Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry
Abstract
1. Introduction
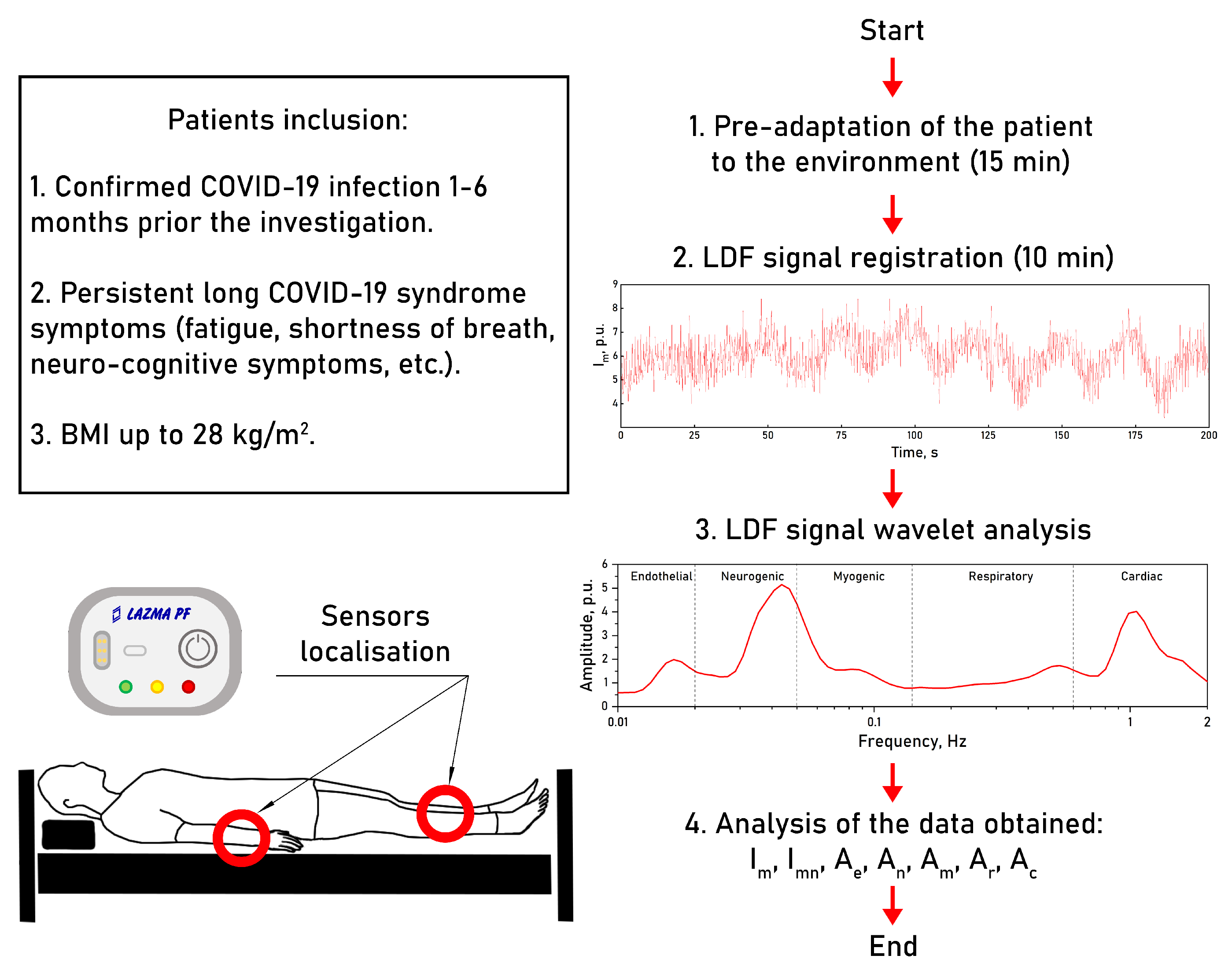
2. Materials and Methods
2.1. Experimental Equipment
2.2. Experimental Protocol
2.3. Data Analysis
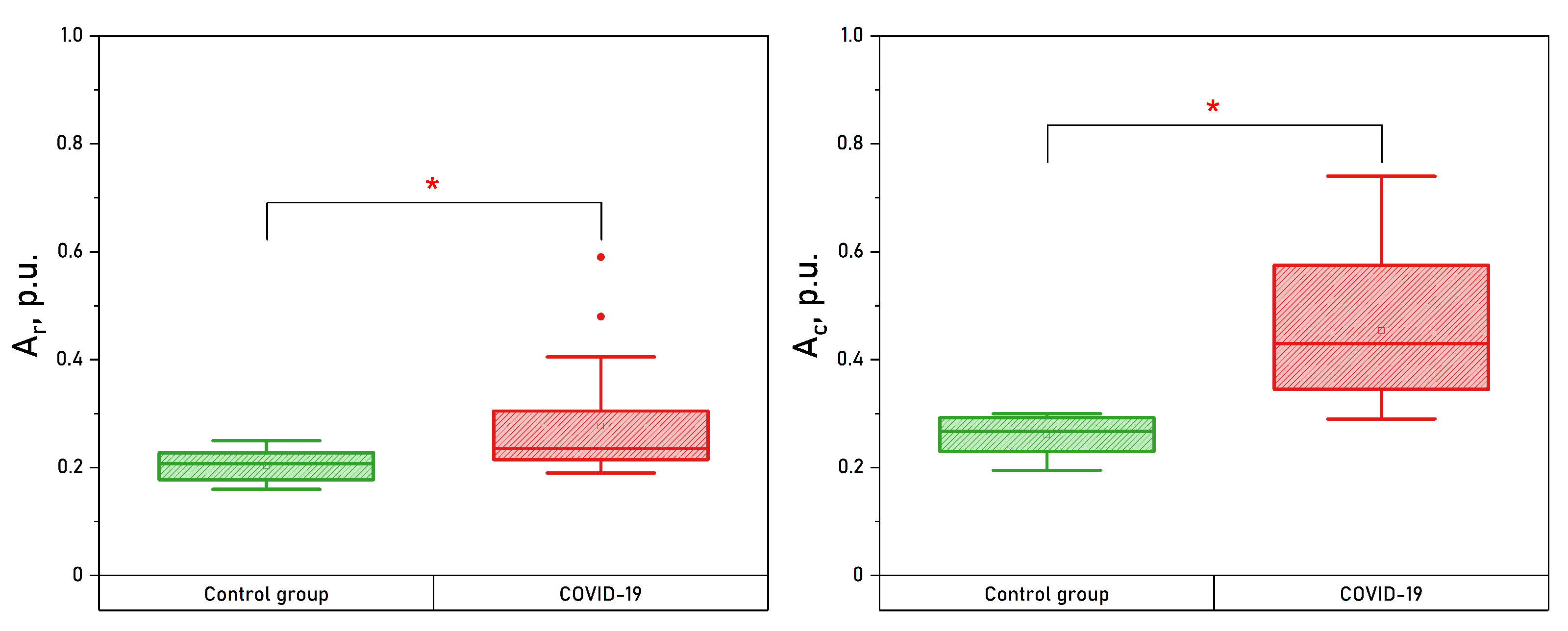
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Paolisso, P.; Sardu, C.; Palomba, L.; D’Onofrio, N.; Cesaro, A.; Barbieri, M.; Rizzo, M.R.; Sasso, F.C.; Scisciola, L.; et al. SARS-COV-2 colonizes coronary thrombus and impairs heart microcirculation bed in asymptomatic SARS-CoV-2 positive subjects with acute myocardial infarction. Crit. Care 2021, 25, 217. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Stanek, A.; Hooshmand, A.; Khamineh, Y.; Ahi, S.; Kazim, S.N.; Ahmad, F.; Muronetz, V.; Samy Abousenna, M.; Zolghadri, S.; et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. J. Clin. Med. 2021, 10, 4802. [Google Scholar] [CrossRef]
- Gąsecka, A.; Filipiak, K.J.; Jaguszewski, M.J. Impaired microcirculation function in COVID-19 and implications for potential therapies. Cardiol. J. 2020, 27, 485–488. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Franke, R.P.; Hufert, F.; Küpper, J.H. COVID-19 and the endothelium. Clin. Hemorheol. Microcirc. 2020, 75, 7–11. [Google Scholar] [CrossRef]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC working group on coronary pathophysiology & microcirculation, ESC working group on thrombosis and the association for acute CardioVascular care (ACVC), in collaboration with the European heart rhythm association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar]
