurosci. 2021, 15, 1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Ge, Y.; Wu, P.-Y.; Lin, J.; Zhao, J.; Song, B. A Clinical-Radiomics Nomogram for Functional Outcome Predictions in Ischemic Stroke. Neurol. Ther. 2021, 10, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, D.; Yan, S.; Xie, Y.; Zhang, S.; Lv, W.; Qin, Y.; Liu, Y.; Liu, C.; Lu, J.; et al. Feasibility of a Clinical-Radiomics Model to Predict the Outcomes of Acute Ischemic Stroke. Korean. J. Radiol. 2022, 23, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, Y.; Ge, Y.; Wu, P.-Y.; Zhao, J.; Wang, H.; Song, B. MRI Whole-Lesion Texture Analysis on ADC Maps for the Prognostic Assessment of Ischemic Stroke. BMC Med. Imaging 2022, 22, 115. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Zhu, J.; Zhuang, Y.; Song, B. Diffusion-Weighted Imaging-Based Radiomics for Predicting 1-Year Ischemic Stroke Recurrence. Front. Neurol. 2022, 13, 1012896. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Zheng, L.; Zhao, J.; Song, B.; Dai, Y. Texture Analysis Based on ADC Maps and T2-FLAIR Images for the Assessment of the Severity and Prognosis of Ischaemic Stroke. Clin. Imaging 2020, 67, 152–159. [Google Scholar] [CrossRef]
- Qiu, Q.; Duan, J.; Deng, H.; Han, Z.; Gu, J.; Yue, N.J.; Yin, Y. Development and Validation of a Radiomics Nomogram Model for Predicting Postoperative Recurrence in Patients With Esophageal Squamous Cell Cancer Who Achieved PCR After Neoadjuvant Chemoradiotherapy Followed by Surgery. Front. Oncol. 2020, 10, 1398. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nohara, Y.; Soejima, H.; Yonehara, T.; Nakashima, N.; Kamouchi, M. Stroke Prognostic Scores and Data-Driven Prediction of Clinical Outcomes After Acute Ischemic Stroke. Stroke 2020, 51, 1477–1483. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P.; Bruno, A.; Connors, J.J.B.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.J.; Singh, S.; Lees, K.R.; Bath, P.M.; Myint, P.K. Validating and Comparing Stroke Prognosis Scales. Neurology 2017, 89, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Soun, J.E.; Chow, D.S.; Nagamine, M.; Takhtawala, R.S.; Filippi, C.G.; Yu, W.; Chang, P.D. Artificial Intelligence and Acute Stroke Imaging. AJNR Am. J. Neuroradiol. 2021, 42, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-H.; Ng, D.K.S.; Chow, D.H.K. An Image Feature Approach for Computer-Aided Detection of Ischemic Stroke. Comput. Biol. Med. 2011, 41, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Toni, D.; Iweins, F.; von Kummer, R.; Busse, O.; Bogousslavsky, J.; Falcou, A.; Lesaffre, E.; Lenzi, G.L. Identification of Lacunar Infarcts before Thrombolysis in the ECASS I Study. Neurology 2000, 54, 684–688. [Google Scholar] [CrossRef]
- Ospel, J.M.; Volny, O.; Qiu, W.; Najm, M.; Kashani, N.; Goyal, M.; Menon, B.K. Displaying Multiphase CT Angiography Using a Time-Variant Color Map: Practical Considerations and Potential Applications in Patients with Acute Stroke. AJNR Am. J. Neuroradiol. 2020, 41, 200–205. [Google Scholar] [CrossRef]
- Verdolotti, T.; Pilato, F.; Cottonaro, S.; Monelli, E.; Giordano, C.; Guadalupi, P.; Benenati, M.; Ramaglia, A.; Costantini, A.M.; Alexandre, A.; et al. ColorViz, a New and Rapid Tool for Assessing Collateral Circulation during Stroke. Brain Sci. 2020, 10, 882. [Google Scholar] [CrossRef]
- Murray, N.M.; Unberath, M.; Hager, G.D.; Hui, F.K. Artificial Intelligence to Diagnose Ischemic Stroke and Identify Large Vessel Occlusions: A Systematic Review. J. NeuroIntervent. Surg. 2020, 12, 156–164. [Google Scholar] [CrossRef]
