Abstract
Blood transfusions are considered a risk factor for adverse outcomes after colorectal surgery. However, it is still unclear if they are the cause (the hen) or the consequence (the egg) of adverse events. A prospective database of 4529 colorectal resections gathered over a 12-month period in 76 Italian surgical units (the iCral3 study), reporting patient-, disease-, and procedure-related variables, together with 60-day adverse events, was retrospectively analyzed identifying a subgroup of 304 cases (6.7%) that received intra- and/or postoperative blood transfusions (IPBTs). The endpoints considered were overall and major morbidity (OM and MM, respectively), anastomotic leakage (AL), and mortality (M) rates. After the exclusion of 336 patients who underwent neo-adjuvant treatments, 4193 (92.6%) cases were analyzed through a 1:1 propensity score matching model including 22 covariates. Two well-balanced groups of 275 patients each were obtained: group A, presence of IPBT, and group B, absence of IPBT. Group A vs. group B showed a significantly higher risk of overall morbidity (154 (56%) vs. 84 (31%) events; OR 3.07; 95%CI 2.13–4.43; p = 0.001), major morbidity (59 (21%) vs. 13 (4.7%) events; OR 6.06; 95%CI 3.17–11.6; p = 0.001), and anastomotic leakage (31 (11.3%) vs. 8 (2.9%) events; OR 4.72; 95%CI 2.09–10.66; p = 0.0002). No significant difference was recorded between the two groups concerning the risk of mortality. The original subpopulation of 304 patients that received IPBT was further analyzed considering three variables: appropriateness of BT according to liberal transfusion thresholds, BT following any hemorrhagic and/or major adverse event, and major adverse event following BT without any previous hemorrhagic adverse event. Inappropriate BT was administered in more than a quarter of cases, without any significant influence on any endpoint. The majority of BT was administered after a hemorrhagic or a major adverse event, with significantly higher rates of MM and AL. Finally, a major adverse event followed BT in a minority (4.3%) of cases, with significantly higher MM, AL, and M rates. In conclusion, although the majority of IPBT was administered with the consequence of hemorrhage and/or major adverse events (the egg), after adjustment accounting for 22 covariates, IPBT still resulted in a definite source of a higher risk of major morbidity and anastomotic leakage rates after colorectal surgery (the hen), calling urgent attention to the implementation of patient blood management programs.
1. Introduction
Preoperative anemia is a very common finding, affecting more than 30% of patient candidates for major digestive surgery [1,2]. Consequently, it is the strongest predictor of blood transfusions (five-fold) in the postoperative period [2]. Postoperative anemia affects up to 90% of patients after major surgery [3]. The immediate and most widely used treatment for postoperative anemia is blood transfusion, entailing the risk of several complications, culminating in a higher incidence of morbidity and mortality [4,5,6]. A recent meta-analysis [7] identified blood transfusions (BTs) as a risk factor for poorer early postoperative outcomes, and previous multicenter prospective studies by the Italian The ColoRectal Anastomotic Leakage (iCral) study group [8,9] showed intra- and/or postoperative BT (IPBT) was independently associated with higher morbidity, anastomotic leakage, and mortality rates after colorectal surgery. However, the results of these studies do not allow one to solve the hen–egg issue in which it is still unclear whether blood transfusions are a definite risk factor for poorer outcomes rather than a marker of bad performers: on the one hand, perioperative blood transfusions may induce immunomodulation (transfusion-related immunomodulation, TRIM) because of the infusion of cytokines, lipids, and allogenic leukocytes, leading to immune activation and resulting in transfusion-related acute lung injury (TRALI) or immune suppression, increasing susceptibility to infectious complications; on the other hand, blood transfusions are generally more frequently administered in patients with major comorbidities, more extensive and longer procedures, more advanced cancer stages, and higher intraoperative blood loss. The iCral study group therefore decided to reappraise the results of its last prospective study (iCral3), trying to solve this hen–egg issue.
2. Materials and Methods
This is a retrospective analysis of the iCral3 study, designed to assess the influence of adherence to an enhanced recovery pathway (ERP) on patient-reported outcome measures and return to intended oncologic therapy after colorectal surgery. Seventy-six Italian surgical centers voluntarily participated in a prospective enrolment carried out from November 2020 to October 2021, upon explicit inclusion and exclusion criteria [10]. Adherence to twenty-six items of the ERP was measured for each enrolled case upon criteria adapted from the 2018 ERAS Society™ [11] and 2019 national [12] guidelines. For the purposes of this study, the population of 4529 enrolled cases was divided in two groups according to the presence (No. = 304; 6.7%) or absence (No. = 4225; 93.3%) of IPBT. Continuous variables were categorized according to their median value. The Mini Nutritional Assessment—Short Form (MNA-SF [13]) was categorized for values < 12, indicating potential malnutrition. Surgical procedures were categorized as standard (anterior resection, right colectomy, and left colectomy) versus non-standard (splenic flexure resection, transverse colectomy, Hartmann’s reversal, subtotal and total colectomy, and other) resections [9]. Biometric data, patient-, disease-, treatment-, and center-related variables (Table 1) were compared among the two groups using cross tabulation and chi-square or Fisher’s exact test where indicated. All analyses were conducted using StatsDirect™ statistical software (StatsDirect Ltd., Wirral, UK); the significance level was set at p < 0.05.

Table 1.
Comparative analysis of study variables in the two groups.
2.1. Outcomes
The study endpoints were overall morbidity (OM, any adverse event), major morbidity (MM, any adverse event grade > II according to Clavien-Dindo [14] and the Japanese Clinical Oncology Group (JCOG) extended criteria [15]), anastomotic leakage (AL), defined according to international consensus [16], and mortality (M, any death) rates at 60 days post-surgery.
2.2. Propensity-Score-Matched Analysis
Neo-adjuvant therapy is a treatment variable exclusively impacting a subgroup of patients; therefore, to avoid bias in the study design, 336 patients who received a neo-adjuvant treatment were excluded (Figure 1) and a cohort of 4193 cases was divided into two groups according to the presence (Group A; No. = 280; 6.7%) or absence (Group B; No. = 3913; 93.3%) of intra- and/or postoperative blood transfusions (IPBTs).