- Yelin, D.; Wirtheim, E.; Vetter, P.; Kalil, A.C.; Bruchfeld, J.; Runold, M.; Guaraldi, G.; Mussini, C.; Gudiol, C.; Pujol, M.; et al. Long-term consequences of COVID-19: Research needs. Lancet Infect. Dis. 2020, 20, 1115–1117. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Mardani, M. Post COVID syndrome. Arch. Clin. Infect. Dis. 2020, 15, e108819. [Google Scholar] [CrossRef]
- Raveendran, A.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Favaron, E.; Ince, C.; Hilty, M.P.; Ergin, B.; van der Zee, P.; Uz, Z.; Garcia, P.D.W.; Hofmaenner, D.A.; Acevedo, C.T.; van Boven, W.J.; et al. Capillary leukocytes, microaggregates, and the response to hypoxemia in the microcirculation of coronavirus disease 2019 patients. Crit. Care Med. 2021, 49, 661. [Google Scholar] [CrossRef] [PubMed]
- Natalello, G.; De Luca, G.; Gigante, L.; Campochiaro, C.; De Lorenzis, E.; Verardi, L.; Paglionico, A.; Petricca, L.; Martone, A.M.; Calvisi, S.; et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: Broadening the spectrum of COVID-19 microvascular involvement. Microvasc. Res. 2021, 133, 104071. [Google Scholar] [CrossRef] [PubMed]
- Karahan, S.; Aydin, K.; Cetinkaya, A.; Sirakaya, H.A. Nailfold Videocapillaroscopy in Patients with COVID-19-associated Pneumonia in Intensive Care Units. J. Coll. Physicians Surg. -JCPSP 2022, 32, 455–460. [Google Scholar] [CrossRef]
