Advanced Imaging for Robotic Bronchoscopy: A Review
Abstract
:1. Introduction
CT-to-Body Divergence
2. Augmented Fluoroscopy
2.1. LungVision™
2.2. CIOS Spin
3. Cone-Beam CT
3.1. CBCT with Conventional and Non-RAB Navigation Platforms
3.2. CBCT with RAB Navigational Platforms
3.2.1. Ion™ Robotic Platform Combined with CBCT
3.2.2. Monarch™ Robotic Platform Combined with CBCT
4. O-Arm CT (OACT)
5. Bronchoscopic Tools to Improve Diagnostic Yield
6. Strategies to Reduce CTBD
7. Therapeutic Potentials with Advanced Bronchoscopy and Augmented Imaging
7.1. Lung Tumor Ablation
7.2. Perspective on the Future of Bronchoscopy-Guided Lung Tumor Ablation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gould, M.K.; Tang, T.; Liu, I.-L.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; DiFiore, J.L.; Sud, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef]
- Meza, R.; Jeon, J.; Toumazis, I.; Ten Haaf, K.; Cao, P.; Bastani, M.; Han, S.S.; Blom, E.F.; Jonas, D.E.; Feuer, E.J.; et al. Evaluation of the Benefits and Harms of Lung Cancer Screening with Low-Dose Computed Tomography: Modeling Study for the US Preventive Services Task Force. JAMA 2021, 325, 988–997. [Google Scholar] [CrossRef]
- Agrawal, A.; Hogarth, D.K.; Murgu, S. Robotic bronchoscopy for pulmonary lesions: A review of existing technologies and clinical data. J. Thorac. Dis. 2020, 12, 3279. [Google Scholar] [CrossRef]
- Chen, A.C.; Pastis, N.J., Jr.; Mahajan, A.K.; Khandhar, S.J.; Simoff, M.J.; Machuzak, M.S.; Cicenia, J.; Gildea, T.R.; Silvestri, G.A. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021, 159, 845–852. [Google Scholar] [CrossRef]
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P., 3rd; Murgu, S. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: Results from the initial multicenter experience. BMC Pulm. Med. 2019, 19, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.C.; Gillespie, C.T. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann. Thorac. Surg. 2018, 106, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.C.; Pastis, N.J.; Machuzak, M.S.; Gildea, T.R.; Simoff, M.J.; Gillespie, C.T.; Mahajan, A.K.; Oh, S.S.; Silvestri, G.A. Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respiration 2020, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Yarmus, L.; Akulian, J.; Wahidi, M.; Chen, A.; Steltz, J.P.; Solomon, S.L.; Yu, D.; Maldonado, F.; Cardenas-Garcia, J.; Molena, D.; et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020, 157, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Bhadra, K.; Calcutt, M.; Folch, E. Virtual or reality: Divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J. Thorac. Dis. 2020, 12, 1595–1611. [Google Scholar] [CrossRef]
- Chen, A.; Pastis, N.; Furukawa, B.; Silvestri, G.A. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest 2015, 147, 1275–1281. [Google Scholar] [CrossRef]
- Reisenauer, J.; Duke, J.D.; Kern, R.; Fernandez-Bussy, S.; Edell, E. Combining Shape-Sensing Robotic Bronchoscopy with Mobile Three Dimensional Imaging to Verify Tool-in-Lesion and Overcome Divergence: A Pilot Study. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.K.; Blaj, M.; Stahl, J.; Speicher, J.; Anciano, C.; Hudson, S.; Kragel, E.A.; Bowling, M.R. Evaluation of Electromagnetic Navigational Bronchoscopy Using Tomosynthesis-Assisted Visualization, Intraprocedural Positional Correction and Continuous Guidance for Evaluation of Peripheral Pulmonary Nodules. J. Bronchol. Interv. Pulmonol. 2023, 30, 16–23. [Google Scholar] [CrossRef]