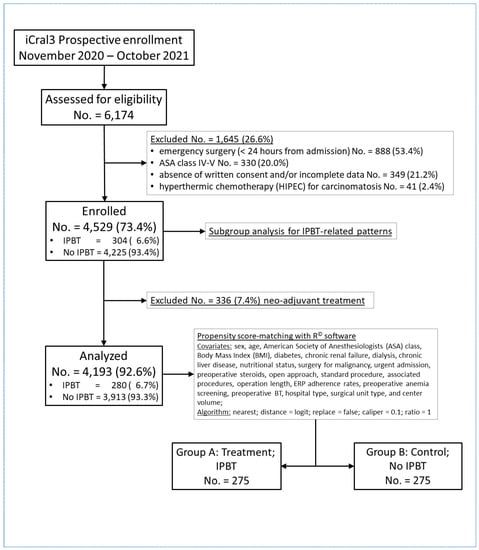
Figure 1.
Study flowchart according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines [17] and to the Reporting and Guidelines in Propensity Score Analysis [18]: iCral: Italian ColoRectal Anastomotic Leakage study group; ASA: American Society of Anesthesiologists; IPBT: intra- and/or postoperative blood transfusion(s); and ERP: enhanced recovery pathway.
The propensity score matching analysis (PSMA) model [19,20] was based on (a) IPBT as the treatment (exposure) variable; (b) group A as the true population of interest; (c) group B as the control population; and (d) the following 22 covariates (confounding variables): sex, age, American Society of Anesthesiologists (ASA) class, body mass index (BMI), diabetes, chronic renal failure, dialysis, chronic liver disease, MNA-SF < 12, surgery for malignancy, urgent admission, preoperative steroids, open approach, standard procedure, associated procedures, operation length, ERP adherence rates, preoperative anemia screening, preoperative BT, hospital type, surgical unit type, and center volume. Adjusted logistic regression was used to estimate the propensity scores in the treatment and control groups.
Based on the conditioning categorical variables selected, each patient was assigned a propensity score estimated by the standardized mean difference (a standardized mean difference less than 0.1 typically indicates a negligible difference between the means of the groups). No outcome variable was included [21]. As balance is the main goal of PSMA, the analysis was performed using the software “R©” (The R Foundation© for Statistical Computing, Vienna, Austria) with the following specifications: (a) seed 100 for the reproducibility of the analysis; (b) method for distance metric = nearest, distance = logit, caliper = 0.1, replace = false (without sampling replacement), ratio = 1; (c) adjusted logistic regression to estimate the association between the exposure/treatment variable and the outcomes. The following R© libraries/programs were used: “matchit”, “glm”, “publish”, “Tablone”, “Plot”, and “cobalt” [22]. Balance in the matched groups was assessed by calculating the standardized mean difference (SMD) and general variance ratio (a variance ratio close to 1 means that variances are equal in the two groups). For outcome modeling, an adjusted logistic regression, based on IPBT as the treatment variable and on the same 22 covariates selected for the PSMA, was performed, presenting odds ratios (ORs) and their 95% confidence intervals (95%CI). The eventual effect of any unobserved confounder was tested via sensitivity analysis [23], using the R© software library “SensitivityR5” and presenting the Γ values (each 0.1 increment of Γ values representing a 10% odds of differential assignment to treatment due to any unobserved variable).
2.3. Subgroup Analysis in the IPBT Population
Considering the population of 304 patients who received one or more IPBT (No. = 304), BT was considered appropriated when administered for Hb levels below liberal [24] transfusion thresholds (≤80 g/L for ASA class I-II, absence of hemodynamic instability, and absence of myocardial ischemia; ≤100 g/L for ASA class III, presence of hemodynamic instability, and/or myocardial ischemia). Furthermore, BT was considered (the egg) secondary to bleeding and/or any major adverse event (B/MAE-BT) if it was administered during the operation and/or within 24 h from it, and/or if there was evidence of any previous hemorrhagic (i.e., abdominal bleeding, trocar/wound site bleeding, or anastomotic bleeding) or major adverse event (MAE). Conversely, any MAE was considered (the hen) secondary to BT when it occurred after any BT without any previous hemorrhagic adverse event (BT-MAE). Again, these three BT categories were further tested for the endpoints, individually and combined in several scenarios, using cross tabulation and the chi-square or Fisher’s exact test where indicated. All analyses were conducted using StatsDirect™ statistical software (StatsDirect Ltd., Wirral, UK); the significance level was set at p < 0.05.
3. Results
The outcomes recorded in the whole population are shown in Table 2.

Table 2.
Comparative analysis of outcomes in the two groups.
