Chemerin and Chemokine-like Receptor 1 Expression in Ovarian Cancer Associates with Proteins Involved in Estrogen Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Tissue Microarray and Immunohistochemistry
2.3. In Silico Analyses
2.4. Statistical Analysis
3. Results
3.1. Intratumoral RARRES2 mRNA Levels in Ovarian Cancer and Metastasis Tissues Are Significantly Reduced When Compared to Normal Ovary
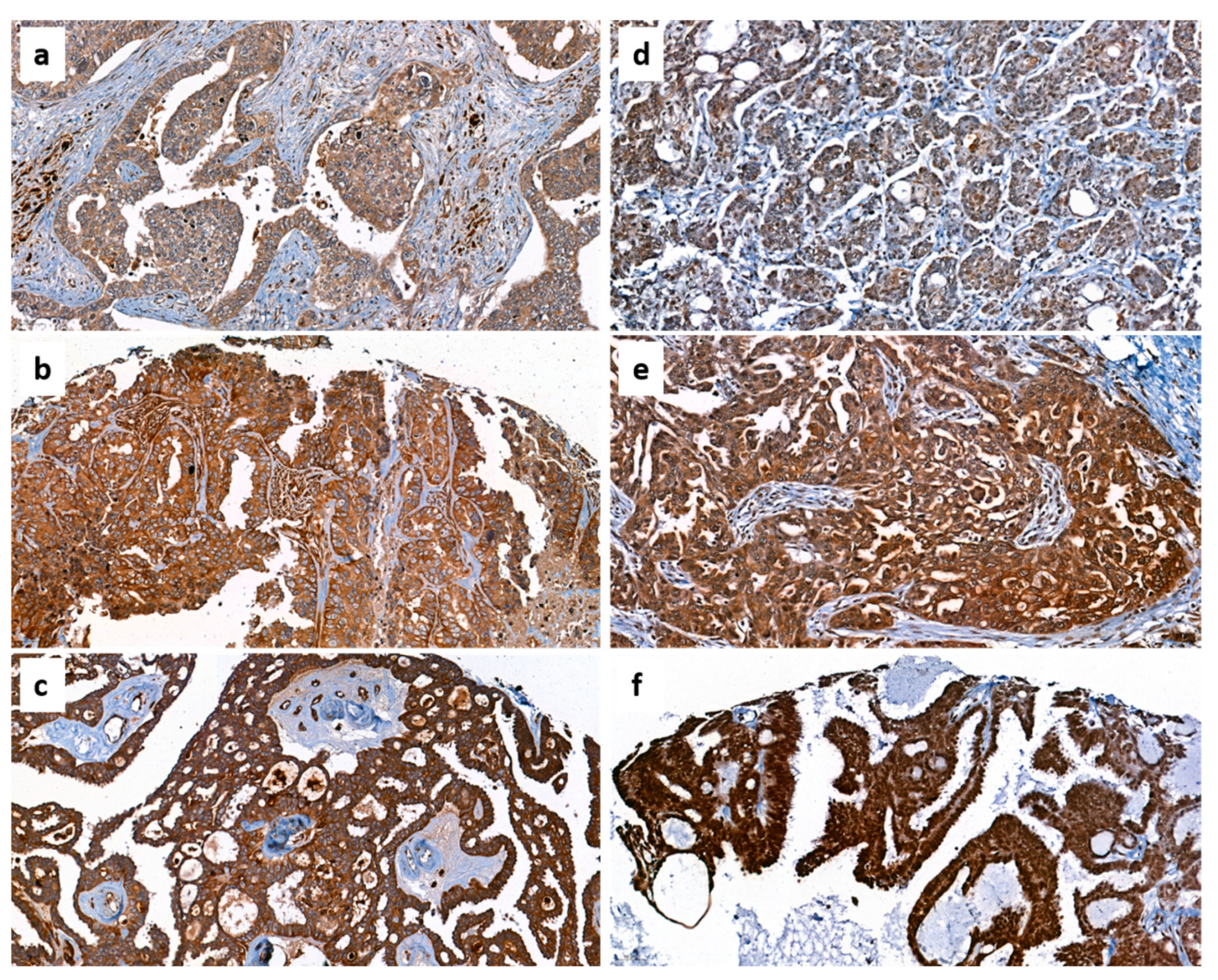
3.2. Protein Levels of Chemerin and CMKLR1 in Ovarian Cancer Tissue
3.3. Protein Levels of Chemerin and CMKLR1 in Ovarian Cancer Tissue Subject to Levels of Ovarian Cancer Markers, Cancer-Related Proteins and Components of Estrogen Signaling Pathways
3.4. Correlation of Chemerin and CMKLR1 Protein Levels with Intratumoral Expression of Proteins Involved in Estrogen Signaling, Ovarian Cancer Markers, and Other Cancer-Related Genes
3.5. Correlation of RARRES2 and CMKLR1 mRNA Levels with Expression of Genes Involved in Sex Steroid Hormone Metabolism and Signaling Assessed by In Silico Analysis
3.6. Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Bäumler, M.; Gallant, D.; Druckmann, R.; Kuhn, W. Ultrasound screening of ovarian cancer. Horm. Mol. Biol. Clin. Investig. 2019, 41. [Google Scholar] [CrossRef]
- Treeck, O.; Buechler, C.; Ortmann, O. Chemerin and Cancer. Int. J. Mol. Sci. 2019, 20, 3750. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.; Zhang, J.; Li, Y.; Zhang, Y. Chemerin promotes proliferation and migration of ovarian cancer cells by upregulating expression of PD-L1. J. Zhejiang Univ. Sci. B 2022, 23, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Gallistl, J.; Schüler-Toprak, S.; Fritsch, J.; Buechler, C.; Ortmann, O.; Treeck, O. Anti-Tumoral Effect of Chemerin on Ovarian Cancer Cell Lines Mediated by Activation of Interferon Alpha Response. Cancers 2022, 14, 4108. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Curran, J.E.; Stocker, C.J.; Zaibi, M.S.; Segal, D.; Konstantopoulos, N.; Morrison, S.; Carless, M.; Dyer, T.D.; Cole, S.A.; et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010, 95, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Rehman, R.; Baig, M.; Khan, T.A. New roles of the multidimensional adipokine: Chemerin. Peptides 2014, 62, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef]
- Nakamura, N.; Naruse, K.; Kobayashi, Y.; Miyabe, M.; Saiki, T.; Enomoto, A.; Takahashi, M.; Matsubara, T. Chemerin promotes angiogenesis in vivo. Physiol. Rep. 2018, 6, e13962. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemerin reveals its chimeric nature. J. Exp. Med. 2008, 205, 2187–2190. [Google Scholar] [CrossRef] [PubMed]
