Association of Alternative Markers of Carbohydrate Metabolism (Fructosamine and 1,5-Anhydroglucitol) with Perioperative Characteristics and In-Hospital Complications of Coronary Artery Bypass Grafting in Patients with Type 2 Diabetes Mellitus, Prediabetes, and Normoglycemia
Abstract
1. Introduction
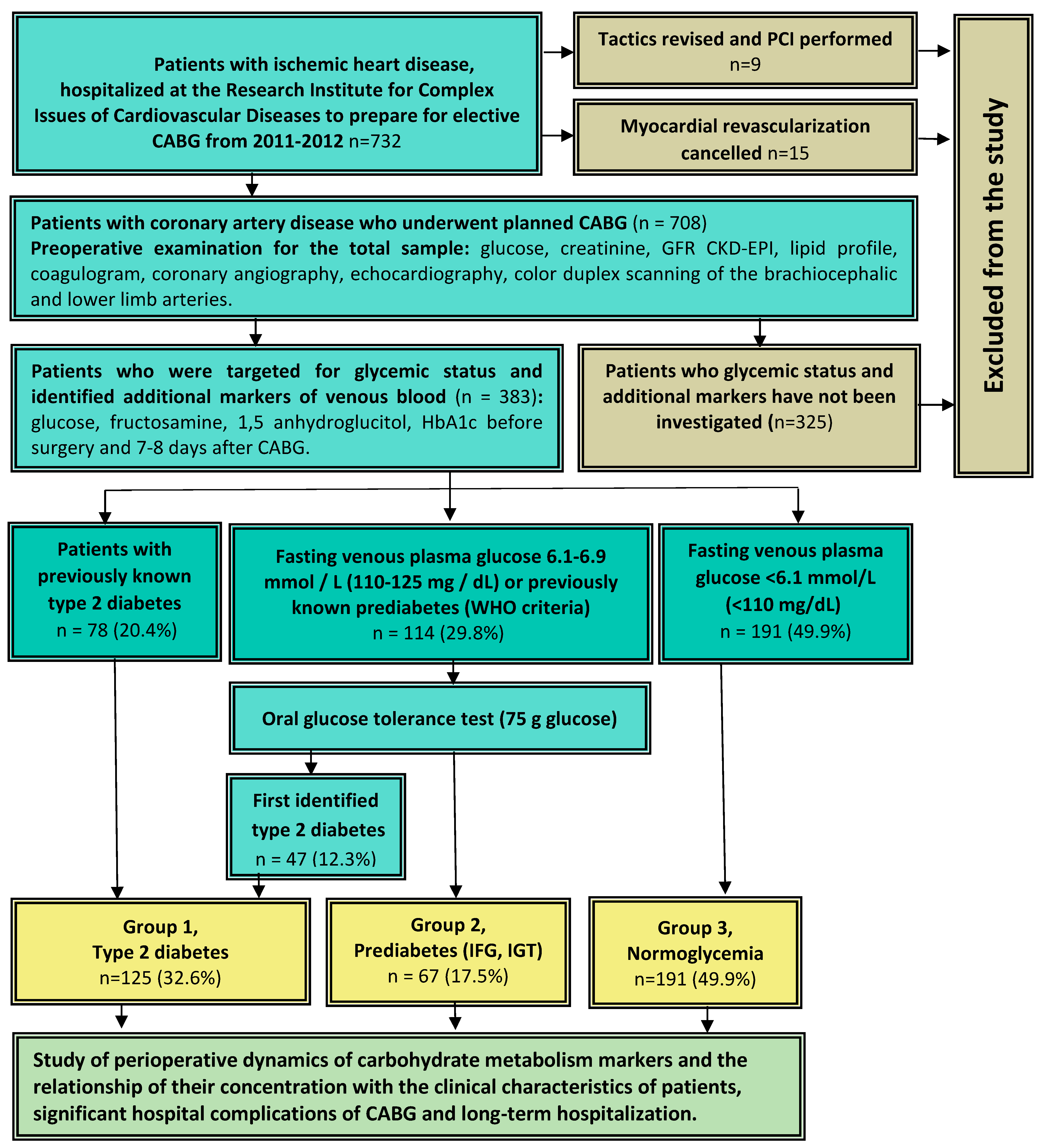
2. Subjects, Materials, and Methods
2.1. Study Population
2.2. Data Collection
2.3. Evaluation of Indicators of Carbohydrate Metabolism
2.4. Hospital Postoperative Complications
2.5. Statistical Analyses
3. Results
3.1. Initial Characteristics of Patients in the Groups of Diabetes Mellitus, Prediabetes, and Normoglycemia
3.2. Perioperative Dynamics of Carbohydrate Metabolism Markers in the Groups of Diabetes Mellitus, Prediabetes, and Normoglycemia
3.3. Correlation of Fructosamine and 1.5 Anhydroglucitol Levels before and after Surgery with Perioperative Characteristics of Patients
3.4. Complications after CABG in the Groups of Diabetes Mellitus, Prediabetes, and Normoglycemia
3.5. Factors Associated with the Development of Hospital Complications of CABG
4. Discussion
Study Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.A.; Berto, B.; Sousa, A.G.; Costa, F.A. Prognosis and Complications of Diabetic Patients Undergoing Isolated Coronary Artery Bypass Surgery. Braz. J. Cardiovasc. Surg. 2016, 31, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Sumin, A.N. Current trends in routine myocardial revascularization. Complex Issues Cardiovasc. Dis. 2021, 10, 25–35. [Google Scholar] [CrossRef]
- Corazzari, C.; Matteucci, M.; Kołodziejczak, M.; Kowalewski, M.; Formenti, A.M.; Giustina, A.; Beghi, C.; Barili, F.; Lorusso, R. Impact of preoperative glycometabolic status on outcomes in cardiac surgery: Systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 164, 1950–1960.e10. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.M.; Lutsey, P.L.; Maruthur, N.; Pankow, J.S.; Steffes, M.; Coresh, J. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation 2015, 132, 269–277. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and Pitfalls of Fructosamine and Glycated Albumin in the Diagnosis and Treatment of Diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef]
- Zaccardi, F.; Kurl, S.; Pitocco, D.; Ronkainen, K.; Laukkanen, J.A. Serum fructosamine and risk of cardiovascular and all-cause mortality: A 24-year prospective population-based study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 236–241. [Google Scholar] [CrossRef]