3.1. Propensity-Score-Matched Analysis
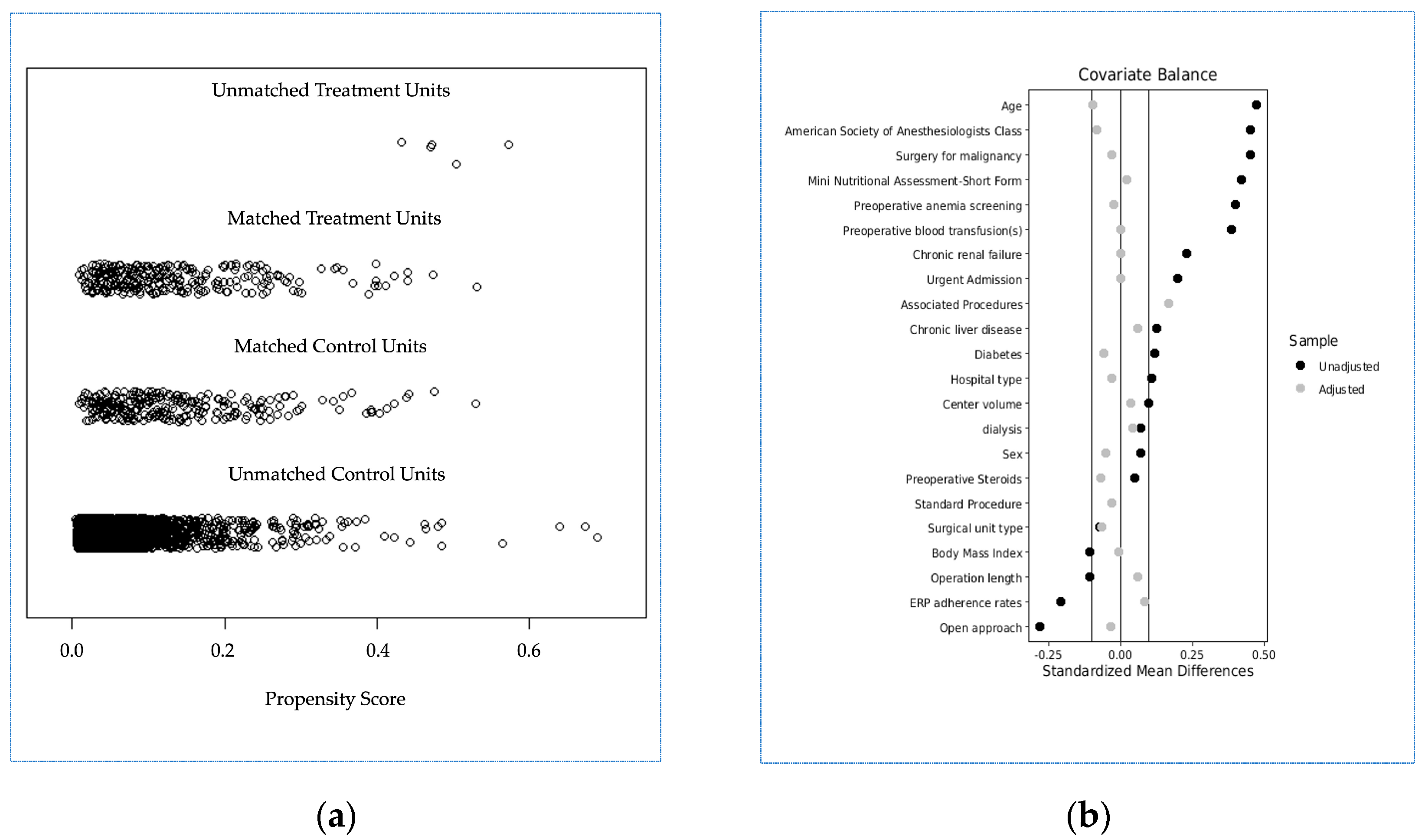
After propensity score matching, 3643 cases were excluded (5 with IPBT and 3638 without IPBT, and two groups of 275 patients each were generated: group A (IPBT, true population of interest) and group B (no IPBT, control population)). A good balance between the two groups was achieved (Table 3 and Figure 2), with a model variance ratio of 1.005.

Table 3.
Variable distribution in treatment and control groups before and after propensity score matching.

Figure 2.
(a) Jitter plot distribution of propensity scores in treatment and control groups; (b) Love plot of covariates’ standardized mean differences between treatment and control groups before and after matching; the vertical lines represent the interval of ±0.1 and within which balance is considered to be acceptable.
After adjusted logistic regression, group A vs. group B (Table 4) showed a significantly higher risk of OM (154 (56.0%) vs. 84 (30.5%) events; OR 3.07; 95%CI 2.13–4.43; p = 0.001), MM (59 (21.4%) vs. 13 (4.7%) events; OR 6.06; 95%CI 3.17–11.6; p = 0.001), and AL (31 (11.3%) vs. 8 (2.9%) events; OR 4.72; 95%CI 2.09–10.66; p = 0.0002). No difference was recorded between the two groups (8 (2.9%) vs. 5 (1.8%) events; OR 1.57; 95%CI 0.42–5.79; p = 0.50) concerning the risk of mortality.

Table 4.
Adjusted multiple regression analysis for endpoints.
Compared to local/regional hospitals, metropolitan/academic hospitals showed a significantly lower risk of OM (125/326 (38.3%) vs. 113/224 (50.4%) events; OR 0.61; 95%CI 0.41–0.92; p = 0.0166) and mortality (3/326 (0.9%) vs. 10/224 (4.5%) events; OR 0.17; 95%CI 0.03–0.90; p = 0.0366). Male vs. female sex was associated with a significantly higher risk of OM (144/309 (46.6%) vs. 94/241 (39.0%) events; OR 1.47; 95%CI 1.0–2.15; p = 0.0487) and MM (50/309 (16.2%) vs. 22/241 (9.1%) events; OR 2.26; 95%CI 1.26–4.08; p = 0.0066). At the same time, operation length > vs. ≤ 180 min was associated with a significantly higher risk of OM (107/214 (50.0%) vs. 131/336 (39.0%) events; OR 1.60; 95%CI 1.08–2.38; p = 0.0183), enrolment > vs. ≤ 44 cases to MM (63/438 (14.4%) vs. 9/112 (8.0%) events; OR 2.36; 95%CI 1.04–5.34; p = 0.0397), and presence vs. absence of chronic renal failure to mortality (5/62 (8.1%) vs. 8/488 (1.6%) events; OR 5.11; 95%CI 1.06–24.54; p = 0.0416).
3.2. Subgroup Analysis in the IPBT Population
Outcome rates according to individual evaluation of the three BT categories (appropriateness; B/MAE-BT; BT-MAE) are shown in Table 5.

Table 5.
Outcome rates according to individual BT categories.
Inappropriate BT was administered in more than a quarter of cases, without any significant influence on any endpoint. On the other hand, the majority of BTs were administered after a hemorrhagic or a major adverse event, with significantly higher rates of MM and AL, but not OM or M. Finally, a BT-MAE was recorded in a minority (4.3%) of cases, showing significantly higher MM, AL, and M rates. Six different scenarios were recorded after matching the three BT categories (Table 6).

Table 6.
Matching scenarios of BT categories.
All of the scenarios related to BT determined a significant variation in MM, AL, and M rates, with the worst scenario represented by a major adverse event following an appropriate BT.