- Cicenia, J.; Bhadra, K.; Sethi, S.; Nader, D.A.; Whitten, P.; Hogarth, D.K. Augmented Fluoroscopy: A New and Novel Navigation Platform for Peripheral Bronchoscopy. J. Bronchol. Interv. Pulmonol. 2021, 28, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, D.K. Use of augmented fluoroscopic imaging during diagnostic bronchoscopy. Future Oncol. 2018, 14, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Aboudara, M.; Roller, L.; Rickman, O.; Lentz, R.J.; Pannu, J.; Chen, H.; Maldonado, F. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology 2020, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Schampaert, S.; de Groot, J.A.H.; Schirmer, C.C.; Van der Bom, I. Cone-Beam CT with Augmented Fluoroscopy Combined with Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J. Bronchol. Interv. Pulmonol. 2018, 25, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, S.K.; Roller, L.; Katsis, J.; Chen, H.; Lentz, R.J.; Rickman, O.B.; Maldonado, F. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-Time Target Acquisition. Respiration 2022, 101, 166–173. [Google Scholar] [CrossRef]
- Pritchett, M.A. Prospective Analysis of a Novel Endobronchial Augmented Fluoroscopic Navigation System for Diagnosis of Peripheral Pulmonary Lesions. J. Bronchol. Interv. Pulmonol. 2021, 28, 107–115. [Google Scholar] [CrossRef]
- Hedstrom, G.; Wagh, A.A. Combining Real-time 3-D imaging and augmented fluoroscopy with robotic bronchoscopy for the diagnosis of peripheral lung lesions. Chest 2022, 162, A2082. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Fuentes, P.; Bott, M.J.; Beattie, J.A.; Lee, R.P.; Chawla, M.; Husta, B.C. Multiplanar 3D fluoroscopy redefines tool-lesion relationship during robotic-assisted bronchoscopy. Respirology 2021, 26, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Potts, K.; Wagh, A. Improvement in peripheral bronchoscopy requiRes. the use of advanced imaging. AME Case Rep. 2022, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Wagh, A.; Ho, E.; Murgu, S.; Hogarth, D.K. Improving diagnostic yield of navigational bronchoscopy for peripheral pulmonary lesions: A review of advancing technology. J. Thorac. Dis. 2020, 12, 7683–7690. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Bhadra, K.; Mattingley, J.S. Electromagnetic Navigation Bronchoscopy with Tomosynthesis-based Visualization and Positional Correction: Three-dimensional Accuracy as Confirmed by Cone-Beam. Computed Tomography. J. Bronchol. Interv. Pulmonol. 2021, 28, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kheir, F.; Thakore, S.R.; Becerra, J.P.U.; Tahboub, M.; Kamat, R.; Abdelghani, R.; Fernandez-Bussy, S.; Kaphle, U.R.; Majid, A. Cone-Beam Computed Tomography-Guided Electromagnetic Navigation for Peripheral Lung Nodules. Respiration 2021, 100, 44–51. [Google Scholar] [CrossRef]
- Casal, R.F.; Sarkiss, M.; Jones, A.K.; Stewart, J.; Tam, A.; Grosu, H.B.; Ost, D.E.; Jimenez, C.A.; Eapen, G.A. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: A prospective pilot study. J. Thorac. Dis. 2018, 10, 6950–6959. [Google Scholar] [CrossRef]
- DiBardino, D.M.; Kim, R.Y.; Cao, Y.; Andronov, M.; Lanfranco, A.R.; Haas, A.R.; Vachani, A.; Ma, K.C.; Hutchinson, C.T. Diagnostic Yield of Cone-beam-Derived Augmented Fluoroscopy and Ultrathin Bronchoscopy Versus Conventional Navigational Bronchoscopy Techniques. J. Bronchol. Interv. Pulmonol. 2022. [Google Scholar] [CrossRef]