- Daly, S.M.; Leahy, M.J. ‘Go with the flow’: A review of methods and advancements in blood flow imaging. J. Biophotonics 2013, 6, 217–255. [Google Scholar] [CrossRef]
- Martini, R.; Bagno, A. The wavelet analysis for the assessment of microvascular function with the laser Doppler fluxmetry over the last 20 years. Looking for hidden informations. Clin. Hemorheol. Microcirc. 2018, 70, 213–229. [Google Scholar] [CrossRef]
- Zharkikh, E.; Dremin, V.; Zherebtsov, E.; Dunaev, A.; Meglinski, I. Biophotonics methods for functional monitoring of complications of diabetes mellitus. J. Biophotonics 2020, 13, e202000203. [Google Scholar] [CrossRef]
- Mizeva, I.A.; Potapova, E.V.; Zharkikh, E.V. Diagnostics of Functional Abnormalities in the Microcirculation System Using Laser Doppler Flowmetry; CRC Press: Boca Raton, FL, USA, 2022; pp. 81–105. [Google Scholar] [CrossRef]
- Mizeva, I.; Makovik, I.; Dunaev, A.; Krupatkin, A.; Meglinski, I. Analysis of skin blood microflow oscillations in patients with rheumatic diseases. J. Biomed. Opt. 2017, 22, 070501. [Google Scholar] [CrossRef]
- Fedorovich, A.; Loktionova, Y.; Zharkikh, E.; Gorshkov, A.Y.; Korolev, A.; Dadaeva, V.; Drapkina, O.; Zherebtsov, E. Skin microcirculation in middle-aged men with newly diagnosed arterial hypertension according to remote laser Doppler flowmetry data. Microvasc. Res. 2022, 144, 104419. [Google Scholar] [CrossRef]