4. Discussion
The comparison of raw data in the subgroups of the whole population (Table 1) fully agrees with the previous findings of the iCral 1 and 2 studies [8,9]; the IPBT subgroup is a reservoir of bad performers (with most of the considered variables showing a significant unfavorable pattern in this subgroup of patients), with significant higher rates of unfavorable outcomes (Table 2). In this setting, it seems that the egg was born before the hen (IPBT may represent the consequence, rather than the cause, of poorer outcomes). Once a nearly perfect balance of the 22 confounding variables was achieved through propensity score matching (Table 3, Figure 2), the paradigm appeared to be totally reversed; the adjusted logistic regression analysis clearly showed (Table 4) that group A (IPBT), compared to group B (no IPBT), is linked to an independent and significant higher risk of OM, MM, and AL (with the lack of statistical significance of the difference concerning the risk of mortality being possibly due to the small number of recorded events). According to these results, it seems that the hen was born before the egg (IPBT may be the cause, rather than the consequence, of poorer outcomes). Assuming that the probabilities of random assignment to the two treatment groups could be different, the sensitivity analysis (Table 4) showed that the relative impact of unknown and/or unmeasured confounding variables should double (Γ = 2.3) for OM and triple (Γ = 3.3) for MM to alter the results and/or their statistical significance. Therefore, the repercussions of this finding on everyday clinical practice are quite relevant: the absolute risk reductions linked to no IPBT recorded in the present study led to small number needed to treat; this could be sufficient to avoid IPBT in 4, 6, and 12 patients to avoid one adverse event, one major adverse event, and one anastomotic leakage, respectively. Another consequence of these findings is that, although the described relationship between blood transfusion and poorer outcomes is not new, a clear understanding of the mechanism by which IPBT may worsen the early outcomes after colorectal surgery is still lacking. Apart from the long-standing and updated concept of TRIM and transient immunosuppression [25,26], a recent retrospective propensity-score-matched study on colorectal cancer surgery patients [27] suggested that the worst early outcomes after surgery for colorectal cancer may be mediated by an exaggerated perioperative systemic inflammatory response in patients receiving perioperative blood transfusions. Moreover, recent experimental evidence [28] suggests a direct link between the gut flora composition (microbiota) and the development of antibody-mediated TRALI in mice. The recent introduction of metabolomics and proteomics to transfusion medicine [29] will possibly clarify how the microbiome and gut microbiota can affect the immune system shaping the antigenicity and contributing to TRIM and the potential transmission of infection by blood donors. As the vast majority of colorectal resections are commonly performed for cancer, representing a particularly vulnerable population and showing significant immunosuppression and altered microbiota [30], further clinical investigation on this issue is warranted.
Most of the other significant findings of the logistic regression analysis, such as higher risk of adverse outcomes in male vs. female sex, metropolitan/academic vs. local/regional hospitals, operation length > vs. ≤ 180 min, and presence vs. absence of chronic renal failure, were expected, having been already recorded in previous studies [8,9]. On the other hand, the finding of a higher risk of major morbidity recorded in high vs. low volume centers seems to confirm that the surgeon’s volume may be more relevant than center volume [31].
Although perioperative BT rates have been declining in the last decade, no change in the risk of mortality after surgery was recorded [32], and there is still a wide variability in perioperative transfusion practices in colorectal surgery [33]. We decided, therefore, to consider liberal (Hb ≤ 80–100 g/L) rather than recommended [24] restrictive (Hb ≤ 70–80 g/L) transfusion thresholds in the analysis of the original subpopulation of patients that received IPBT. Even considering liberal thresholds, inappropriate IPBT was still administered in more than a quarter of cases (Table 5), although this did not determine any significant difference in the outcomes. Anyway, the majority of IPBTs were administered after hemorrhagic and/or major adverse events with a small subgroup of patients (4.3%), in which the BT preceded the major adverse event without any previous hemorrhagic event (Table 5), showing the highest rates of adverse outcomes (Table 6). Applying the long-time-honored 20–80 rule, also known as the “Pareto Principle” [34,35], it could be argued that improving transfusion appropriateness and eliminating this small subgroup of patients may allow for a significant improvement in the outcomes. This is the main aim of the recent call toward the urgent need for patient blood management (PBM) program [36,37] implementation by the World Health Organization [38] and the Italian Surgical Association [39]. Actually, a recent pre- vs. post-PBM implementation study regarding colorectal cancer surgery from Korea [40] showed a significant decrease in the total transfusion rate, Hb threshold before transfusion (Hb trigger), anastomotic leakage rate, and postoperative length of stay. For these reasons, the iCral study group is currently enrolling patients in its fourth observational multicenter prospective study [41], designed to test the effect of adherence to a combined ERP-PBM pathway on blood transfusion rates and outcomes.
The main strength of this study is its methodology: a large database gathered during a prospective multicenter study was analyzed using a PSMA perfectly responding to the EQUATOR (Enhancing the Quality and Transparency of Health Research) network reporting guidelines [18]. Although observational studies cannot be regarded as a replacement for randomized studies, data generated from large observational cohorts have been increasingly used to evaluate important clinical questions where data from randomized trials are limited or do not exist [42], mainly because of lower barriers and cost regarding subject recruitment. PSMA offers an alternative approach for estimating treatment effects with observational data when randomized trials are not feasible or unethical, or when researchers need to assess treatment effects based on real life data, collected through the observation of systems as they operate in normal practice without any intervention implemented via randomized assignment rules, responding to the frequent need to draw conditioned casual inferences from quasi-experimental studies. To account for the conditional probability of treatment selection, thus reducing confounding bias, PSMA presents analytical and interpretation challenges that need to be addressed to maintain the reproducibility of its results, which in recent years has been recognized as a crucial element of high-quality research [43]. The relevant quality of the PSMA used in the present study is based on (1) rigorous patient selection from the parent population, performed adhering to explicit criteria; (2) the inclusion of 22 conditioning variables (covariates), such as hospital type, unit type, and accrual volume, to account for the potential heterogeneity of multicenter, clustered data and adherence to the ERP to account for the potential heterogeneity of medical, anesthesiologic, and surgical perioperative management; (3) a clear, sheer, and restrictive balance algorithm (Figure 1), particularly regarding caliper = 0.1, matching ratio = 1:1, and complete balance assessment; (4) complete description of the software package and of its related analytic details; (5) evaluation of the treatment effect through an adjusted multiple regression model including the same 22 covariates used for matching; and (6) a sensitivity analysis to account for unmeasured confounders.