- Kowalczuk-Wieteska, A.M.; Wróbel, M.; Rokicka, D.; Szymborska-Kajanek, A.; Foremny, J.; Nadziakiewicz, P.; Zembala, M.; Strojek, K. Determination of the value of glycated hemoglobin HbA1c and fructosamine in assessing the risk of perioperative complications after cardiac surgery in patients with type 2 diabetes. Kardiochirurgia Torakochirurgia Pol. 2016, 13, 305–308. [Google Scholar] [CrossRef]
- Migała, M.; Chałubińska-Fendler, J.; Zielińska, M. 1,5-Anhydroglucitol as a Marker of Acute Hyperglycemia in Cardiovascular Events. Rev. Diabet. Stud. 2022, 18, 68–75. [Google Scholar] [CrossRef]
- Teng, H.I.; Chen, H.Y.; Tsai, C.T.; Huang, W.C.; Chen, Y.Y.; Hsueh, C.H.; Hau, W.K.; Lu, T.M. The clinical impact of serum 1,5-anhydro-D-glucitol levels on coronary artery calcification and adverse outcomes assessed by coronary optical coherence tomography in diabetic patients. Front. Cardiovasc. Med. 2022, 9, 997649. [Google Scholar] [CrossRef]
- Ouchi, S.; Shimada, K.; Miyazaki, T.; Takahashi, S.; Sugita, Y.; Shimizu, M.; Murata, A.; Kadoguchi, T.; Kato, T.; Aikawa, T.; et al. Low 1,5-anhydroglucitol levels are associated with long-term cardiac mortality in acute coronary syndrome patients with hemoglobin A1c levels less than 7.0. Cardiovasc. Diabetol. 2017, 16, 151. [Google Scholar] [CrossRef]
- Takahashi, S.; Shimada, K.; Miyauchi, K.; Miyazaki, T.; Sai, E.; Ogita, M.; Tsuboi, S.; Tamura, H.; Okazaki, S.; Shiozawa, T.; et al. Low and exacerbated levels of 1,5-anhydroglucitol are associated with cardiovascular events in patients after first-time elective percutaneous coronary intervention. Cardiovasc. Diabetol. 2016, 15, 145. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; pp. 1–50. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Sumin, A.N.; Bezdenezhnykh, N.A.; Bezdenezhnykh, A.V.; Osokina, A.V.; Kuz’mina, A.A.; Tsepokina, A.V.; Barbarash, O.L. Screening for Glucose Metabolism Disorders, Assessment the Disse Insulin Resistance Index and Hospital Prognosis of Coronary Artery Bypass Surgery. J. Pers. Med. 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Shohat, N.; Tarabichi, M.; Tischler, E.H.; Jabbour, S.; Parvizi, J. Serum Fructosamine: A Simple and Inexpensive Test for Assessing Preoperative Glycemic Control. J. Bone Joint Surg. Am. 2017, 99, 1900–1907. [Google Scholar] [CrossRef]
- Shohat, N.; Tarabichi, M.; Tan, T.L.; Goswami, K.; Kheir, M.; Malkani, A.L.; Shah, R.P.; Schwarzkopf, R.; Parvizi, J. 2019 John Insall Award: Fructosamine is a better glycaemic marker compared with glycated haemoglobin (HbA1C) in predicting adverse outcomes following total knee arthroplasty: A prospective multicentre study. Bone Joint J. 2019, 101-B (Supple. C), 3–9. [Google Scholar] [CrossRef]