- Fredriksson, I.; Larsson, M.; Strömberg, T. Model-based quantitative laser Doppler flowmetry in skin. J. Biomed. Opt. 2010, 15, 057002. [Google Scholar] [CrossRef]
- Kozlov, I.; Zherebtsov, E.; Podmasteryev, K.; Dunaev, A. Digital laser Doppler flowmetry: Device, signal processing technique, and clinical testing. Biomed. Eng. 2021, 55, 12–16. [Google Scholar] [CrossRef]
- Iwasaki, W.; Nogami, H.; Takeuchi, S.; Furue, M.; Higurashi, E.; Sawada, R. Detection of site-specific blood flow variation in humans during running by a wearable laser Doppler flowmeter. Sensors 2015, 15, 25507–25519. [Google Scholar] [CrossRef]
- Sabioni, L.; De Lorenzo, A.; Lamas, C.; Muccillo, F.; Castro-Faria-Neto, H.C.; Estato, V.; Tibirica, E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc. Res. 2021, 134, 104119. [Google Scholar] [CrossRef] [PubMed]
- Glazkov, A.; Ulbashev, D.; Borshchev, G.; Pulin, A.; Glazkova, P.; Kulikov, D. Skin microcirculation reactivity to local thermal hyperaemia in patients with COVID-19—A pilot observational study. Clin. Hemorheol. Microcirc. 2023, 83, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Gille-Johnson, P. Microvascular dysfunction in patients with critical COVID-19, a pilot study. Shock 2021, 56, 964. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, V.; Rybakov, Y.L.; Gukasov, V.; Evtushenko, G. A System of Local Analyzers for Noninvasive Diagnostics of the General State of the Tissue Microcirculation System of Human Skin. Biomed. Eng. 2022, 55, 379–382. [Google Scholar] [CrossRef]