- Chung, J.; Kim, Y.; Cha, J.; Choi, E.; Kim, B.M.; Seo, W.; Kim, G.; Bang, O.Y. Characterization of Clot Composition in Acute Cerebral Infarct Using Machine Learning Techniques. Ann. Clin. Transl. Neurol. 2019, 6, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Jeong, H.-G.; Kim, D.Y.; Yang, W.; Lee, S.-H. Prediction of Stroke Subtype and Recanalization Using Susceptibility Vessel Sign on Susceptibility-Weighted Magnetic Resonance Imaging. Stroke 2017, 48, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Y.; Liu, C.; Zhang, M.; Yan, S.; Liebeskind, D.S.; Lou, M. Overestimation of Susceptibility Vessel Sign: A Predictive Marker of Stroke Cause. Stroke 2017, 48, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yoon, W.; Kim, T.S.; Kim, H.S.; Heo, T.W.; Park, M.S. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am. J. Neuroradiol. 2015, 36, 1756–1762. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kondo, T. Time-Dependent Organic Changes of Intravenous Thrombi in Stasis-Induced Deep Vein Thrombosis Model and Its Application to Thrombus Age Determination. Forensic. Sci. Int. 2010, 195, 143–147. [Google Scholar] [CrossRef]
- Thijs, V.N.; Lansberg, M.G.; Beaulieu, C.; Marks, M.P.; Moseley, M.E.; Albers, G.W. Is Early Ischemic Lesion Volume on Diffusion-Weighted Imaging an Independent Predictor of Stroke Outcome? Stroke 2000, 31, 2597–2602. [Google Scholar] [CrossRef]
- Nighoghossian, N.; Hermier, M.; Adeleine, P.; Derex, L.; Dugor, J.F.; Philippeau, F.; Ylmaz, H.; Honnorat, J.; Dardel, P.; Berthezène, Y.; et al. Baseline Magnetic Resonance Imaging Parameters and Stroke Outcome in Patients Treated by Intravenous Tissue Plasminogen Activator. Stroke 2003, 34, 458–463. [Google Scholar] [CrossRef]
- Tanriverdi, Z.; Gocmen, R.; Oguz, K.K.; Topcuoglu, M.A.; Arsava, E.M. Elevations in Tissue Fluid-Attenuated Inversion Recovery Signal Are Related to Good Functional Outcome after Thrombolytic Treatment. J. Stroke Cerebrovasc. Dis. 2016, 25, 480–483. [Google Scholar] [CrossRef]
- Heiss, W.-D.; Kidwell, C.S. Imaging for Prediction of Functional Outcome and Assessment of Recovery in Ischemic Stroke. Stroke 2014, 45, 1195–1201. [Google Scholar] [CrossRef]
- Kranz, P.G.; Eastwood, J.D. Does Diffusion-Weighted Imaging Represent the Ischemic Core? An Evidence-Based Systematic Review. Am. J. Neuroradiol. 2009, 30, 1206–1212. [Google Scholar] [CrossRef]
- Guadagno, J.V.; Warburton, E.A.; Jones, P.S.; Day, D.J.; Aigbirhio, F.I.; Fryer, T.D.; Harding, S.; Price, C.J.; Green, H.A.; Barret, O.; et al. How Affected Is Oxygen Metabolism in DWI Lesions?: A Combined Acute Stroke PET-MR Study. Neurology 2006, 67, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, Y.; He, X.; Xu, Y.; Shu, Z.; Hu, X.; Chen, J.; Jiang, H.; Gong, X. Prediction of Malignant Acute Middle Cerebral Artery Infarction via Computed Tomography Radiomics. Front. Neurosci. 2020, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture Analysis of Medical Images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The Facts and the Challenges of Image Analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Boss, S.M.; Moustafa, R.R.; Moustafa, M.A.; El Sadek, A.; Mostafa, M.M.; Aref, H.M. Lesion Homogeneity on Diffusion-Weighted Imaging Is a Marker of Outcome in Acute Ischemic Stroke. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 59. [Google Scholar] [CrossRef]