- Fujiwara, T.; Yoshida, M.; Akashi, N.; Yamada, H.; Tsukui, T.; Nakamura, T.; Sakakura, K.; Wada, H.; Arao, K.; Katayama, T.; et al. Lower 1,5-anhydroglucitol is associated with adverse clinical events after percutaneous coronary intervention. Heart Vessel. 2016, 31, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.R.; Alayesh, Y.M.; Algarni, A.M.; Alotaibi, O.D.; Aladnani, A.A.; Fernandez, J.A.; Bennett, M.R. Effect of Acute Stress Glycemic Control and Long-Term Glycemic Control on the Incidence of Post-Operative Infection in Diabetics Undergoing Cardiac Surgery. Cureus 2021, 13, e14031. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, V.A. Commentary: Breaking the perioperative glucose control barrier is like breaking the sound barrier-it takes a team! J. Thorac. Cardiovasc. Surg. 2022, 164, 1961–1962. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef] [PubMed]



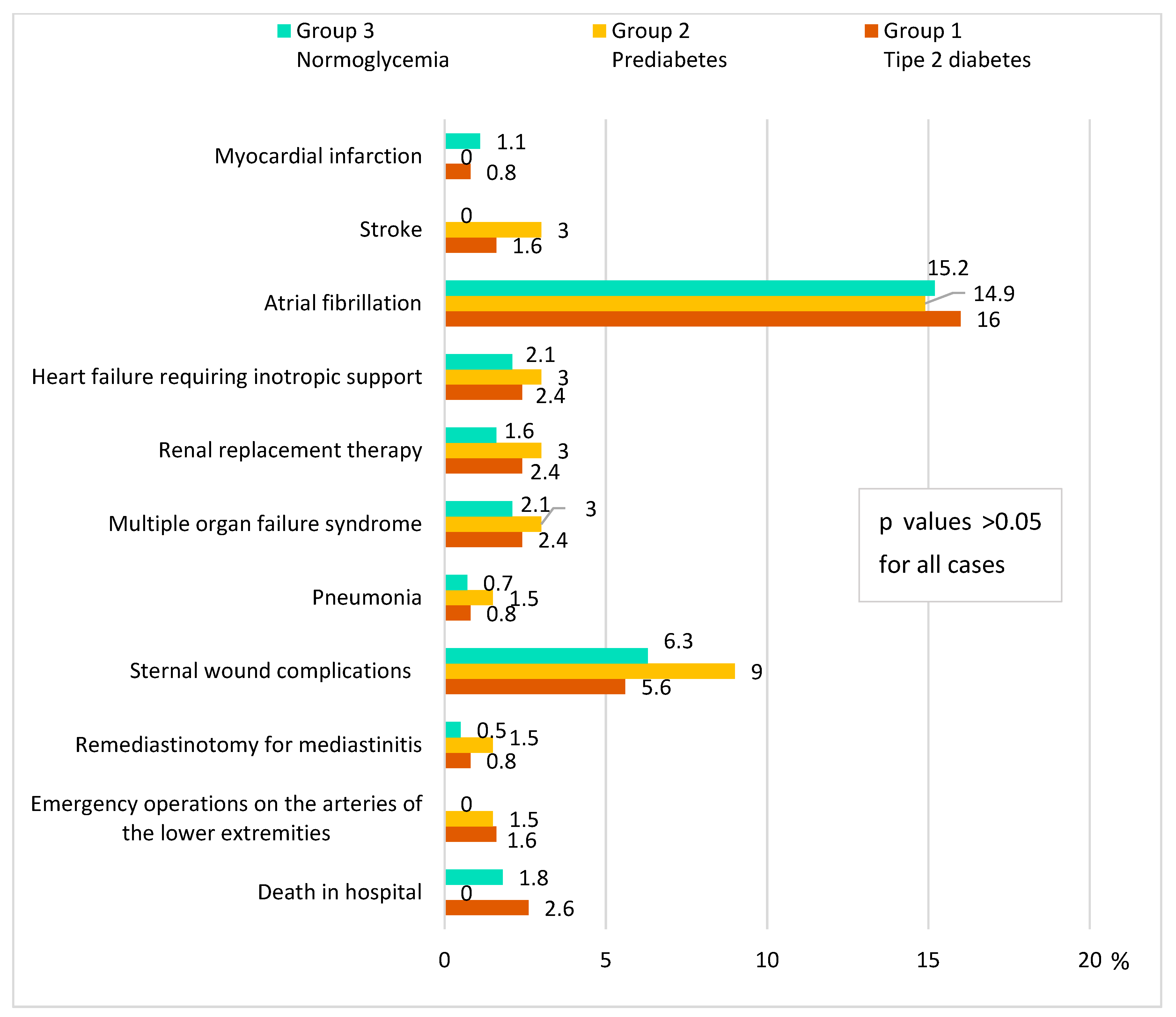

| Parameter | Group 1 Type 2 Diabetes n = 125 | Group 2 Prediabetes n = 67 | Group 3 Normoglycemia n = 191 | p |
|---|---|---|---|---|
| Men (n, %) | 79 (63.2) | 51 (76.1) | 154 (80.6) | <0.001 * |
| Age, years (Me [LQ; UQ]) | 59.0 [54.5; 63.0] | 58.0 [54.5; 63.0] | 59.0 [54.0; 65.0] | 0.493 |
| BMI, kg/m 2 (Me [LQ; UQ]) | 29.8 [27.2; 32.6] | 29.3 [26.8; 32.3] | 27.0 [24.2; 30.9] | <0.001 * |
| Overweight or obesity (BMI ≥25 kg/m2, n, %) | 113 (90.4) | 57 (85.1) | 132 (69.1) | <0.001 * 0.011 # |
| Obesity (BMI ≥30 kg/m2) (n, %) | 62 (49.6) | 29 (43.3) | 58 (30.4) | <0.001 * |
| Arterial hypertension (n, %) | 117 (93.6) | 61 (91.0) | 165 (86.4) | 0.111 |
| Angina class III-IV (n, %) | 48 (38.4) | 26 (38.8) | 71 (37.1) | 0.802 |
| Heart failure class NYHA III–IV (n, %) | 41 (32.8) | 19 (28.3) | 52 (27.2) | 0.803 |
| Ventricular arrhythmias (n, %) | 21 (16.8) | 9 (13.4) | 26 (13.6) | 0.751 |
| Supraventricular arrhythmias (n, %) | 14 (11.2) | 6 (8.9) | 13 (6.8) | 0.100 |
| Intermittent claudication (n, %) | 18 (14.4) | 8 (13.4) | 27 (14.1) | 0.445 |
| Smoking/smoking (n, %) | 29 (23.2) | 20 (29.9) | 79 (41.4) | <0.001 * |
| History of myocardial infarction (n, %) | 81 (64.8) | 41 (61.2) | 120 (62.8) | 0.665 |
| History of stroke (n, %) | 9 (7.2) | 3 (4.5) | 15 (7.8) | 0.647 |
| Previous PCI (n, %) | 16 (8.4) | 6 (7.5) | 16 (8.3) | 0.342 |
| Previous CABG (n, %) | 2 (1.6) | 0 (0) | 2 (1.1) | 0.582 |
| Surgery on the carotid arteries (n, %) | 10 (6.4) | 2 (3.0) | 1 (0.5) | 0.002 * |