- Saha, M.; Dremin, V.; Rafailov, I.; Dunaev, A.; Sokolovski, S.; Rafailov, E. Wearable laser doppler flowmetry sensor: A feasibility study with smoker and non-smoker volunteers. Biosensors 2020, 10, 201. [Google Scholar] [CrossRef]
- Zharkikh, E.V.; Loktionova, Y.I.; Dremin, V.V.; Podmasteryev, K.V.; Sidorov, V.V.; Rafailov, E.U.; Dunaev, A.V. Comparison of wearable and bedside laser Doppler flowmetry and fluorescence spectroscopy monitors. Opt. Technol. Biol. Med. 2022, 12192, 114–117. [Google Scholar] [CrossRef]
- Rajan, V.; Varghese, B.; van Leeuwen, T.G.; Steenbergen, W. Review of methodological developments in laser Doppler flowmetry. Lasers Med. Sci. 2009, 24, 269–283. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Krupatkin, A. Cardiac and respiratory oscillations of the blood flow in microvessels of the human skin. Hum. Physiol. 2008, 34, 323–329. [Google Scholar] [CrossRef]
- Dunaev, A.; Sidorov, V.; Krupatkin, A.; Rafailov, I.; Palmer, S.; Stewart, N.; Sokolovski, S.; Rafailov, E. Investigating tissue respiration and skin microhaemocirculation under adaptive changes and the synchronization of blood flow and oxygen saturation rhythms. Physiol. Meas. 2014, 35, 607. [Google Scholar] [CrossRef] [PubMed]