- De Henau, O.; Degroot, G.-N.; Imbault, V.; Robert, V.; De Poorter, C.; Mcheik, S.; Galés, C.; Parmentier, M.; Springael, J.-Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Serafin, D.S.; Allyn, B.; Sassano, M.F.; Timoshchenko, R.G.; Mattox, D.; Brozowski, J.M.; Siderovski, D.P.; Truong, Y.K.; Esserman, D.; Tarrant, T.K.; et al. Chemerin-activated functions of CMKLR1 are regulated by G protein-coupled receptor kinase 6 (GRK6) and β-arrestin 2 in inflammatory macrophages. Mol. Immunol. 2019, 106, 12–21. [Google Scholar] [CrossRef]
- Goralski, K.B.; Jackson, A.E.; McKeown, B.T.; Sinal, C.J. More Than an Adipokine: The Complex Roles of Chemerin Signaling in Cancer. Int. J. Mol. Sci. 2019, 20, 4778. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Wang, P.; Salazar, N.; Zheng, Y.; Nease, L.; Rosalez, J.; Leong, W.-I.; Virdi, G.; Rennier, K.; Shin, W.J.; et al. Chemerin Suppresses Breast Cancer Growth by Recruiting Immune Effector Cells into the Tumor Microenvironment. Front. Immunol. 2019, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Wang, Y.; Leung, T.H.Y.; Liu, S.S.; Cheung, A.N.Y.; Ngan, H.Y.S. Differential expression of estrogen receptor subtypes and variants in ovarian cancer: Effects on cell invasion, proliferation and prognosis. BMC Cancer 2017, 17, 606. [Google Scholar] [CrossRef]
- O’Donnell, A.J.M.; Macleod, K.G.; Burns, D.J.; Smyth, J.F.; Langdon, S.P. Estrogen receptor-alpha mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr. Relat. Cancer 2005, 12, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Papaioannidou, P. Estrogen receptor beta and ovarian cancer: A key to pathogenesis and response to therapy. Arch. Gynecol. Obstet. 2016, 293, 1161–1168. [Google Scholar] [CrossRef]
- Halon, A.; Nowak-Markwitz, E.; Maciejczyk, A.; Pudelko, M.; Gansukh, T.; Györffy, B.; Donizy, P.; Murawa, D.; Matkowski, R.; Spaczynski, M.; et al. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res. 2011, 31, 711–718. [Google Scholar]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Estrogen receptor β is associated with expression of cancer associated genes and survival in ovarian cancer. BMC Cancer 2018, 18, 981. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Moehle, C.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Effect of estrogen receptor β agonists on proliferation and gene expression of ovarian cancer cells. BMC Cancer 2017, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Pfeiler, G.; Mitter, D.; Lattrich, C.; Piendl, G.; Ortmann, O. Estrogen receptor {beta}1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells. J. Endocrinol. 