| Intervention on the arteries lower limbs or amputation (n, %) | 2 (1.6) | 0 (0) | 1 (0.5) | 0.413 |
| EuroSCORE II, % (Me [LQ; UQ]) | 2.1 [1.3; 3.5] | 1.5 [0.9; 2.3] | 1.7 [1.2; 2.6] | 0.008 # 0.023 * |
| EuroSCORE II, points (Me [LQ; UQ]) | 3 [2; 5] | 3 [1; 3] | 3 [2; 4] | 0.006 * 0.011 # |
| Length of hospital stay after CABG, days (Me [LQ; UQ]) | 13.0 [11.0; 17.0] | 13.0 [9.0; 15.0] | 12.0 [10.0; 14.0] | <0.001 * |
| Preoperative medicine therapy (n, %) | ||||
| β-Blockers | 124 (99.2) | 66 (98.5) | 186 (97.4) | 0.621 |
| Angiotensin converting enzyme inhibitors | 109 (87.2) | 59 (88.1) | 169 (88.5) | 0.731 |
| Angiotensin 2 receptor antagonists | 9 (7.2) | 2 (3.0) | 5 (4.5) | 0.293 |
| Mineralocorticoid receptor antagonists | 22 (17.6) | 12 (17.9) | 32 (16.8) | 0.765 |
| Thiazide-like diuretics | 16 (12.9) | 5 (7.4) | 18 (9.4) | 0.182 |
| Loop diuretics | 10 (8.0) | 4 (6.0) | 11 (5.8) | 0.306 |
| Calcium channel blockers | 97 (77.6) | 42 (62.6) | 111 (58.1) | 0.043 |
| HMG-CoA reductase inhibitors (statins) | 96 (76.8) | 49 (73.1) | 143 (74.9) | 0.923 |
| Only oral hypoglycemic drugs | 41 (21.3) | - | - | - |
| Metformin | 72 (37.6) | - | - | - |
| Sulfonylurea | 2 8 (22.4) | - | - | - |
| DPP-4 inhibitors or GLP-1 receptor agonists | 5 (4.0) | - | - | - |
| Insulin therapy before hospitalizations | 19 (15.2) | - | - | - |
| Insulin therapy in time hospitalizations | 52 (41.6) | - | - | - |
| Parameter | Group 1 Type 2 Diabetes n = 125 | Group 2 Prediabetes n = 67 | Group 3 Normoglycemia n = 191 | p |
|---|---|---|---|---|
| Characteristic of CABG | ||||
| Use of cardiopulmonary bypass (n, %) | 118 (94.4) | 59 (88.1) | 169 (88.5) | 0.179 |
| Isolated CABG (n, %) | 111 (88.8) | 63 (94.0) | 178 (93.2) | 0.128 |
| Combined operations (n, %) | 14 (11.2) | 4 (6.0) | 11 (5.8) | 0.128 |
| Carotid endarterectomy (n, %) | 2 (1.6) | 3 (4.5) | 3 (1.6) | 0.322 |
| Ventriculoplasty (n, %) | 8 (6.4) | 1 (1.4) | 5 (2.6) | 0.024 |
| Radio frequency ablation (n, %) | 7 (5.6) | 2 (3.0) | 4 (2.1) | 0.238 |
| Mitral valve (n, %) | 0 (0) | 0 (0) | 1 (0.5) | 0.604 |
| Aortic valve (n, %) | 0 (0) | 1 (1.5) | 2 (1.1) | 0.451 |
| Cardiopulmonary bypass duration, minutes (Me [ LQ; UQ]) | 100.0 [81.0; 118.0] | 92.5 [75.0; 109.0] | 95.0 [78.0; 109.0] | 0.115 |
| Aortic clamp time, minutes (Me [LQ; UQ]) | 66.5 [51.5; 78.0] | 58.0 [49.0; 67.0] | 60 [49.0; 72.0] | 0.064 |
| Total operation time, minutes (Me [LQ; UQ]) | 246.0 [204.0; 300.0] | 240.0 [201.0; 270.0] | 240.0 [198.0; 264.0] | 0.114 |