- Folch, E.E.; Pritchett, M.A.; Reisenauer, J.; Casal, R.F.; Simoff, M.J.; Keyes, C.; Diaz-Mendoza, J.I.; Fernandez-Bussy, S.; Majid, A.; Parkih, M. Prospective, multicenter analysis of shape-sensing robotic-assisted bronchoscopy: Updates from the precise study. Chest 2022, 162, A2655–A2656. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022, 161, 572–582. [Google Scholar] [CrossRef]
- Benn, B.S.; Romero, A.O.; Lum, M.; Krishna, G. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021, 199, 177–186. [Google Scholar] [CrossRef]
- Styrvoky, K.; Schwalk, A.; Pham, D.; Chiu, H.T.; Rudkovskaia, A.; Madsen, K.; Carrio, S.; Kurian, E.M.; De Las Casas, L.; Abu-Hijleh, M. Shape-Sensing Robotic-Assisted Bronchoscopy with Concurrent use of Radial Endobronchial Ultrasound and Cone Beam Computed Tomography in the Evaluation of Pulmonary Lesions. Lung 2022, 200, 755–761. [Google Scholar] [CrossRef]
- Cumbo-Nacheli, G.; Velagapudi, R.K.; Enter, M.; Egan, J.P., 3rd; Conci, D. Robotic-assisted Bronchoscopy and Cone-beam CT: A Retrospective Series. J. Bronchol. Interv. Pulmonol. 2022, 29, 303–306. [Google Scholar] [CrossRef]
- Chan, J.W.Y.; Chang, A.T.C.; Yu, P.S.Y.; Lau, R.W.H.; Ng, C.S.H. Robotic Assisted-Bronchoscopy with Cone-Beam. CT ICG Dye Marking for Lung Nodule Localization: Experience Beyond USA. Front. Surg. 2022, 9, 943531. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, K.; Takahashi, Y.; Kaga, K.; Senmaru, N.; Kotani, Y.; Matsui, Y. Video-assisted thoracoscopic surgery using mobile computed tomography: New method for locating of small lung nodules. J. Cardiothorac. Surg. 2014, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Cho, R.J.; Senitko, M.; Wong, J.; Dincer, E.H.; Khosravi, H.; Abraham, G.E., 3rd. Feasibility of Using the O-Arm Imaging System During ENB-rEBUS-guided Peripheral Lung Biopsy: A Dual-center Experience. J. Bronchol. Interv. Pulmonol. 2021, 28, 248–254. [Google Scholar] [CrossRef]
- Chambers, J.; Knox, D.; Leclair, T. O-arm CT for Confirmation of Successful Navigation During Robotic Assisted Bronchoscopy. J. Bronchol. Interv. Pulmonol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gildea Thomas, R.; Folch Erik, E.; Pritchett, M.A.; LeMense, G.P.; Linden, P.A.; Arenberg, D.A.; Rickman, O.B.; Mahajan, A.K.; Singh, J. NAVIGATE Study Investigators. The Impact of Biopsy Tool Choice and Rapid On-Site Evaluation on Diagnostic Accuracy for Malignant Lesions in the Prospective: Multicenter NAVIGATE Study. J. Bronchol. Interv. Pulmonol. 2021, 28, 174–183. [Google Scholar] [CrossRef]
- Bhadra, K.; Setser, R.M.; Condra, W.; Pritchett, M.A. Lung Navigation Ventilation Protocol to Optimize Biopsy of Peripheral Lung Lesions. J. Bronchol. Interv. Pulmonol. 2022, 29, 7. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Lau, K.; Skibo, S.; Bhadra, K. Anesthesia considerations to reduce motion and atelectasis during advanced guided bronchoscopy. BMC Pulm. Med. 2021, 21, 240. [Google Scholar] [CrossRef]
- Salahuddin, M.; Sarkiss, M.; Sagar, A.-E.S.; Vlahos, I.; Chang, C.H.; Shah, A.; Sabath, B.F.; Lin, J.; Song, J.; Moon, T.; et al. Ventilatory Strategy to Prevent Atelectasis During Bronchoscopy Under General Anesthesia: A Multicenter Randomized Controlled Trial (Ventilatory Strategy to Prevent Atelectasis -VESPA- Trial). Chest 2022, 162, 1393–1401. [Google Scholar] [CrossRef]