- Aalkjær, C.; Boedtkjer, D.; Matchkov, V. Vasomotion–what is currently thought? Acta Physiol. 2011, 202, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Bulow, J.; Lassen, N. Vasomotion in human skin before and after local heating recorded with laser Doppler flowmetry. A method for induction of vasomotion. Int. J. Microcirc. Clin. Exp. 1989, 8, 205–215. [Google Scholar]
- Sakurai, T.; Terui, N. Effects of sympathetically induced vasomotion on tissue-capillary fluid exchange. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1761–H1767. [Google Scholar] [CrossRef]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID-19 syndrome: Is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front. Cardiovasc. Med. 2021, 8, 1702. [Google Scholar] [CrossRef]
- Invernizzi, A.; Pellegrini, M.; Messenio, D.; Cereda, M.; Olivieri, P.; Brambilla, A.M.; Staurenghi, G. Impending central retinal vein occlusion in a patient with coronavirus disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 2020, 28, 1290–1292. [Google Scholar] [CrossRef]
- Invernizzi, A.; Schiuma, M.; Parrulli, S.; Torre, A.; Zicarelli, F.; Colombo, V.; Marini, S.; Villella, E.; Bertoni, A.; Antinori, S.; et al. Retinal vessels modifications in acute and post-COVID-19. Sci. Rep. 2021, 11, 19373. [Google Scholar] [CrossRef]

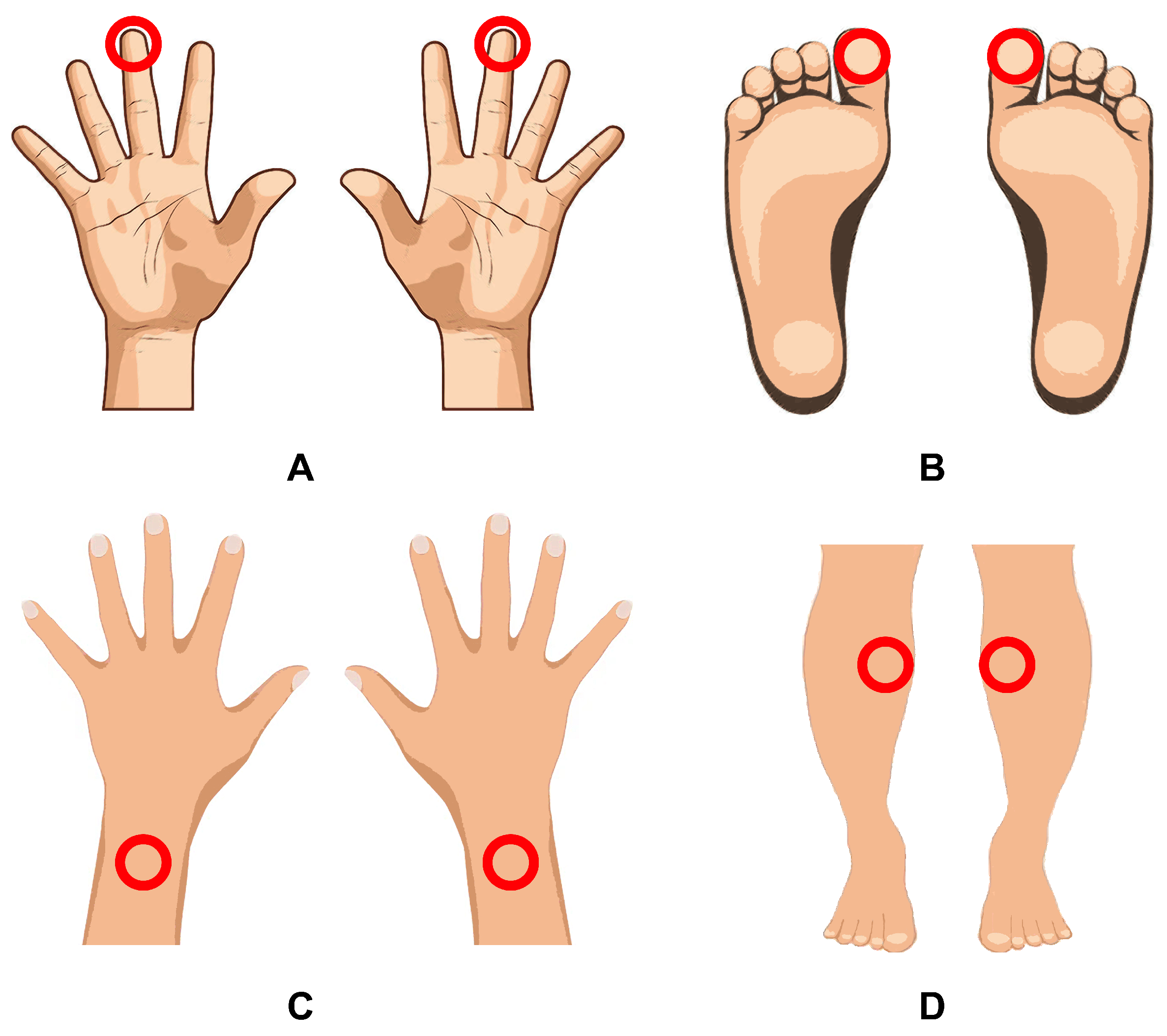
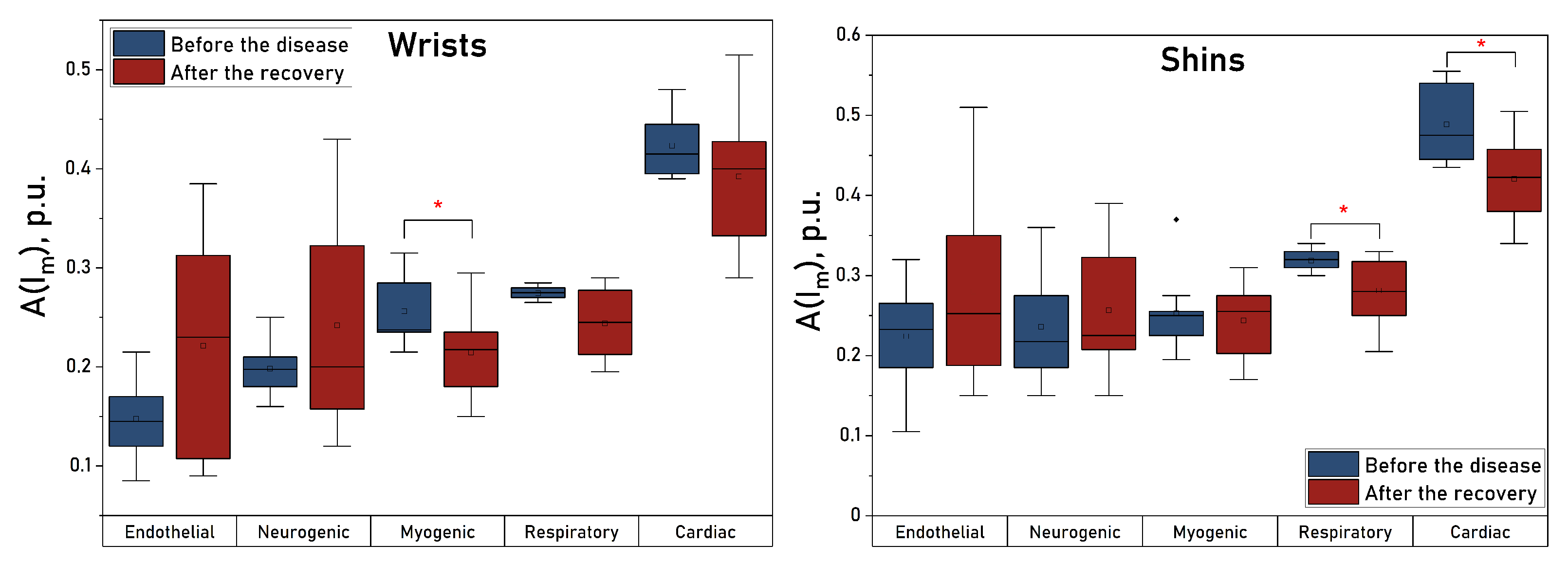

| Parameter | Wrists before | Wrists after | Shins before | Shins after | Fingers before | Fingers after | Toes before | Toes after |