| Intraoperative blood loss, ml (Me [LQ; UQ]) | 500.0 [500.0; 600.0] | 500.0 [500.0; 500.0] | 500.0 [500.0; 500.0] | 0.117 |
| Number of shunts (Me [LQ; UQ]) | 3.0 [2.0; 3.0] | 2.0 [2.0; 3.0] | 2.0 [2.0; 3.0] | 0.089 |
| Complete revascularization (n, %) | 1 17 (93.6) | 61 (91.0) | 172 (90.1) | 0.534 |
| Preoperative laboratory fasting blood values (Me [LQ; UQ]) | ||||
| Total cholesterol, mmol/L/ | 5.2 [4.2; 6.2] | 4.6 [3.8; 5.8] | 5.0 [4.2; 6.0] | 0.079 |
| HDL cholesterol, mmol/L | 0.9 [0.8; 1.1] | 0.96 [ 0.8; 1.1] | 1.0 [0.9; 1.2] | 0.095 |
| LDL cholesterol mmol/L | 3.0 [2.3; 4.1] | 2.5 [ 6.3; 2.1] | 2.9 [2.3; 3.7] | 0.150 |
| Triglycerides, mmol/L | 2.1 [1.5; 2.7] | 1.9 [1.2; 2.2] | 1.6 [1.2; 2.2] | <0.001 * |
| Creatinine, µmol/L | 83.0 [68.0; 95.0] | 85.0 [71.0; 104.0] | 83.0 [74.0; 106.0] | 0.098 |
| GFR CKD—EPI, mL/min/1.73 m2 | 82.5 [69.2; 99.7] | 80.3 [62, 3; 100.0] | 82.4 [66.3; 103.5] | 0.297 |
| Fibrinogen, g/L | 4.8 [3.8; 6.0] | 4.4 [3.5; 5.3] | 4.4 [3.5; 5.6] | 0.187 |
| Preoperative echocardiography (Me [LQ; UQ]) | ||||
| End-diastolic LV volume (mL) | 160.0 [136.0;194.5] | 160.0 [140.0;185.0] | 154.0 [132.5; 185.0] | 0.123 |
| End-diastolic LV dimension (cm) | 5.6 [5.3; 6.2] | 5.7 [5.3; 6.0] | 5.5 [5.1; 6.0] | 0.324 |
| End-systolic LV volume (mL) | 67.5 [51.0; 104.0] | 66.0 [51.0; 88.0] | 59.5 [44.0; 91.0] | 0.034 |
| End-systolic LV dimension (cm) | 4.0 [3.5; 4.9] | 3.9 [3.5; 4.4] | 3.7 [3.3; 4.5] | 0.019 |
| Left atrium (cm) | 4.3 [4.0; 4.6] | 4.3 [3.9; 4.5] | 4.2 [3.8; 4.4] | 0.014 * |
| LV ejection fraction (%) | 58.0 [48.0; 64.0] | 60.0 [52.0; 64.0] | 62.0 [52.0; 65.0] | 0.107 |
| LV myocardial mass by Deveraux and Reichek, g | 312.3 [258.5; 372.0] | 292.5 [255.8; 353.9] | 292.5 [241.1; 370.0] | 0.029 |
| LV myocardial mass index, g/m2 | 165.0 [140.0; 188.0] | 153.4 [129.9; 188.0] | 155.0 [126.2; 188.1] | 0.119 |
| Stroke volume (mL) | 90.2 [80.0; 103.0] | 90.1 [79.0; 102.0] | 89.0 [76.0; 103.0] | 0.279 |
| Parameter (Me [LQ; UQ]) | Group 1 Type 2 Diabetes n = 125 | Group 2 Prediabetes n = 67 | Group 3 Normoglycemia n = 191 | p |
|---|---|---|---|---|
| Glucose 1st point (before surgery), mmol/L | 7.6 [6.4; 9.9] | 6.2 [6.0; 6.5] | 5.2 [4.9; 5.5] | <0.001 * # $ |
| Glucose 2nd point (7–8 days after surgery), mmol/L | 6,9 [5.6; 8.35] | 5.7 [5.2; 6.4] | 5.6 [5.0; 6.3] | <0,001 * $ |
| p1st–2nd point (for glucose) | 0.003 | 0.011 | <0.001 | |
| Fructosamine 1st point (before surgery), µmol/L | 317 [287;366] | 284 [259; 297] | 245 [194; 285] | <0.001 * # $ |
| Fructosamine 2nd point (7–8 days after surgery), µmol/L | 291 [236.5; 372.5] | 233 [205; 268] | 234 [186; 270] | <0.001 * $ |