2007, 193, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Viswanadhapalli, S.; Garcia, L.; Zhou, M.; Nair, B.C.; Kost, E.; Rao Tekmal, R.; Li, R.; Rao, M.K.; Curiel, T.; et al. Therapeutic utility of natural estrogen receptor beta agonists on ovarian cancer. Oncotarget 2017, 8, 50002–50014. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Expression of estrogen-related receptors in ovarian cancer and impact on survival. J. Cancer Res. Clin. Oncol. 2021, 147, 2555–2567. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mori, T.; Sawada, M.; Kuroboshi, H.; Tatsumi, H.; Yoshioka, T.; Matsushima, H.; Iwasaku, K.; Kitawaki, J. Estrogen-related receptor-γ regulates estrogen receptor-α responsiveness in uterine endometrial cancer. Int. J. Gynecol. Cancer 2012, 22, 1509–1516. [Google Scholar] [CrossRef]
- Tanida, T.; Matsuda, K.I.; Yamada, S.; Hashimoto, T.; Kawata, M. Estrogen-related Receptor β Reduces the Subnuclear Mobility of Estrogen Receptor α and Suppresses Estrogen-dependent Cellular Function. J. Biol. Chem. 2015, 290, 12332–12345. [Google Scholar] [CrossRef] [PubMed]
- Ranhotra, H.S. The estrogen-related receptors: Orphans orchestrating myriad functions. J. Recept. Signal Transduct. Res. 2012, 32, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, P.; Dong, B.; Sehouli, J. Key regulator of cellular metabolism, estrogen-related receptor α, a new therapeutic target in endocrine-related gynecological tumor. Cancer Manag. Res. 2018, 10, 6887–6895. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Cornuau, M.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum. Reprod. 2012, 27, 1790–1800. [Google Scholar] [CrossRef]
- Tang, M.; Huang, C.; Wang, Y.-F.; Ren, P.-G.; Chen, L.; Xiao, T.-X.; Wang, B.-B.; Pan, Y.-F.; Tsang, B.K.; Zabel, B.A.; et al. CMKLR1 deficiency maintains ovarian steroid production in mice treated chronically with dihydrotestosterone. Sci. Rep. 2016, 6, 21328. [Google Scholar] [CrossRef]
- Mirlacher, M.; Simon, R. Recipient block TMA technique. Methods Mol. Biol. 2010, 664, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Mirlacher, M.; Sauter, G. Immunohistochemical analysis of tissue microarrays. Methods Mol. Biol. 2010, 664, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Vorschlag zur einheitlichen Definition eines Immunreaktiven Score (IRS) für den immunhistochemischen Ostrogenrezeptor-Nachweis (ER-ICA) im Mammakarzinomgewebe. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Spaulding, D.C.; Spaulding, B.O. Epidermal growth factor receptor expression and measurement in solid tumors. Semin. Oncol. 2002, 29, 45–54. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Tarpin, C.; Bardou, V.-J.; Bertucci, F.; Ginestier, C.; Braud, A.-C.; Puig, B.; Geneix, J.; Hassoun, J.; Birnbaum, D.; et al. Immunophenotypic analysis of inflammatory breast cancers: Identification of an ‘inflammatory signature’. J. Pathol. 2004, 202, 265–273. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Rytelewska, E.; Kiezun, M.; Kisielewska, K.; Gudelska, M.; Dobrzyn, K.; Kaminska, B.; Kaminski, T.; Smolinska, N. Chemerin as a modulator of ovarian steroidogenesis in pigs: An in vitro study. Theriogenology 2021, 160, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Mellouk, N.; Bongrani, A.; Plotton, I.; Langer, I.; Ramé, C.; Petit, C.; Guérif, F.; Froment, P.; Dupont, J. Involvement of chemerin and CMKLR1 in the progesterone decrease by PCOS granulosa cells. Reproduction 2021, 162, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, S.; Zhao, M.; Sheng, B.; Zhu, H.; Zhu, X. Prognostic value of progesterone receptor expression in ovarian cancer: A meta-analysis. Oncotarget 2017, 8, 36845–36856. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Rak, A.; Ptak, A. Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicol. Lett. 2018, 291, 61–69. [Google Scholar] [CrossRef]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Sun, J.; Yan, C.; Xu, D.; Zhang, Z.; Li, K.; Li, X.; Zhou, M.; Hao, D. Immuno-genomic characterisation of high-grade serous ovarian cancer reveals immune evasion mechanisms and identifies an immunological subtype with a favourable prognosis and improved therapeutic efficacy. Br. J. Cancer 2022, 126, 1570–1580. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Rennier, K.; Shin, W.J.; Krug, E.; Virdi, G.; Pachynski, R.K. Chemerin Reactivates PTEN and Suppresses PD-L1 in Tumor Cells via Modulation of a Novel CMKLR1-mediated Signaling Cascade. Clin. Cancer Res. 2020, 26, 5019–5035. [Google Scholar] [CrossRef]


| Characteristics | Number of Patients | (%) |
|---|---|---|
| 208 | 100 | |
| FIGO stage | ||
| FIGO I | 22 | 10.58 |
| FIGO II | 8 | 3.85 |
| FIGO III | 65 | 31.25 |
| FIGO IV | 50 | 24.04 |
| Unknown | 63 | 30.29 |
| Histological subtype | ||
| Serous | 135 | 64.90 |
| Mucinous | 6 | 2.88 |
| Endometroid | 10 | 4.81 |
| Clear cell | 3 | 1.44 |
| Undifferentiated | 54 | 25.96 |
| Histological grade | ||
| G2 | 53 | 25.48 |
| G3 | 122 | 58.65 |
| Unknown | 33 | 15.87 |