- Lin, J.; Sabath, B.F.; Sarkiss, M.; Jimenez, C.A.; Casal, R.F. Lateral Decubitus Positioning for Mobile CT-guided Robotic Bronchoscopy: A Novel Technique to Prevent Atelectasis. J. Bronchol. Interv. Pulmonol. 2022, 29, 220. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Antoni, D.; Charloux, A.; Falcoz, P.E.; Quoix, E. Comorbidities in the management of patients with lung cancer. Eur. Respir. J. 2017, 49, 1601721. [Google Scholar] [CrossRef] [Green Version]
- Berzenji, L.; Schil, P.E.V. Surgery or stereotactic body radiotherapy for early-stage lung cancer: Two sides of the same coin? Eur. Respir. J. 2019, 53, 1900711. [Google Scholar] [CrossRef] [Green Version]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Daly, M.E.; Kennedy, E.B.; Antonoff, M.B.; Broderick, S.; Feldman, J.; Jolly, S.; Meyers, B.; Rocco, G.; Rusthoven, C.; et al. Stereotactic Body Radiotherapy for Early-Stage Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J. Clin. Oncol. 2018, 36, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.; Visser, O.; Lagerwaard, F.J.; Belderbos, J.; Slotman, B.J.; Senan, S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: A population-based time-trend analysis. J. Clin. Oncol. 2010, 28, 5153–5159. [Google Scholar] [CrossRef]
- Palma, D.; Lagerwaard, F.; Rodrigues, G.; Haasbeek, C.; Senan, S. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: Stereotactic radiotherapy outcomes and systematic review. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Donington, J.; Ferguson, M.; Mazzone, P.; Handy, J., Jr.; Schuchert, M.; Fernando, H.; Loo, B., Jr.; Lanuti, M.; de Hoyos, A.; Detterbeck, F.; et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012, 142, 1620–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, A.; Yoshida, E.J.; Bui, K.; Katrivesis, J.; Fernando, D.; Nelson, K.; Abi-Jaoudeh, N. Patient and facility demographics related outcomes in early-stage non-small cell lung cancer treated with radiofrequency ablation: A National Cancer Database analysis. J. Vasc. Interv. Radiol. 2018, 29, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, D.E.; Fernando, H.C.; Hillman, S.; Ng, T.; Tan, A.D.; Sharma, A.; Rilling, W.S.; Hong, K.; Putnam, J.B. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015, 121, 3491–3498. [Google Scholar] [CrossRef] [Green Version]
- De Baere, T.; Tselikas, L.; Woodrum, D.; Abtin, F.; Littrup, P.; Deschamps, F.; Suh, R.; Aoun, H.D.; Callstrom, M. Evaluating cryoablation of metastatic lung tumors in patients—Safety and efficacy: The ECLIPSE Trial--interim analysis at 1 year. J. Thorac. Oncol. 2015, 10, 1468–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lencioni, R.; Crocetti, L.; Cioni, R.; Uh, R.; Glenn, D.; Regge, D.; Helmberger, T.; Gillams, A.R.; Frilling, A.; Ambrogi, M.; et al. Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008, 9, 621–628. [Google Scholar] [CrossRef]
- Genshaft, S.J.; Suh, R.D.; Abtin, F.; Baerlocher, M.O.; Dariushnia, S.R.; Devane, A.M.; Himes, E.; Lisberg, A.; Padia, S.; Patel, S.; et al. Society of Interventional Radiology Quality Improvement Standards on Percutaneous Ablation of Non-Small Cell Lung Cancer and Metastatic Disease to the Lungs. J. Vasc. Interv. Radiol. 2021, 32, 1242.e1–1242.e10. [Google Scholar] [CrossRef]
- Tsushima, K.; Koizumi, T.; Tanabe, T.; Nakagawa, R.; Yoshikawa, S.; Yasuo, M.; Kubo, K. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur. Respir. J. 2007, 29, 1193–1200. [Google Scholar] [CrossRef]