| p1st–2nd point (for FA) | 0.030 | 0.001 | 0.038 | |
| 1.5 Anhydroglucitol 1st point (before surgery), mcg/mL | 17.72 [14.2; 21.8] | 23.37 [19.99; 26.08] | 23.64 [19.8; 28.9] | <0.001 * $ |
| 1.5 Anhydroglucitol 2nd point (7–8 days after surgery), mcg/mL | 17.44 [14.95; 20.62] | 21.34 [15.80; 26.25] | 20.93 [17.64; 24.22] | <0.001 * |
| p1st–2nd point (for 1.5 AG) | 0.247 | 0.674 | 0.092 | |
| HbA1c 1st point (before surgery), % | 7.3 [5.9; 8.2] | 5.4 [5.3; 6.0] | 5.1 [5.0; 5.6] | <0.001 * # $ |
| HbA1c 2nd point (7–8 days after surgery), % | 7.2 [5.8; 8.5] | 5.4 [5.1; 6.0] | 5.1 [5.1; 5.8] | <0.001 * # $ |
| p1st–2nd point (for HbA1c) | 0.181 | 0.601 | 0.100 |
| Correlates | Spearman-R | p-Value |
|---|---|---|
| Fructosamine 1st point (before surgery) | ||
| Fructosamine 1st point and Type 2 diabetes | 0.505 | <0.001 |
| Fructosamine 1st point and EuroSCORE (points) | 0.196 | <0.001 |
| Fructosamine 1st point and EuroSCORE (%) | 0.152 | 0.002 |
| Fructosamine 1st point and Off-pump | 0.113 | 0.033 |
| Fructosamine 1st point and Multiplicity of cardioplegia | 0.197 | <0.001 |
| Fructosamine 1st point and Cardiopulmonary bypass duration | 0.204 | <0.001 |
| Fructosamine 1st point and Aortic clamp time | 0.177 | <0.001 |
| Fructosamine 1st point and Number of distal anastomoses | 0.129 | 0.016 |
| Fructosamine 1st point and Number of shunts | 0.124 | 0.012 |
| Fructosamine 1st point and Total operation duration | 0.120 | 0.027 |
| Fructosamine 1st point and Body mass index | 0.176 | <0.001 |
| Fructosamine 1st point and Overweight or obesity | 0.175 | <0.001 |
| Fructosamine 1st point and Left atrium | 0.117 | 0.028 |
| Fructosamine 1st point and Flow velocity | −0.193 | 0.030 |
| Fructosamine 1st point and LOS after CABG | 0.206 | <0.001 |
| Fructosamine 1st point and Triglycerides | 0.215 | <0.001 |
| Fructosamine 1st point and Fibrinogen | 0.155 | 0.002 |
| Fructosamine 1st point and Glucose 1st point | 0.480 | <0.001 |
| Fructosamine 1st point and Glucose 2nd point | 0.249 | <0.001 |
| Fructosamine 1st point and HbA1c 1st point | 0.379 | <0.001 |
| Fructosamine 1st point and HbA1c 2nd point | 0.280 | <0.001 |
| Fructosamine 1st point and Fructosamine 2nd point | 0.265 | <0.001 |
| Fructosamine 2nd point (7–8 days after surgery) | ||
| Fructosamine 2nd point and Type 2 diabetes | 0.351 | <0.001 |
| Fructosamine 2nd point and Body mass index | 0.134 | 0.019 |
| Fructosamine 2nd point and Overweight or obesity | 0.145 | 0.011 |
| Fructosamine 2nd point and Intraoperative blood loss | 0.120 | 0.037 |