|---|---|---|---|---|---|---|---|---|
| , p.u. | ||||||||
| , p.u. | ||||||||
| , p.u. | ||||||||
| , p.u. | ||||||||
| , p.u. | * | |||||||
| , p.u. | * | |||||||
| , p.u. | * |
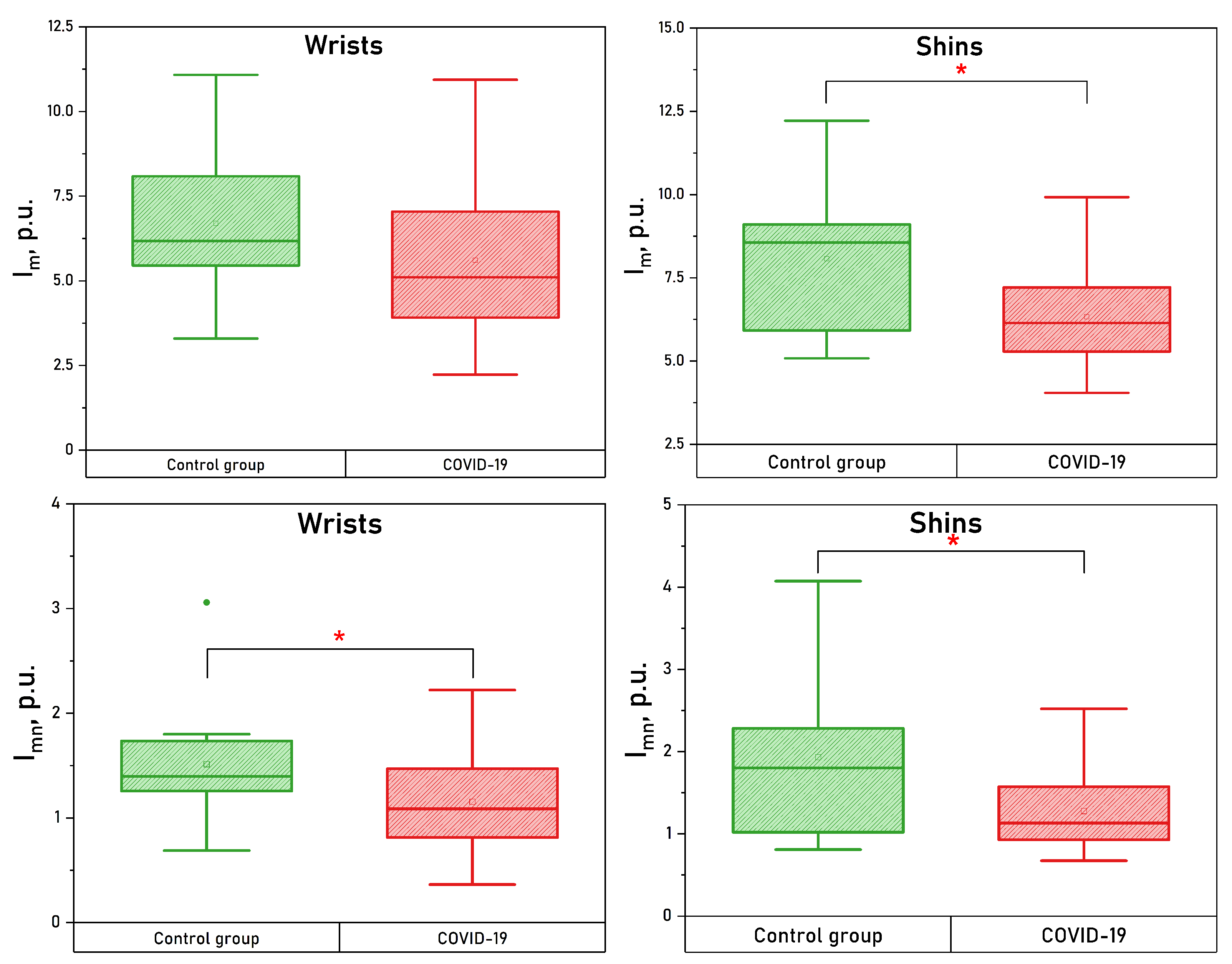
| Parameter | Wrists CONTROL | Wrists COVID-19 | Shins CONTROL | Shins COVID-19 |
|---|---|---|---|---|
| , p.u. | * | |||
| , p.u. | * | * | ||
| , p.u. | ||||
| , p.u. | * | |||
| , p.u. | ||||
| , p.u. | * | |||
| , p.u. | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharkikh, E.V.; Loktionova, Y.I.; Fedorovich, A.A.; Gorshkov, A.Y.; Dunaev, A.V. Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics 2023, 13, 920. https://doi.org/10.3390/diagnostics13050920
Zharkikh EV, Loktionova YI, Fedorovich AA, Gorshkov AY, Dunaev AV. Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics. 2023; 13(5):920. https://doi.org/10.3390/diagnostics13050920
Chicago/Turabian StyleZharkikh, Elena V., Yulia I. Loktionova, Andrey A. Fedorovich, Alexander Y. Gorshkov, and Andrey V. Dunaev. 2023. "Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry" Diagnostics 13, no. 5: 920. https://doi.org/10.3390/diagnostics13050920
APA StyleZharkikh, E. V., Loktionova, Y. I., Fedorovich, A. A., Gorshkov, A. Y., & Dunaev, A. V. (2023). Assessment of Blood Microcirculation Changes after COVID-19 Using Wearable Laser Doppler Flowmetry. Diagnostics, 13(5), 920. https://doi.org/10.3390/diagnostics13050920