The other strength of this study is the large number of enrolled patients in a well-defined time-lapse in a large number of centers, representing a very wide sample of surgical units performing colorectal resections in Italy. While the multicenter nature of the parent database may be a definite source of clustering bias, it is undoubtedly representative of real-life data.
However, this study is subject to several limitations, and its results should be interpreted with caution. Several potential confounders were not measured or recorded in the parent study: the number and age of transfused packed red blood cells [44,45], pre- and postoperative Hb levels, iron and Hb status before and after BT [3], the management of preoperative and postoperative anemia through high-dose i.v. iron preparations [46,47], and, as reported above, the composition of blood donors and recipients’ microbiome [48]. Finally, although data quality control was performed and repeated at various levels, we could not rule out potential measurement errors caused by the participating investigators.
5. Conclusions
This retrospective PSMA of a large prospective multicenter database confirmed that IPBTs are a definite risk factor for morbidity and anastomotic leakage after colorectal resections even after a well-balanced matching of 22 potential confounders. Although most IPBTs are administered in response to intraoperative blood loss and early postoperative hemorrhagic adverse events, in a minority of cases a major adverse event is triggered by IPBT. In this setting, the avoidance of inappropriate (or unnecessary) BT through the implementation of PBM programs in colorectal surgery may significantly influence the incidence of perioperative adverse outcomes.
Author Contributions
M.C., iCral study group coordinator, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: M.C., S.G., L.A.M., P.C., M.B., P.D., G.G., F.P. and M.S. Acquisition, analysis, or interpretation of data: M.C., S.G., F.M., L.A.M., P.C., M.B., P.D., G.G., F.P. and M.S. Drafting of the manuscript: M.C., S.G. and F.M. Critical revision of the manuscript for important intellectual content: M.C., S.G., F.M., L.A.M., P.C., M.B., P.D., G.G., F.P. and M.S. Statistical analysis: F.M., S.G. and M.C. All other co-authors participated in data acquisition and quality control, and read and approved the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice E6 (R2) principles. The study protocol was approved by the coordinating center’s ethics committee (Comitato Etico Regionale delle Marche—C.E.R.M. #2020/192, approved on 30 July 2020) and was registered at ClinicalTrials.gov (NCT04397627). Thereafter, all participating centers obtained authorization from the local institutional review board.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Individual participant-level anonymized datasets are available upon reasonable request by contacting the study coordinator.
Conflicts of Interest
Catarci reports personal fees from Baxter Spa outside the submitted work. Guadagni, Masedu, Montemurro, Ciano, Benedetti, Delrio, Garulli, Pirozzi, Scatizzi, and all other co-authors have no competing interests to declare.
Appendix A
† iCral3 study group investigators with shared authorship are as follows: Maria Sole Mattei, MD, Elena Belloni, MD, Matteo Di Carlo, MD, Daniela Apa, MD, General Surgery Unit; Sandro Pertini Hospital, ASL Roma 2; Marco Clementi, MD, General Surgery Unit, University of L’Aquila; Ugo Pace, MD, Andrea Fares Bucci, MD, Colorectal Surgical Oncology, Istituto Nazionale per lo Studio e la Cura dei Tumori, “Fondazione Giovanni Pascale IRCCS-Italia”, Napoli; Francesco Monari, MD, General Surgery Unit, Infermi Hospital, Rimini; Antonio Sciuto, MD, General Surgery Unit, ASL Napoli 2 Nord, Pozzuoli (NA); Lorenzo Pandolfini, MD, Alessandro Falsetto, MD, General Surgery Unit, Santa Maria Annunziata and Serristori Hospital, Firenze; Giacomo Ruffo, MD, Elisa Bertocchi, MD, Gaia Masini, MD, General Surgery Unit, IRCCS Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella (VR); Massimo Giuseppe Viola, MD, Amedeo Altamura, MD, Francesco Rubichi, MD, General Surgery Unit, Cardinale G. Panico Hospital, Tricase (LE); Ferdinando Ficari, MD, Francesco Giudici, MD, Fabio Cianchi, MD, General Surgery and IBD Unit, Careggi University Hospital, Firenze; Felice Borghi, MD, Oncologic Surgery Unit, Candiolo Cancer Institute, FPO-IRCCS, Candiolo (TO); Desirée Cianflocca, MD, Marco Migliore, MD, General and Oncologic Surgery Unit, Department of Surgery, Santa Croce e Carle Hospital, Cuneo; Raffaele De Luca, MD, Department of Surgical Oncology, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; Alessandro Rizzo, MD, Department of Medical Oncology, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; Anna Albano, MS, Trial Office (AA), IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; Alberto Patriti, MD, Marcella Lodovica Ricci, MD, Department of Surgery, Marche Nord Hospital, Pesaro e Fano (PU); Walter Siquini, MD, Alessandro Cardinali, MD, General Surgery Unit, S. Lucia Hospital, Macerata; Stefano D’Ugo, MD, PhD, FEBS, FACS, Marcello Spampinato, MD, PhD, FEBS (HPB), General Surgery Unit, “V. Fazzi” Hospital, Lecce; Stefano Scabini, MD, Alessandra Aprile, MD, Domenico Soriero, MD, General and Oncologic Surgery Unit, IRCCS “San Martino” National Cancer Center, Genova; Marco