Clinical Features and Prognostic Impact of Pancreatic Ductal Adenocarcinoma without Dilatation of the Main Pancreatic Duct: A Single-Center Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
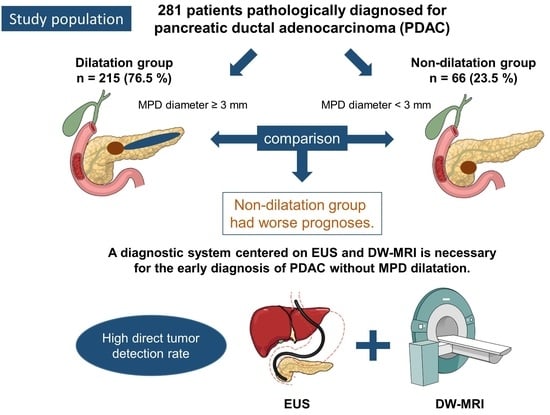
2.1. Patients
2.2. Evaluations
2.3. Imaging and Pathological Diagnosis
2.3.1. Imaging Diagnosis
2.3.2. Pathological Diagnosis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Reasons for Medical Examination
3.3. Imaging Findings
3.4. Pathological Diagnosis
3.5. Clinical Stage and Resectability Classification
3.5.1. Prognosis
3.5.2. Factors Affecting PDAC Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan MoH, Labour, Welfare). Cancer Statistics. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#mortality (accessed on 15 July 2022).
- Center for Cancer Control and Information Services, National Cancer Center, Japan. Monitoring of Cancer Incidence in Japan—Survival 2009–2011 Report. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#survival (accessed on 15 July 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 mm) pancreatic adenocarcinomas: Analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pour, P.M.; Sayed, S.; Sayed, G. Hyperplastic, preneoplastic and neoplastic lesions found in 83 human pancreases. Am. J. Clin. Pathol. 1982, 77, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Cancer Information Service NCC, Japan (Ministry of Health, Labour and Welfare, National, Registry). C. Cancer Statistics. 2021. Available online: https://www.ncc.go.jp/en/icc/cancer-info/index.html (accessed on 15 July 2022).
- Working Group on Revision of the Manual for Abdominal Ultrasound in Cancer Screening and Health Checkups, Ultrasound Screening Committee of the Japanese Society of Gastrointestinal Cancer Screening et al Manual for abdominal ultrasound in cancer screening and health checkups. J. Gastrointest. Cancer 2014, 52, 471–493. [Google Scholar]
- Greenberg, P.L.; Attar, E.; Bennett, J.M.; Bloomfield, C.D.; De Castro, C.M.; Deeg, H.J.; Foran, J.M.; Gaensler, K.; Garcia-Manero, G.; Gore, S.D.; et al. NCCN clinical practice guidelines in oncology: Myelodysplastic syndromes. J. Natl. Compr. Canc. Netw. 2011, 9, 30–56. [Google Scholar] [CrossRef] [Green Version]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours (UICC); John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- Minjoung, M.K.; Ruth, S.; Sean, M.; Gary, A.A.; Lucy, E.B.; Greg, P.R.; Georgios, L. Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakaizumi, A.; Ioka, T.; Oshikawa, O.; Uehara, H.; Nakao, M.; Yamamoto, K.; Ishikawa, O.; Ohigashi, H.; Kitamra, T. Main pancreatic duct dilatation: A sign of high risk for pancreatic cancer. Jpn. J. Clin. Oncol. 2002, 32, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, K.; Watanabe, H.; Ikeda, S. Differences between solid and duct-ectatic types of pancreatic ductal carcinomas. Cancer 1992, 69, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Obana, T.; Takasawa, O.; Sawai, T. A case of a small pancreatic cancer with an intact main pancreatic duct. Nihon Shokakibyo Gakkai Zasshi 2009, 106, 1220–1226. [Google Scholar] [PubMed]
- Tsutsumi, M. Development and characteristics of pancreatic exocrine tumors. Pathol. Clin. Care 2004, 22, 768–774. [Google Scholar]