- Lovett, J.K.; Coull, A.J.; Rothwell, P.M. Early Risk of Recurrence by Subtype of Ischemic Stroke in Population-Based Incidence Studies. Neurology 2004, 62, 569–573. [Google Scholar] [CrossRef]
- Kolmos, M.; Christoffersen, L.; Kruuse, C. Recurrent Ischemic Stroke—A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2021, 30, 105935. [Google Scholar] [CrossRef]
- Parekh, V.; Jacobs, M.A. Radiomics: A New Application from Established Techniques. Expert. Rev. Precis. Med. Drug. Dev. 2016, 1, 207–226. [Google Scholar] [CrossRef]
- Chen, L.-L.; Wang, W.-T.; Zhang, S.; Liu, H.-M.; Yuan, X.-Y.; Yang, X.; Gu, P. Cohort Study on the Prognosis of Acute Cerebral Infarction in Different Circulatory Systems at 1-Year Follow-Up. BMC Cardiovasc. Disord. 2021, 21, 521. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Oh, C.W.; Bae, H.-J.; Han, M.-K.; Park, H.; Bang, J.S. The Prognostic Factors That Influence Long-Term Survival in Acute Large Cerebral Infarction. J. Korean Neurosurg. Soc. 2011, 49, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Blagus, R.; Lusa, L. SMOTE for High-Dimensional Class-Imbalanced Data. BMC Bioinform. 2013, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Synhaeve, N.E.; Arntz, R.M.; van Alebeek, M.E.; van Pamelen, J.; Maaijwee, N.A.M.; Rutten-Jacobs, L.C.A.; Schoonderwaldt, H.C.; de Kort, P.L.M.; van Dijk, E.J.; de Leeuw, F.-E. Women Have a Poorer Very Long-Term Functional Outcome after Stroke among Adults Aged 18–50 Years: The FUTURE Study. J. Neurol. 2016, 263, 1099–1105. [Google Scholar] [CrossRef]
- Eren, F.; Ozguncu, C.; Ozturk, S. Short-Term Prognostic Predictive Evaluation in Female Patients With Ischemic Stroke: A Retrospective Cross-Sectional Study. Front. Neurol. 2022, 13, 329. [Google Scholar] [CrossRef]
- Ursprung, S.; Beer, L.; Bruining, A.; Woitek, R.; Stewart, G.D.; Gallagher, F.A.; Sala, E. Radiomics of Computed Tomography and Magnetic Resonance Imaging in Renal Cell Carcinoma-a Systematic Review and Meta-Analysis. Eur. Radiol. 2020, 30, 3558–3566. [Google Scholar] [CrossRef]
- Stanzione, A.; Gambardella, M.; Cuocolo, R.; Ponsiglione, A.; Romeo, V.; Imbriaco, M. Prostate MRI Radiomics: A Systematic Review and Radiomic Quality Score Assessment. Eur. J. Radiol. 2020, 129, 109095. [Google Scholar] [CrossRef]
- Granzier, R.W.Y.; van Nijnatten, T.J.A.; Woodruff, H.C.; Smidt, M.L.; Lobbes, M.B.I. Exploring Breast Cancer Response Prediction to Neoadjuvant Systemic Therapy Using MRI-Based Radiomics: A Systematic Review. Eur. J. Radiol. 2019, 121, 108736. [Google Scholar] [CrossRef]
- Cronin, P.; Kelly, A.M.; Altaee, D.; Foerster, B.; Petrou, M.; Dwamena, B.A. How to Perform a Systematic Review and Meta-Analysis of Diagnostic Imaging Studies. Acad. Radiol. 2018, 25, 573–593. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef]
- McCague, C.; Ramlee, S.; Reinius, M.; Selby, I.; Hulse, D.; Piyatissa, P.; Bura, V.; Crispin-Ortuzar, M.; Sala, E.; Woitek, R. Introduction to Radiomics for a Clinical Audience. Clin. Radiol. 2023, 78, 83–98. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies that investigated MRI radiomics features in patients with AIS Studies that assessed the clinical outcome based on RA features in AIS patients | Unavailable data on RA and predictive model performance CT-, CTA- or US-based RA studies Non-original investigations (reviews, editorials, letters or opinions) |