Caricato, MD, FACS, Gabriella Teresa Capolupo, MD, FACS, Colorectal Surgery Unit, Policlinico Campus BioMedico, Roma; Giusto Pignata, MD, Jacopo Andreuccetti, MD, Ilaria Canfora, MD, Second General Surgery Unit 2, Spedali Civili di Brescia; Andrea Liverani, MD, Andrea Scarinci, MD, General Surgery Unit, Regina Apostolorum Hospital, Albano Laziale (RM); Roberto Campagnacci, MD, Angela Maurizi, MD, General Surgery Unit, “C. Urbani” Hospital, Jesi (AN); Pierluigi Marini, MD, Grazia Maria Attinà, MD, General and Emergency Surgery Unit, San Camillo-Forlanini Hospital, Roma; Ugo Elmore, MD, Giulia Maggi, MD, Department of Gastrointestinal Surgery Unit, San Raffaele Research Hospital and “Vita-Salute” San Raffaele University, Milano; Francesco Corcione, MD, Umberto Bracale, MD, Roberto Peltrini, MD, Maria Michela Di Nuzzo, MD, Minimally Invasive General and Oncologic and Surgery Unit, “Federico II” University, Napoli; Roberto Santoro, MD, Pietro Amodio, MD, General Oncologic Surgery Unit, Belcolle Hospital, Viterbo; Massimo Carlini, MD, FACS, Domenico Spoletini, MD, PhD, FACS, Rosa Marcellinaro, MD, Giorgio Lisi, MD, General Surgery Unit, S. Eugenio Hospital, ASL Roma 2; Antonio Giuliani, MD, Giovanni Del Vecchio, MD, General Surgery Unit, S. Carlo Hospital, Potenza; Mario Sorrentino, MD, Massimo Stefanoni, MD, General Surgery Unit, Latisana-Palmanova Hospital, Friuli Centrale University (UD); Giovanni Ferrari, MD, Carmelo Magistro, MD, General Oncologic and Mininvasive Surgery Unit, Great Metropolitan Niguarda Hospital, Milano; Gianandrea Baldazzi, MD, Diletta Cassini, MD, General Surgery Unit, ASST Ovest Milanese, Nuovo Ospedale di Legnano, Legnano (MI); Alberto Di Leo, MD, Lorenzo Crepaz, MD, General and Minimally Invasive Surgery Unit, San Camillo Hospital, Trento; Augusto Verzelli, MD, Andrea Budassi, MD, General Surgery Unit, Profili Hospital, Fabriano (AN); Giuseppe Sica, MD, Bruno Sensi, MD, Minimally Invasive Surgery Unit, Policlinico Tor Vergata University Hospital, Roma; Stefano Rausei, MD, Silvia Tenconi, MD, General Surgery Unit, Gallarate Hospital (VA); Davide Cavaliere, MD, Leonardo Solaini, MD, Giorgio Ercolani, MD, General and Oncologic Surgery Unit, AUSL Romagna, Forlì (FC); Gian Luca Baiocchi, MD, FACS, Sarah Molfino, MD, General Surgery Unit 3, Department of Clinical and Experimental Sciences, University of Brescia; Marco Milone, MD, Giovanni Domenico De Palma, MD, General and Endoscopic Surgery Unit, “Federico II” University, Napoli; Giovanni Ciaccio, MD, Paolo Locurto, MD, General Surgery Unit, S. Elia Hospital, Caltanissetta; Giovanni Domenico Tebala, MD, FACS, FRCS, Antonio Di Cintio, MD, General Surgery Unit, S. Maria Hospital, Terni; Luigi Boni, MD, FACS, Elisa Cassinotti, MD, General Surgery Unit, Fondazione IRCCS Ca’ Granda, Policlinico Maggiore Hospital, Milano; Stefano Mancini, MD, Andrea Sagnotta, MD, PhD, General and Oncologic Surgery Unit, San Filippo Neri Hospital, ASL Roma 1; Mario Guerrieri, MD, Monica Ortenzi, MD, Surgical Clinic, Torrette Hospital, University of Ancona; Roberto Persiani, MD, Alberto Biondi, MD, General Surgery Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma; Andrea Lucchi, MD, FACS, Alban Cacurri, MD, General Surgery Unit, “Ceccarini” Hospital, Riccione (RN); Dario Parini, MD, Maurizio De Luca, MD, General Surgery Unit, S. Maria della Misericordia Hospital, Rovigo; Antonino Spinelli, MD, Francesco Carrano, MD, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI) and IRCCS Humanitas Research Hospital, Rozzano (MI); Michele Genna, MD, Francesca Fior, MD, General Surgery Unit, University Hospital, Verona; Vincenzo Bottino, MD, Antonio Ferronetti, MD, General and Oncologic Surgery Unit, Evangelico Betania Hospital, Napoli; Andrea Coratti, MD, Giuseppe Giuliani, MD, Roberto Benigni, MD, General and Emergency Surgery Unit, Misericordia Hospital, Grosseto; Dario Scala, MD, Graziella Marino, MD, Battistino Puppio, MD, Abdominal Oncologic Surgery Unit, IRCCS CROB Basilicata Referral Cancer Center, Rionero in Vulture (PZ); Andrea Muratore, MD, Patrizia Marsanic, MD, Nicoletta Sveva Pipitone Federico, MD, General Surgery Unit, “E. Agnelli” Hospital, Pinerolo (TO); Maurizio Pavanello, MD, Carlo Di Marco, MD, General Surgery Unit, AULSS2 Marca Trevigiana, Conegliano Veneto (TV); Umberto Rivolta, MD, Camillo Leonardo Bertoglio, MD, PhD, General Surgery Unit, Fornaroli Hospital, ASST Ovest Milanese, Magenta (MI); Micaela Piccoli, MD, FACS, Francesca Pecchini, MD, General Surgery Unit, Civil Hospital, Baggiovara (MO); Carlo Talarico, MD, Vincenzo Greco, MD, General Surgery Unit, Villa dei Gerani Hospital, Vibo Valentia (VV); Alessandro Carrara, MD, Michele Motter, MD, Giuseppe Tirone, MD, First General Surgery Unit, S. Chiara Hospital, Trento; Mauro Totis, MD, Nicolò Tamini, MD, Colorectal Surgery Unit, San Gerardo Hospital, ASST Monza; Franco Roviello, MD, Riccardo Piagnerelli, MD, General and Oncologic Surgery Unit, AOU Senese, Siena; Alessandro Anastasi, MD, Giuseppe Canonico, MD, General Surgery Unit, San Giovanni di Dio Hospital, Firenze; Gianluca Guercioni, MD, Simone Cicconi, MD, General Surgery Unit, “C. e G. Mazzoni” Hospital, Ascoli Piceno; Giuseppe Maria Ettorre, MD, Marco Colasanti, MD, General and Transplant Surgery Unit, San Camillo-Forlanini Hospital, Roma; Mauro Montuori, MD, Enrico Pinotti, MD, General and Mininvasive Surgery Unit, S. Pietro Hospital, Ponte