| Fructosamine 2nd point and Off-pump | −0.128 | 0.032 |
| Fructosamine 2nd point and Posterior wall of the LV | 0.135 | 0.018 |
| Fructosamine 2nd point and LV myocardial mass by Deveraux and Reichek | 0.121 | 0.033 |
| Fructosamine 2nd point and Flow velosity | −0.184 | 0.049 |
| Fructosamine 2nd point and Triglycerides | 0.172 | 0.005 |
| Fructosamine 2nd point and Glucose 1st point | 0.324 | <0.001 |
| Fructosamine 2nd point and Glucose 2nd point | 0.935 | <0.001 |
| Fructosamine 2nd point and HbA1c 1st point | 0.268 | <0.001 |
| Fructosamine 2nd point and HbA1c 2nd point | 0.172 | <0.001 |
| 1.5 Anhydroglucitol 1st point (before surgery) | ||
| 1.5 Anhydroglucitol 1st point and Type 2 diabetes | −0.458 | <0.001 |
| 1.5 Anhydroglucitol 1st point and Intima media thickness | −0.194 | 0.016 |
| 1.5 Anhydroglucitol 1st point and LV end-diastolic volume | 0.183 | 0.020 |
| 1.5 Anhydroglucitol 1st point and Glucose 1st point | −0.527 | <0.001 |
| 1.5 Anhydroglucitol 1st point and Glucose 2nd point | −0.309 | <0.001 |
| 1.5 Anhydroglucitol 1st point and 1.5 anhydroglucitol 2nd point | 0.586 | <0.001 |
| 1.5 Anhydroglucitol 1st point and Fructosamine 1st point | −0.198 | 0.012 |
| 1.5 Anhydroglucitol 1st point and Fructosamine 2nd point | −0.302 | <0.001 |
| 1.5 Anhydroglucitol 2nd point (7–8 days after surgery) | ||
| 1.5 Anhydroglucitol 2nd point and Type 2 diabetes | −0.387 | 0.004 |
| 1.5 Anhydroglucitol 2nd point and Overweight or obesity | −0.277 | 0.047 |
| 1.5 Anhydroglucitol 2nd point and Off-pump | −0.346 | 0.011 |
| 1.5 Anhydroglucitol 2nd point and Combined operations | −0.273 | 0.048 |
| 1.5 Anhydroglucitol 2nd point and Glucose 1st point | −0.433 | 0.001 |
| 1.5 Anhydroglucitol 2nd point and Glucose 2nd point | −0.349 | 0.012 |
| 1.5 Anhydroglucitol 2nd point and Fructosamine 1st point | −0.414 | 0.003 |
| 1.5 Anhydroglucitol 2nd point and Fructosamine 2nd point | −0.336 | 0.016 |
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Step 1 a | Age | 0.074 | 0.026 | 8.077 | 1 | 0.004 | 1.077 |
| Constant | −2.677 | 1.519 | 3.104 | 1 | 0.078 | 0.069 | |
| Step 2 b | Age | 0.074 | 0.026 | 7.946 | 1 | 0.005 | 1.076 |
| Fructosamine | 0.007 | 0.003 | 5.226 | 1 | 0.022 | 1.007 | |
| Constant | −4.561 | 1.744 | 6.840 | 1 | 0.009 | 0.010 | |
| a. Variable(s) entered on step 1: Age | |||||||
| b. Variable(s) entered on step 2: Fructosamine | |||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Step 1 a | Age | 0.096 | 0.025 | 15.230 | 1 | 0.000 | 1.101 |
| Constant | −7.052 | 1.559 | 20.450 | 1 | 0.000 | 0.001 | |