| Marker/Protein | Antibody Clone | Pretreatment | Dilution | Pattern |
|---|---|---|---|---|
| Chemerin (RARRES2) | LS-B13333 (Biozol) | none | 1:100 | cytoplasmic (non-specific) nuclear |
| CMKLR1 | LS-B12924 (Biozol) | none | 1:100 | membranous/cytoplasmic |
| ERβ | PPG5/10 (Novus Biologicals) | none | 1:20 | nuclear/cytoplasmic |
| ERα | 6F11 (Novocastra) | CC1 64 min | 1:35 | nuclear |
| CA-125 | OC125 (Cell Marque) | CC1 52 min | 1:1 | cytoplasmic/membranous |
| CEA (polyclonal) | A 0115 (Dako) | P1 8 min | 1:500 | cytoplasmic |
| CA72.4 | B72.3 (Alexis Biochemicals) | CC1 36 min | 1:50 | cytoplasmic |
| EGFR | E30 (Dako) | P1 4 min | 1:100 | membranous |
| p53 | sc-263 (Santa Cruz) | CC1 36 min | 1:2000 | nuclear |
| Ki-67 | MIB-1/M7240 (Dako) | CC1 64 min | 1:100 | nuclear |
| PR | NCL-L-PGR-312 (Clone 16) (Novocastra) | CC1 64 min | 1:50 | nuclear |
| Her2/neu | A0485 (Dako) | CC1 36 min | 1:250 | membranous |
| Chemerin | CMKLR1 | Chemerin | CMKLR1 | ||||
|---|---|---|---|---|---|---|---|
| ERα | low | 1.902 (0.7822) | 2.143 (0.7810) | TP53 | low | 1.910 (0.7926) | 2.090 (0.7781) |
| high | 2.049 (0.7400) | 2.195 (0.7490) | high | 1.984 (0.7512) | 2.238 (0.7559) | ||
| p = 0.2878 | p = 0.7393 | p = 0.5472 | p = 0.2451 | ||||
| ERβ (n) | low | 1.895 (0.7665) | 2.119 (0.7736) | HER2 | low | 1.893 (0.7585) | 2.139 (0.7640) |
| high | 2.429 (0.5345) | 2.571(0.5345) | high | 2.133 (0.8193) | 2.200 (0.8052) | ||
| p = 0.0683 | p = 0.1372 | p = 0.1355 | p = 0.6702 | ||||
| ERβ (cm) | low | 1.838 (0.7423) | 2.060(0.7576) | EGFR | low | 1.905 (0.7739) | 2.119 (0.7757) |
| high | 2.212 (0.7809) | 2.424 (0.7513) | high | 2.200 (0.6959) | 2.450 (0.6863) | ||
| p = 0.0143 | p = 0.0133 | p = 0.1069 | p = 0.0752 | ||||
| PR | low | 1.941 (0.7675) | 2.169 (0.7655) | ERRα | low | 1.671 (0.7082) | 1.753 (0.6827) |
| high | 2.231 (0.7250) | 2.308 (0.7511) | high | 2.187 (0.7478) | 2.533 (0.6438) | ||
| p = 0.1925 | p = 0.5397 | p < 0.0001 | p < 0.0001 | ||||
| CEA | low | 1.908 (0.7889) | 2.107 (0.7671) | ERRβ | low | 1.524 (0.6016) | 1.524 (0.6016) |
| high | 2.143 (0.6547) | 2.429 (0.7464) | high | 2.000 (0.7776) | 2.246 (0.7477) | ||
| p = 0.1839 | p = 0.0684 | p = 0.0091 | p < 0.0001 | ||||
| CA125 | low | 1.950 (0.8256) | 2.000 (0.8584) | ERRγ | low | 1.632 (0.7609) | 1.632 (0.8307) |
| high | 1.940 (0.7663) | 2.180 (0.7571) | high | 1.970 (0.7611) | 2.220 (0.7343) | ||
| p = 0.9701 | p = 0.3678 | p = 0.0691 | p = 0.0031 | ||||
| CA72.4 | low | 1.955 (0.7756) | 2.188 (0.7656) | CMKLR1 | low | 1.314 (0.5827) | |
| high | 1.925 (0.7642) | 2.100 (0.7779) | high | 2.127 (0.7226) | |||