San Pietro (BG); Pierpaolo Mariani, MD, Roberta Carminati, MD, General Surgery Unit, Pesenti Fenaroli Hospital, Alzano Lombardo (BG); Nicolò de Manzini, MD, Edoardo Osenda, MD, Surgical Clinic, University of Trieste; Annibale Donini, MD, Luigina Graziosi, MD, General and Emergency Surgery Unit, University of Perugia; Mariano Fortunato Armellino, MD, Ciro De Martino, MD, Giovanna Ioia, MD, General and Emergency Surgery Unit, S. Giovanni di Dio e Ruggi d’Aragona Hospital, Salerno; Lucio Taglietti, MD, Arianna Birindelli, MD, General Surgery Unit, ASST Valcamonica, Esine (BS); Gabriele Anania, MD, Matteo Chiozza, MD, General and Laparoscopic Surgery Unit, University Hospital, Ferrara; Mariantonietta Di Cosmo, MD, Daniele Zigiotto, MD, General and Upper GI Surgery Unit, University Hospital, Verona; Carlo Vittorio Feo, MD, Fioralba Pindozzi, MD, General Surgery Unit, Delta Hospital, Lagosanto (FE); Paolo Millo, MD, Manuela Grivon, MD, General Surgery Unit, “U. Parini” Regional Hospital, Aosta; Corrado Pedrazzani, MD, Cristian Conti, MD, General and HPB Surgery Unit, University Hospital, Verona; Silvio Guerriero, MD, Lorenzo Organetti, MD, General Surgery Unit, “A. Murri” Hospital, Fermo; Andrea Costanzi, MD, Michela Monteleone, MD, General Surgery Unit, S. Leopoldo Hospital, Merate (LC); Nereo Vettoretto, MD, Emanuele Botteri, MD, General Surgery Unit, Spedali Civili of Brescia, Montichiari (BS); Federico Marchesi, MD, Giorgio Dalmonte, MD, Surgical Clinic, University of Parma; Massimo Basti, MD, Diletta Frazzini, MD, General Surgery Unit, Spirito Santo Hospital, Pescara; Graziano Longo, MD, Simone Santoni, MD, General Surgery Unit, Policlinico Casilino, Roma; Moreno Cicetti, MD, Gabriele La Gioia, MD, General Surgery Unit, S. Maria della Misericordia Hospital, Urbino (PU); Italy.
References
- Beattie, W.S.; Karkouti, K.; Wijeysundera, D.N.; Tait, G. Risk associated with preoperative anemia in noncardiac surgery: A single-center cohort study. Anesthesiology 2009, 110, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Rao Baikady, R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.E.; Kim, T.J.; Khanuja, H.S. Perioperative blood transfusions in orthopaedic surgery. J. Bone Joint Surg. Am. 2014, 96, 1836–1844. [Google Scholar] [CrossRef]
- Kumar, A. Perioperative management of anemia: Limits of blood transfusion and alternatives to it. Clevel. Clin. J. Med. 2009, 76 (Suppl. 4), S112–S118. [Google Scholar] [CrossRef]
- Saleh, A.; Small, T.; Chandran Pillai, A.L.; Schiltz, N.K.; Klika, A.K.; Barsoum, W.K. Allogenic blood transfusion following total hip arthroplasty: Results from the nationwide inpatient sample, 2000 to 2009. J. Bone Joint Surg. Am. 2014, 96, e155. [Google Scholar] [CrossRef]
- Pang, Q.Y.; An, R.; Liu, H.L. Perioperative transfusion and the prognosis of colorectal cancer surgery: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 7–17. [Google Scholar] [CrossRef]
- Italian ColoRectal Anastomotic Leakage (iCral) Study Group. Risk factors for adverse events after elective colorectal surgery: Beware of blood transfusions. Updates Surg. 2020, 72, 811–819. [Google Scholar] [CrossRef]
- Catarci, M.; Ruffo, G.; Viola, M.G.; Pirozzi, F.; Delrio, P.; Borghi, F.; Garulli, G.; Baldazzi, G.; Marini, P.; Sica, G.; et al. ERAS program adherence-institutionalization, major morbidity and anastomotic leakage after elective colorectal surgery: The iCral2 multicenter prospective study. Surg. Endosc. 2022, 36, 3965–3984. [Google Scholar] [CrossRef]
- The Italian Colorectal Anastomotic Leakage (iCral) Study Group. Patient-reported outcomes and return to intended oncologic therapy after colorectal enhanced recovery pathway: The iCral3 prospective study. Ann. Surg. Open, 2023; in press. [Google Scholar]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, N.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Ficari, F.; Borghi, F.; Catarci, M.; Scatizzi, M.; Alagna, V.; Bachini, I.; Baldazzi, G.; Bardi, U.; Benedetti, M.; Beretta, L.; et al. Enhanced recovery pathways in colorectal surgery: A consensus paper by the Associazione Chirurghi Ospedalieri Italiani (ACOI) and the PeriOperative Italian Society (POIS). Il Giornale di Chirurgia 2019, 40 (Suppl. 4), 1–40. [Google Scholar]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Kurokawa, Y.; Nakamura, K.; Ito, H.; Kanemitsu, Y.; Masuda, N.; Tsubosa, Y.; Satoh, T.; Yokomizo, A.; Fukuda, H.; et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg. Today 2016, 46, 668–685. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Yao, X.I.; Wang, X.; Speicher, P.J.; Hwang, E.S.; Cheng, P.; Harpole, D.H.; Berry, M.F.; Schrag, D.; Pang, H.H. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J. Natl. Cancer Inst. 2017, 109, djw323. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R. The power of a sensitivity analysis and its limit. In Design of Observational Studies, 2nd ed.; Springer Series in Statistics; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 317–336. [Google Scholar]
- Carson, J.L.; Stanworth, S.J.; Dennis, J.A.; Trivella, M.; Roubinian, N.; Fergusson, D.A.; Triulzi, D.; Dorée, C.; Hébert, P.C. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst. Rev. 2021, 12, CD002042. [Google Scholar]
- Opelz, G.; Sengar, D.P.; Mickey, M.R.; Terasaki, P.I. Effect of blood transfusions on subsequent kidney transplants. Transplant. Proc. 1973, 5, 253–259. [Google Scholar] [PubMed]