- Japan Pancreas Society. General Rules for the Study of Pancreatic Cancer, 7th ed.; Kanehara & Co, Ltd.: Tokyo, Japan, 2016. [Google Scholar]
- Tanaka, S.; Nakaizumi, A.; Ioka, T.; Takakura, R.; Uehara, H.; Nakao, M.; Suzuki, R.; Fukuda, J.; Ishikawa, O.; Ohigashi, H. Periodic ultrasonography checkup for the early detection of pancreatic cancer. Preliminary report. Pancreas 2004, 28, 268–272. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: A prospective study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef]
- Haneki, H.; Atomi, H. Usefulness and limitations of external ultrasound. Tan Sui 2005, 26, 573–578. [Google Scholar]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Yasuda, I.; Iwashita, T.; Doi, S.; Nakashima, M.; Moriwaki, H. Role of EUS in the early detection of small pancreatic cancer. Dig. Endosc. 2011, 23 (Suppl. 1), 22–25. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Nishihara, K.; Tsuneyoshi, M. Non-icteric pancreas head carcinoma fares worse than icteric pancreas head carcinoma. J. Surg. Oncol. 1992, 49, 253–258. [Google Scholar] [CrossRef]
- Miura, S.; Takikawa, T.; Kikuta, K.; Hamada, S.; Kume, K.; Yoshida, N.; Tanaka, Y.; Matsumoto, R.; Ikeda, M.; Kataoka, F.; et al. Focal Parenchymal Atrophy of the Pancreas Is Frequently Observed on Pre-Diagnostic Computed Tomography in Patients with Pancreatic Cancer: A Case-Control Study. Diagnostics 2021, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Nakahodo, J.; Kikuyama, M.; Nojiri, S.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Horiguchi, S.I.; Honda, G. Focal parenchymal atrophy of pancreas: An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology 2020, 20, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Uchiyama, M.; Matsui, J. The “K-Sign”-A Nove CT Finding Suggestive before the Appearance of Pancreatic cancer. Cancers 2021, 13, 4222. [Google Scholar] [CrossRef] [PubMed]
- Toshima, F.; Watanabe, R.; Inoue, D.; Yoneda, N.; Yamamoto, T.; Sasahira, N.; Sasaki, T.; Matsuyama, M.; Minehiro, K.; Tateishi, U. CT abnormalities of the pancreas associated with the subsequent diagnosis of clinical stage I pancreatic ductal adenocarcinoma more than 1 year later: A case-control study. AJR Am. J. Roentgenol. 2021, 217, 1353–1364. [Google Scholar] [CrossRef]
- Miura, S.; Kume, K.; Kikuta, K.; Hamada, S.; Takikawa, T.; Yoshida, N.; Hongo, S.; Tanaka, Y.; Matsumoto, R.; Sano, T.; et al. Focal Parenchymal Atrophy and Fat Replacement Are Clues for Early Diagnosis of Pancreatic Cancer with Abnormalities of the Main Pancreatic Duct. Tohoku J. Exp. Med. 2020, 252, 63–71. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef]
- Puli, S.R.; Bechtold, M.L.; Buxbaum, J.L.; Eloubeidi, M.A. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas 2013, 42, 20–26. [Google Scholar] [CrossRef]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Haba, S.; Yamao, K.; Bhatia, V.; Mizuno, N.; Hara, K.; Hijioka, S.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J. Gastroenterol. 2013, 48, 973–981. [Google Scholar] [CrossRef]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Kamada, H.; Tsutsui, K.; Ono, M.; Aritomo, Y.; Masaki, T.; Kushida, Y.; Haba, R.; Nakatsu, T.; Kuriyama, S. Utility of pancreatic duct brushing for diagnosis of pancreatic carcinoma. J. Gastroenterol. 