| p = 0.8374 | p = 0.5349 | p < 0.0001 | |||||
| Ki-67 | low | 1.936 (0.7488) | 2.083 (0.7592) | Chemerin | low | 1.560 (0.6440) | |
| high | 2.031 (0.8224) | 2.406 (0.7560) | high | 2.447 (0.6527) | |||
| p = 0.5490 | p = 0.0304 | p < 0.0001 | |||||
| Ovarian Cancer | Serous Ovarian Cancer | |||
|---|---|---|---|---|
| Chemerin | CMKLR1 | Chemerin | CMKLR1 | |
| ERα | n.s. | n.s. | n.s. | n.s. |
| PR | p < 0.0001 rho = 0.7952 | n.s. | p < 0.0001 rho = 0.8175 | n.s. |
| Ki67 (MKI67) | n.s. | n.s. | n.s. | n.s. |
| CA-125 (MUC16) | n.s. | n.s. | n.s. | n.s. |
| Her2 | n.s. | n.s. | n.s. | n.s. |
| EGFR | n.s. | n.s. | n.s. | n.s. |
| p53 | n.s. | n.s. | n.s. | n.s. |
| CEA (CEACAM1, 3,4,6,7 and 8) | p = 0.0498 rho = 0.1549 | n.s. | p = 0.0428 rho = 0.1868 | n.s. |
| CA72-4 | n.s. | n.s. | n.s. | n.s. |
| ERβ (n) | n.s. | p = 0.0009 rho = 0.2641 | p = 0.0213 rho = 0.2127 | p = 0.0039 rho = 0.2630 |
| ERβ (cm) | p = 0.0137 rho = 0.2009 | p = 0.007 rho = 0.216 | p = 0.0029 rho = 0.2731 | p = 0.003 rho = 0.2700 |
| ERRα | p < 0.0001 rho = 0.384 | p < 0.0001 rho = 0.5207 | p < 0.0001 rho = 0.3989 | p < 0.0001 rho = 0.4709 |
| ERRβ | p < 0.0001 rho = 0.3343 | p < 0.0001 rho = 0.4239 | p = 0.0007 rho = 0.3082 | p < 0.0001 rho = 0.3665 |
| ERRγ | p < 0.0001 rho = 0.3830 | p < 0.0001 rho = 0.4198 | p < 0.0001 rho = 0.4534 | p < 0.0001 rho = 0.4869 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, F.; Schueler-Toprak, S.; Buechler, C.; Ortmann, O.; Treeck, O. Chemerin and Chemokine-like Receptor 1 Expression in Ovarian Cancer Associates with Proteins Involved in Estrogen Signaling. Diagnostics 2023, 13, 944. https://doi.org/10.3390/diagnostics13050944
Weber F, Schueler-Toprak S, Buechler C, Ortmann O, Treeck O. Chemerin and Chemokine-like Receptor 1 Expression in Ovarian Cancer Associates with Proteins Involved in Estrogen Signaling. Diagnostics. 2023; 13(5):944. https://doi.org/10.3390/diagnostics13050944
Chicago/Turabian StyleWeber, Florian, Susanne Schueler-Toprak, Christa Buechler, Olaf Ortmann, and Oliver Treeck. 2023. "Chemerin and Chemokine-like Receptor 1 Expression in Ovarian Cancer Associates with Proteins Involved in Estrogen Signaling" Diagnostics 13, no. 5: 944. https://doi.org/10.3390/diagnostics13050944
APA StyleWeber, F., Schueler-Toprak, S., Buechler, C., Ortmann, O., & Treeck, O. (2023). Chemerin and Chemokine-like Receptor 1 Expression in Ovarian Cancer Associates with Proteins Involved in Estrogen Signaling. Diagnostics, 13(5), 944. https://doi.org/10.3390/diagnostics13050944