- Vamvakas, E.C.; Blajchman, M.A. Transfusion-related immunomodulation (TRIM): An update. Blood Rev. 2007, 21, 327–348. [Google Scholar] [CrossRef] [PubMed]
- McSorley, S.T.; Tham, A.; Dolan, R.D.; Steele, C.W.; Ramsingh, J.; Roxburgh, C.; Horgan, P.G.; McMillan, D.C. Perioperative Blood transfusion is associated with postoperative systemic inflammatory response and poorer outcomes following surgery for colorectal cancer. Ann. Surg. Oncol. 2020, 27, 833–843. [Google Scholar] [CrossRef]
- Kapur, R.; Kim, M.; Rebetz, J.; Hallström, B.; Björkman, J.T.; Takabe-French, A.; Kim, N.; Liu, J.; Shanmugabhavananthan, S.; Milosevic, S.; et al. Gastrointestinal microbiota contributes to the development of murine transfusion-related acute lung injury. Blood Adv. 2018, 2, 1651–1663. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Zolla, L. Proteomic analysis of red blood cells and the potential for the clinic: What have we learned so far? Expert Rev. Proteom. 2017, 14, 243–252. [Google Scholar] [CrossRef]
- Goubran, H.; Seghatchian, J.; Radosevic, J.; Ragab, G.; Burnouf, T. The microbiome and transfusion in cancer patients. Transfus. Apher. Sci. 2017, 56, 330–335. [Google Scholar] [CrossRef]
- García-Granero, E.; Navarro, F.; Cerdán Santacruz, C.; Frasson, M.; García-Granero, A.; Marinello, F.; Flor-Lorente, B.; Espí, A. Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: An institutional analysis of 800 patients. Surgery 2017, 162, 1006–1016. [Google Scholar] [CrossRef]
- Nordestgaard, A.T.; Rasmussen, L.S.; Sillesen, M.; Steinmetz, J.; Eid, A.I.; Meier, K.; Kaafarani, H.M.A.; Velmahos, G.C. Red blood cell transfusion in surgery: An observational study of the trends in the USA from 2011 to 2016. Anaesthesia 2020, 75, 455–463. [Google Scholar] [CrossRef]
- Aquina, C.T.; Blumberg, N.; Probst, C.P.; Becerra, A.Z.; Hensley, B.J.; Noyes, K.; Monson, J.R.; Fleming, F.J. Large Variation in Blood Transfusion Use After Colorectal Resection: A Call to Action. Dis. Colon Rectum 2016, 59, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Pareto, V. Cours d’Économie Politique Professé a l’Université de Lausanne; F. Rouge: Lausanne, Switzerland, 1896; Volume I. [Google Scholar]
- Juran, J.J. Quality Control Handbook; McGraw-Hill: New York, NY, USA, 1951. [Google Scholar]
- Hofmann, A.; Farmer, S.; Towler, S.C. Strategies to preempt and reduce the use of blood products: An Australian perspective. Curr. Opin. Anaesthesiol. 2012, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Isbister, J.P. The three-pillar matrix of patient blood management. ISBT Sci. Ser. 2015, 10 (Suppl. 1), 286–294. [Google Scholar] [CrossRef]
- World Health Organization. The Urgent Need to Implement Patient Blood Management: Policy Brief; Electronic Version; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003574-4. [Google Scholar]
- Catarci, M.; Borghi, F.; Ficari, F.; Scatizzi, M. Perioperative anemia and its implications. II Giornale di Chirurgia—JISA 2022, 42, e01. [Google Scholar] [CrossRef]
- Shin, S.H.; Piozzi, G.N.; Kwak, J.M.; Baek, S.J.; Kim, J.; Kim, S.H. Effect of a Patient Blood Management system on perioperative transfusion practice and short-term outcomes of colorectal cancer surgery. Blood Transfus. 2022, 20, 475–482. [Google Scholar]
- Italian ColoRectal Anastomotic Leakage (iCral) Study Group. Enhanced Recovery and Patient Blood Management in Colorectal Surgery: The Italian ColoRectal Anastomotic Leakage Study Group (iCral 4). NCT05227014; 2022; ClinicalTrials.gov—NIH—US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT05227014 (accessed on 28 December 2022).
- Silverman, S.L. From randomized controlled trials to observational studies. Am. J. Med. 2009, 122, 114–120. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef]
- Kim, Y.; Amini, N.; Gani, F.; Wagner, D.; Johnson, D.J.; Scott, A.; Ejaz, A.; Margonis, G.A.; Xu, L.; Buettner, S.; et al. Age of transfused blood impacts perioperative outcomes among patients who undergo major gastrointestinal surgery. Ann. Surg. 2017, 265, 103–110. [Google Scholar] [CrossRef]
- Halabi, W.J.; Jafari, M.D.; Nguyen, V.Q.; Carmichael, J.C.; Mills, S.; Pigazzi, A.; Stamos, M.J. Blood transfusions in colorectal cancer surgery: Incidence, outcomes, and predictive factors: An American College of Surgeons National Surgical Quality Improvement Program analysis. Am. J. Surg. 2013, 206, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.M.; Meland, E.; McGinn, S.; Anderson, J.H. Correction of iron-deficiency anaemia in colorectal surgery reduces perioperative transfusion rates: A before and after study. Int. J. Surg. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Khalafallah, A.A.; Yan, C.; Al-Badri, R.; Robinson, E.; Kirkby, B.E.; Ingram, E.; Gray, Z.; Khelgi, V.; Robertson, I.K.; Kirkby, B.P. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: A prospective, open-label, randomised controlled trial. Lancet Haematol. 2016, 3, e415–e425. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, S.; Busch, M.P.; Murphy, E.L.; Shan, H.; Ness, P.; Glynn, S.A.; National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III). The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): A research program striving to improve blood donor and transfusion recipient outcomes. Transfusion 2014, 54, 942–955. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).