2007, 42, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Matsumoto, K.; Kurumi, H.; Koda, H.; Yamashita, T.; Onoyama, T.; Kawata, S.; Horie, Y.; Isomoto, H. Efficacy and safety of pancreatic juice cytology by using synthetic secretin in the diagnosis of pancreatic ductal adenocarcinoma. Dig. Endosc. 2018, 30, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Kikuyama, M.; Kawaguchi, S. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (SPACE). Clin. J. Gastroenterol. 2017, 10, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Takenaka, M.; Omoto, S. A case of pancreatic carcinoma in situ diagnosed by repeated pancreatic juice cytology. Oncology 2017, 93, S98–S101. [Google Scholar] [CrossRef]
- Iiboshi, T.; Hanada, K.; Fukuda, T. Value of cyto-diagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, J.; Serikawa, M.; Hanada, K.; Eguchi, N.; Sasaki, T.; Fujimoto, Y.; Sugiyama, S.; Yamaguchi, A.; Noma, B.; Kamigaki, M.; et al. Clinical analysis of early-stage pancreatic cancer and proposal for a new diagnostic algorithm: A multicenter observational study. Diagnostics 2021, 11, 287. [Google Scholar] [CrossRef]
- Iwata, T.; Kitamura, K.; Yamamiya, A.; Ishii, Y.; Sato, Y.; Nomoto, T.; Ikegami, A.; Yoshida, H. Evaluation of diagnostic cytology via endoscopic naso-pancreatic drainage for pancreatic tumor. World J. Gastrointest. Endosc. 2014, 6, 366–372. [Google Scholar] [CrossRef]
- Groot, V.P.; Mosier, S.; Javed, A.A.; Teinor, J.A.; Gemenetzis, G.; Ding, D.; Haley, L.M.; Yu, J.; Burkhart, R.A.; Hasanain, A.; et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 4973–4984. [Google Scholar] [CrossRef]
- Lee, B.; Lipton, L.; Cohen, J.; Tie, J.; Javed, A.A.; Li, L.; Goldstein, D.; Burge, M.; Cooray, P.; Nagrial, A.; et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann. Oncol. 2019, 30, 1472–1478. [Google Scholar] [CrossRef]
- Sivapalan, L.; Thorn, G.J.; Gadaleta, E.; Kocher, H.M.; Ross-Adams, H.; Chelala, C. Longitudinal profiling of circulating tumour DNA for tracking tumour dynamics in pancreatic cancer. BMC Cancer 2022, 22, 369. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.A.; Shapiro, B.; Sklaroff, D.M.; Yaros, M.J. Free DNA in the Serum of Cancer Patients and the Effect of Therapy. Cancer Res. 1977, 37, 646–650. [Google Scholar] [PubMed]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaoka, K.; Ohno, E.; Kawabe, N.; Kuzuya, T.; Funasaka, K.; Nakagawa, Y.; Nagasaka, M.; Ishikawa, T.; Watanabe, A.; Tochio, T.; et al. Current Status of the Diagnosis of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics 2023, 13, 215. [Google Scholar] [CrossRef]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Minami, T.; Ikemoto, J.; Kanemitsu, K.; Hino, F. Effective screening for early diagnosis of pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 929–939. [Google Scholar] [CrossRef]









| Dilatation Group (n = 215) | Non-Dilatation Group (n = 66) | p-Value | |
|---|---|---|---|
| Sex, n (male/female) | 124/91 | 39/27 | 0.82 |
| Age, mean ± SD (range) | 74 ± 7 (43–97) | 74 ± 5 (45–94) | 0.9 |
| Tumor size, mm (range) | 30.1 (17–43) | 33.7 (18–48) | 0.75 |
| Location | |||
| head/body/tail, n (%) | 141 (66)/59 (27)/15 (7.0) | 22 (33)/4 (6)/40 (61) | <0.001 |
| Risk factors, n (%) | |||
| DM | 50 (23) | 19 (29) | 0.62 |
| Tobacco use | 53 (25) | 17(26) | 0.87 |