| Study, Year | Sample, Age, Sex | AIS Type | Tx | Onset-to-MRI Time | Outcome Criteria | Clinical Factors | MRI Markers | MRI Seq | RA Features | Predictive Models | AUC, 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quan et al. [33], 2021 | 110, 62, 70.9% male | first AIS in MCA territory, onset ≤72 h | ivT, MT: 12 p | 26.5 ± 15.7 | 90 days unfavorable outcome mRS > 2 | Age, gender, admission NIHSS | DWI-ASPECT score, ODs | FLAIR ADC | 6, TA, wavelet | Clinical | 0.79, 0.68–0.89 |
| Clinical + MRI | 0.78, 0.68–0.88 | ||||||||||
| ADC radiomics | 0.77, 0.62–0.83 | ||||||||||
| FLAIR radiomics | 0.73. 0.62–0.83 | ||||||||||
| ADC + FLAIR radiomics | 0.81, 0.73–0.89 | ||||||||||
| RA + Clinical + MRI | 0.92, 0.87–0.97 | ||||||||||
| Wang et al. [34], 2021 | 399, 67, 63.9% male | NR | NR | within 24 h after AIS onset | 90 days outcome mRS > 2 | Age, 24-h NIHSS | Hemorrhage | DWI | 11, TA | Clinical model | 0.77, 0.71–0.84 |
| Radiomics model | 0.70, 0.64–0.77 | ||||||||||
| Clinical + radiomics | 0.80, 0.75–0.86 | ||||||||||
| Zhou et al. [35], 2022 | 311, 58, 72.7% male | Pen artery: 43.1%, cMCA: 28.6%, cACA: 5.5%, cPCA = 8.4%, ≥2 territories: 14.5% | NR | <24 h: 6.1%24–72 h: 93.9% | 6-month good outcome (mRS ≤ 2), poor outcome (mRS > 2) | Age, gender, stroke history, DM, b-mRS, b-NIHSS | - | DWI, ADC | 7, first-order statistics, TA | Clinical model | 0.82, 0.77–0.87 |
| Radiomics model | 0.76, 0.70–0.82 | ||||||||||
| Clinical + radiomics | 0.86, 0.82–0.91 | ||||||||||
| Zhang et al. [36], 2022 | 103, 65, 64% male | Unilateral anterior circulation | NR | NR | 90 days outcome mRS > 2 | Atrial fibrillation | - | ADC | 7, TA, wavelet, LGT | ADC | 0.60, 049–0.71 |
| tADC | 0.83, 075–0.91 | ||||||||||
| tADC + clinical | 0.86, 079–0.93 | ||||||||||
| Wang et al. [37], 2022 | 1003, 67, 67.9% m | Ant-circ: 68.5%, Post-circ: 28.5%, Both: 3% | NR | 72 h of AIS onset | 90 d outcome 1y AIS recurrence | NR | - | DWI | 100, TA, wavelet | Radiomics model | 0.77, 0.75–0.80 |
| Clinical + radiomics | 0.84, 0.82–0.87 | ||||||||||
| Wang et al. [38], 2020 | 116, 64, 72% male | NR | NR | NR | 90 days outcome mRS > 2, stroke severity | - | - | FLAIR, ADC | 15, first-order statistics, TA | RA features were not predictive of mRS. ADC-entropy and T2-FLAIR 0.75 quantile predicted AIS severity (AUC = 0.7, p = 0.01). | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragoș, H.M.; Stan, A.; Pintican, R.; Feier, D.; Lebovici, A.; Panaitescu, P.-Ș.; Dina, C.; Strilciuc, S.; Muresanu, D.F. MRI Radiomics and Predictive Models in Assessing Ischemic Stroke Outcome—A Systematic Review. Diagnostics 2023, 13, 857. https://doi.org/10.3390/diagnostics13050857
Dragoș HM, Stan A, Pintican R, Feier D, Lebovici A, Panaitescu P-Ș, Dina C, Strilciuc S, Muresanu DF. MRI Radiomics and Predictive Models in Assessing Ischemic Stroke Outcome—A Systematic Review. Diagnostics. 2023; 13(5):857. https://doi.org/10.3390/diagnostics13050857
Chicago/Turabian StyleDragoș, Hanna Maria, Adina Stan, Roxana Pintican, Diana Feier, Andrei Lebovici, Paul-Ștefan Panaitescu, Constantin Dina, Stefan Strilciuc, and Dafin F. Muresanu. 2023. "MRI Radiomics and Predictive Models in Assessing Ischemic Stroke Outcome—A Systematic Review" Diagnostics 13, no. 5: 857. https://doi.org/10.3390/diagnostics13050857
APA StyleDragoș, H. M., Stan, A., Pintican, R., Feier, D., Lebovici, A., Panaitescu, P.-Ș., Dina, C., Strilciuc, S., & Muresanu, D. F. (2023). MRI Radiomics and Predictive Models in Assessing Ischemic Stroke Outcome—A Systematic Review. Diagnostics, 13(5), 857. https://doi.org/10.3390/diagnostics13050857