| Step 2 b | Age | 0.112 | 0.027 | 17.889 | 1 | 0.000 | 1.119 |
| Aortic occlusion time | 0.026 | 0.008 | 9.725 | 1 | 0.002 | 1.026 | |
| Constant | −9.760 | 1.911 | 26.086 | 1 | 0.000 | 0.000 | |
| Step 3 c | Age | 0.137 | 0.029 | 21.972 | 1 | 0.000 | 1.147 |
| Aortic occlusion time | 0.043 | 0.011 | 15.942 | 1 | 0.000 | 1.044 | |
| Shunt count | −0.913 | 0.314 | 8.470 | 1 | 0.004 | 0.401 | |
| Constant | −10.029 | 1.975 | 25.774 | 1 | 0.000 | 0.000 | |
| a. Variable(s) entered on step 1: Age. | |||||||
| b. Variable(s) entered on step 2: Aortic occlusion time | |||||||
| c. Variable(s) entered on step 3: Shunt count | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumin, A.N.; Bezdenezhnykh, N.A.; Bezdenezhnykh, A.V.; Kuzmina, A.A.; Dyleva, Y.A.; Barbarash, O.L. Association of Alternative Markers of Carbohydrate Metabolism (Fructosamine and 1,5-Anhydroglucitol) with Perioperative Characteristics and In-Hospital Complications of Coronary Artery Bypass Grafting in Patients with Type 2 Diabetes Mellitus, Prediabetes, and Normoglycemia. Diagnostics 2023, 13, 969. https://doi.org/10.3390/diagnostics13050969
Sumin AN, Bezdenezhnykh NA, Bezdenezhnykh AV, Kuzmina AA, Dyleva YA, Barbarash OL. Association of Alternative Markers of Carbohydrate Metabolism (Fructosamine and 1,5-Anhydroglucitol) with Perioperative Characteristics and In-Hospital Complications of Coronary Artery Bypass Grafting in Patients with Type 2 Diabetes Mellitus, Prediabetes, and Normoglycemia. Diagnostics. 2023; 13(5):969. https://doi.org/10.3390/diagnostics13050969
Chicago/Turabian StyleSumin, Alexey N., Natalia A. Bezdenezhnykh, Andrey V. Bezdenezhnykh, Anastasiya A. Kuzmina, Yuliya A. Dyleva, and Olga L. Barbarash. 2023. "Association of Alternative Markers of Carbohydrate Metabolism (Fructosamine and 1,5-Anhydroglucitol) with Perioperative Characteristics and In-Hospital Complications of Coronary Artery Bypass Grafting in Patients with Type 2 Diabetes Mellitus, Prediabetes, and Normoglycemia" Diagnostics 13, no. 5: 969. https://doi.org/10.3390/diagnostics13050969
APA StyleSumin, A. N., Bezdenezhnykh, N. A., Bezdenezhnykh, A. V., Kuzmina, A. A., Dyleva, Y. A., & Barbarash, O. L. (2023). Association of Alternative Markers of Carbohydrate Metabolism (Fructosamine and 1,5-Anhydroglucitol) with Perioperative Characteristics and In-Hospital Complications of Coronary Artery Bypass Grafting in Patients with Type 2 Diabetes Mellitus, Prediabetes, and Normoglycemia. Diagnostics, 13(5), 969. https://doi.org/10.3390/diagnostics13050969