| IPMN | 36 (17) | 13 (20) | 0.27 |
| Chronic pancreatitis | 9 (4.2) | 1 (1.5) | 0.13 |
| Heavy alcohol consumption | 41 (19) | 12 (18) | 0.85 |
| Obesity (>BMI 30 kg/m2) | 5 (2.3) | 2 (3.0) | 0.92 |
| Family history of pancreatic cancer | 7 (3.2) | 2 (3.0) | 1 |
| Examination Opportunities | Dilatation Group (n = 215) (%) | Non-Dilatation Group (n = 66) (%) | p-Value |
|---|---|---|---|
| Symptoms | 130/215 (60) | 40/66 (61) | 0.77 |
| Abdominal pain | 54/130 (42) | 16/40 (40) | 1 |
| Back pain | 10/130 (7.7) | 2/40 (5.0) | 1 |
| Nausea | 9/130 (6.9) | 2/40 (5.0) | 1 |
| Diarrhea | 3/130 (2.3) | 2/40 (5.0) | 1 |
| Jaundice | 52/130 (40) | 6/40 (15) | 0.014 |
| Weight loss | 1/130 (0.77) | 4/40 (10) | 0.008 |
| Other | 24/130 (18) | 10/40 (25) | 0.052 |
| Abnormalities identified on medical check-up | 14/215 (6.5) | 8/66 (12) | 1 |
| Abnormal findings on US | 12/14 (86) | 3/8 (38) | 0.055 |
| Elevated tumor marker levels | 1/14 (7.1) | 4/8 (50) | 0.11 |
| Others | 1/14 (7.1) | 1/5 (20) | 0.4 |
| Abnormalities identified during screening for other diseases | 58/215 (27) | 15/66 (23) | 0.74 |
| Abnormal imaging findings | 54/58 (92) | 15/15 (100) | 1 |
| Plain CT | 9/54 (17) | 7/15 (47) | 0.088 |
| CECT | 26/54 (48) | 7/15 (47) | 0.47 |
| US | 18/54 (33) | 0/15 (0) | 0.25 |
| MRI | 0/54 (0) | 0/15 (0) | 1 |
| EUS | 1/54 (1.9) | 0/15 (0) | 0.055 |
| PET–CT | 0/54 (0) | 1/15 (6.7) | 0.21 |
| Elevated pancreatic enzymes | 2/58 (3.4) | 0/15 (0) | 1 |
| Elevated tumor marker levels | 2/58 (3.4) | 1/15 (6.7) | 0.46 |
| Abnormalities during follow-up of pancreatic diseases | 10/215 (4.7) | 3/66 (4.5) | 0.75 |
| Other | 1/215 (0.47) | 0/66 (0) | 1 |
| Dilatation Group (n = 215) (%) | Non-Dilatation Group (n = 66) (%) | p-Value | |
|---|---|---|---|
| Imaging performed, US/CT/MRI/EUS | 151/207/133/179 | 48/54/37/49 | |
| Detection of pancreatic tumors | |||
| US | 129/151 (85) | 20/48 (42) | <0.001 |
| CECT | 195/207 (94) | 50/54 (93) | 0.75 |
| MRI T1 | 93/133 (70) | 24/37 (65) | 0.85 |
| MRI T2 | 78/133 (59) | 18/37 (49) | 0.58 |
| DW-MRI | 119/133 (89) | 32/37 (86) | 0.8 |
| EUS | 178/179 (99) | 48/49 (98) | 0.38 |
| Indirect imaging findings | |||
| MPD dilatation | |||
| US | 106/151 (70) | ||
| CECT | 197/207 (95) | ||
| MRI (MRCP) | 122/133 (92) | ||
| EUS | 161/179 (90) | ||
| MPD stenosis | |||
| US | 89/151 (59) | 0/48 (0) | <0.001 |
| CECT | 194/207 (94) | 0/54 (0) | <0.001 |
| MRI (MRCP) | 118/133 (89) | 1/37 (2.7) | <0.001 |
| EUS | 156/179 (87) | 2/49 (4.1) | <0.001 |
| Dilatation Group (n = 215) (%) | Non-Dilatation Group (n = 66) (%) | p-Value | ||
|---|---|---|---|---|
| ERCP | 131/215 (61) | 14/66 (21) | <0.001 | |
| Biopsy of CBD stenosis | 30/61 (49) | 4/6 (67) | 0.67 | |
| Brushing cytology of CBD stenosis | 30/79 (38) | 3/11 (27) | 0.74 | |
| ENBD | 20/73 (27) | 0/6 (0) | 0.33 | |
| Single aspiration of pancreatic juice | 15/37 (41) | 0/1 (0) | 1 | |
| Brushing cytology of MPD stenosis | 27/47 (57) | 1/1 (100) | 1 | |
| SPACE | 18/32 (56) | 0/2 (0) | 0.21 | |
| Confirmation of malignancy | 82/131 (63) | 8/14 (57) | 0.78 | |
| EUS-FNA | 154/215 (72) | 45/66 (68) | 0.36 | |
| Biopsy | 142/154 (92) | 44/45 (98) | 0.3 | |
| Gastrointestinal biopsy | 20/21 (95) | 7/7 (100) | 1 | |
| Liver tumor biopsy | 3/4 (75) | 4/4 (100) | 1 | |
| Cytology of ascites | 3/6 (50) | 5/7 (71) | 0.59 |
| (A) | |||
|---|---|---|---|
| Clinical Stage | Dilatation Group (n = 215) (%) | Non-Dilatation Group (n = 66) (%) | p-Value |
| 0 | 3 (1.4) | 1 (1.5) | 1 |
| IA | 5 (2.3) | 2 (3.0) | 0.67 |
| IB | 8 (3.7) | 1 (1.5) | 0.69 |
| IIA | 67 (31) | 10 (15) | <0.001 |
| IIB | 12 (5.6) | 2 (3.0) | 0.74 |
| III | 41 (19) | 8 (12) | 0.33 |
| IV | 79 (37) | 42 (64) | <0.001 |
| (B) | |||
| Resectability Classification | Dilatation Group (n = 215) (%) | Non-Dilatation Group (n = 66) (%) | p-Value |
| R | 62 (29) | 14 (21) | 0.16 |
| BR-PV | 34 (16) | 1 (1.5) | 0.004 |
| BR-A | 5 (2.3) | 2 (3.0) | 1 |
| UR-LA | 35 (16) | 7 (11) | 0.68 |
| UR-M | 79 (37) | 42 (64) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Hr (95% Ci) | p-Value | Hr (95% Ci) | p-Value | |
| Tumor location | ||||
| Head/Body | 1 | 1 | ||
| Tail | 1.59 (1.11–2.25) | 0.011 | 1.28 (0.78–2.09) | 0.331 |
| Jaundice | ||||
| No | 1 | |||
| Yes | 1.15 (0.75–1.76) | 0.515 | ||
| Weight loss | ||||
| No | 1 | |||
| Yes | 0.38 (0.05–2.57) | 0.306 | ||
| Clinical stage | ||||
| 0-I | 1 | 1 | ||
| II–III | 1.60 (0.69–3.71) | 0.268 | 1.28 (0.51–3.18) | 0.599 |
| IV | 2.05 (1.42–3.00) | 0.016 | 2.83 (1.00–8.00) | 0.049 |
| Resectability classification | ||||
| R | 1 | 1 | ||
| BR-PV or BR-A | 1.54 (0.89–2.66) | 0.124 | 1.58 (0.86–2.89) | 0.138 |
| UR-LA or UR-M | 2.19 (1.48–3.22) | <0.001 | 1.25 (0.65–2.42) | 0.503 |
| Treatment | ||||
| Surgery | 1 | 1 | ||
| Chemotherapy or CRT | 1.58 (0.97–2.58) | 0.068 | 0.74 (0.41–1.34) | 0.318 |
| Best supportive care | 4.20 (2.63–6.70) | <0.001 | 2.92 (1.73–4.93) | <0.001 |
| Presence of MPD dilatation (≥3 mm) | ||||
| Yes | 1 | 1 | ||
| No | 0.51 (0.34–0.78) | 0.001 | 0.77 (0.53–1.14) | 0.753 |
| Type | I | II | III | IV | V |
|---|---|---|---|---|---|
| n | 1 | 10 | 11 | 4 | 40 |
| Bile duct obstruction | 0 | 4 | 8 | 1 | 0 |
| Duodenal invasion | 0 | 4 | 4 | 0 | 0 |
| Duodenal obstruction | 0 | 4 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayanagi, T.; Sekino, Y.; Kasuga, N.; Ishii, K.; Nagase, H.; Nakajima, A. Clinical Features and Prognostic Impact of Pancreatic Ductal Adenocarcinoma without Dilatation of the Main Pancreatic Duct: A Single-Center Retrospective Analysis. Diagnostics 2023, 13, 963. https://doi.org/10.3390/diagnostics13050963
Takayanagi T, Sekino Y, Kasuga N, Ishii K, Nagase H, Nakajima A. Clinical Features and Prognostic Impact of Pancreatic Ductal Adenocarcinoma without Dilatation of the Main Pancreatic Duct: A Single-Center Retrospective Analysis. Diagnostics. 2023; 13(5):963. https://doi.org/10.3390/diagnostics13050963
Chicago/Turabian StyleTakayanagi, Takuya, Yusuke Sekino, Noriki Kasuga, Ken Ishii, Hajime Nagase, and Atsushi Nakajima. 2023. "Clinical Features and Prognostic Impact of Pancreatic Ductal Adenocarcinoma without Dilatation of the Main Pancreatic Duct: A Single-Center Retrospective Analysis" Diagnostics 13, no. 5: 963. https://doi.org/10.3390/diagnostics13050963
APA StyleTakayanagi, T., Sekino, Y., Kasuga, N., Ishii, K., Nagase, H., & Nakajima, A. (2023). Clinical Features and Prognostic Impact of Pancreatic Ductal Adenocarcinoma without Dilatation of the Main Pancreatic Duct: A Single-Center Retrospective Analysis. Diagnostics, 13(5), 963. https://doi.org/10.3390/diagnostics13050963