- Henne, E.; Ferguson, J.S.; Mest, R.; Herth, F.J. Thermal Vapor Ablation for Lung Lesions in a Porcine Model. Respiration 2015, 90, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Casal, R.F.; Walsh, G.; McArthur, M.; Hill, L.R.; Landaeta, M.F.; Armas Villalba, A.J.; Ong, P.; Debiane, L.; Vakil, E.; Grosu, H.B.; et al. Bronchoscopic Laser Interstitial Thermal Therapy: An Experimental Study in Normal Porcine Lung Parenchyma. J. Bronchol. Interv. Pulmonol. 2018, 25, 322–329. [Google Scholar] [CrossRef]
- Sebek, J.; Kramer, S.; Rocha, R.; Yu, K.C.; Bortel, R.; Beard, W.L.; Biller, D.S.; Hodgson, D.S.; Ganta, C.K.; Wibowo, H.; et al. Bronchoscopically delivered microwave ablation in an in vivo porcine lung model. ERJ Open Res. 2020, 6, 00146–02020. [Google Scholar] [CrossRef]
- Ferguson, J.S.; Henne, E. Bronchoscopically Delivered Thermal Vapor Ablation of Human Lung Lesions. J. Bronchol. Interv. Pulmonol. 2019, 26, 108–113. [Google Scholar] [CrossRef]
- Tanabe, T.; Koizumi, T.; Tsushima, K.; Ito, M.; Kanda, S.; Kobayashi, T.; Yasuo, M.; Yamazaki, Y.; Kubo, K.; Honda, T.; et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010, 137, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chen, J.; Jiang, Y.; Un, J.; Hogarth, D.K.; Herth, F.J. Microwave ablation via a flexible catheter for the treatment of nonsurgical peripheral lung cancer: A pilot study. Thorac. Cancer 2022, 13, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.Y.; Lau, R.W.H.; Ngai, J.C.L.; Tsoi, C.; Chu, C.M.; Mok, T.S.K.; Ng, C.S. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance—A novel technique and initial experience with 30 cases. Transl. Lung Cancer Res. 2021, 10, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Spiers, A.; Pritchett, M.; Krimsky, W. P1.05-06 Bronchoscopic Image-Guided Microwave Ablation of Peripheral Lung Tumours—Early Results. J. Thorac. Oncol. 2018, 13, S542. [Google Scholar] [CrossRef] [Green Version]
- Pritchett, M.; Reisenauer, J.; Kern, R.; Wilson, D.S.; Meyers, E.E.; Szapary, P.O.; Laeseke, P.F. Image-Guided Transbronchial Microwave Ablation of Peripheral Primary Lung Tumors with A Flexible Probe: First In Us Experience. Chest 2020, 158, A1452–A1453. [Google Scholar] [CrossRef]
- Zeng, C.; Fu, X.; Yuan, Z.; Hu, S.; Wang, X.; Ping, W.; Cai, Y.; Wang, J. Application of electromagnetic navigation bronchoscopy-guided microwave ablation in multiple pulmonary nodules: A single-centre study. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac071. [Google Scholar] [CrossRef] [PubMed]
- Ye, X. Microwave Ablation in Ground Glass Nodules. Clinical Trial Registration NCT03490890. Available online: https://clinicaltrials.gov/ct2/show/NCT03490890 (accessed on 3 February 2023).
- Chen, C. Efficiency and Safety of Microwave Ablation Plus Immune Checkpoint Inhibitor for Patients with Multiple Primary Lung Cancer: A Open, Multi-Center, Phase II Clinical Trial. Clinical Trial Registration NCT05053802, clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05053802 (accessed on 3 February 2023).
- Ethicon, Inc. A Prospective Multicenter Study of Transbronchial Microwave Ablation Using Robotic-Assisted Bronchoscopy in Subjects with Oligometastatic Tumors in the Lung. Clinical Trial Registration NCT05299606. Available online: https://clinicaltrials.gov/ct2/show/NCT05299606 (accessed on 3 February 2023).
- Majid, A. Feasibility and Efficacy of the AveCure Flexible Microwave Ablation Probe for Peripheral Lung Nodule. Clinical Trial Registration NCT05281237. Available online: https://clinicaltrials.gov/ct2/show/NCT05281237 (accessed on 3 February 2023).
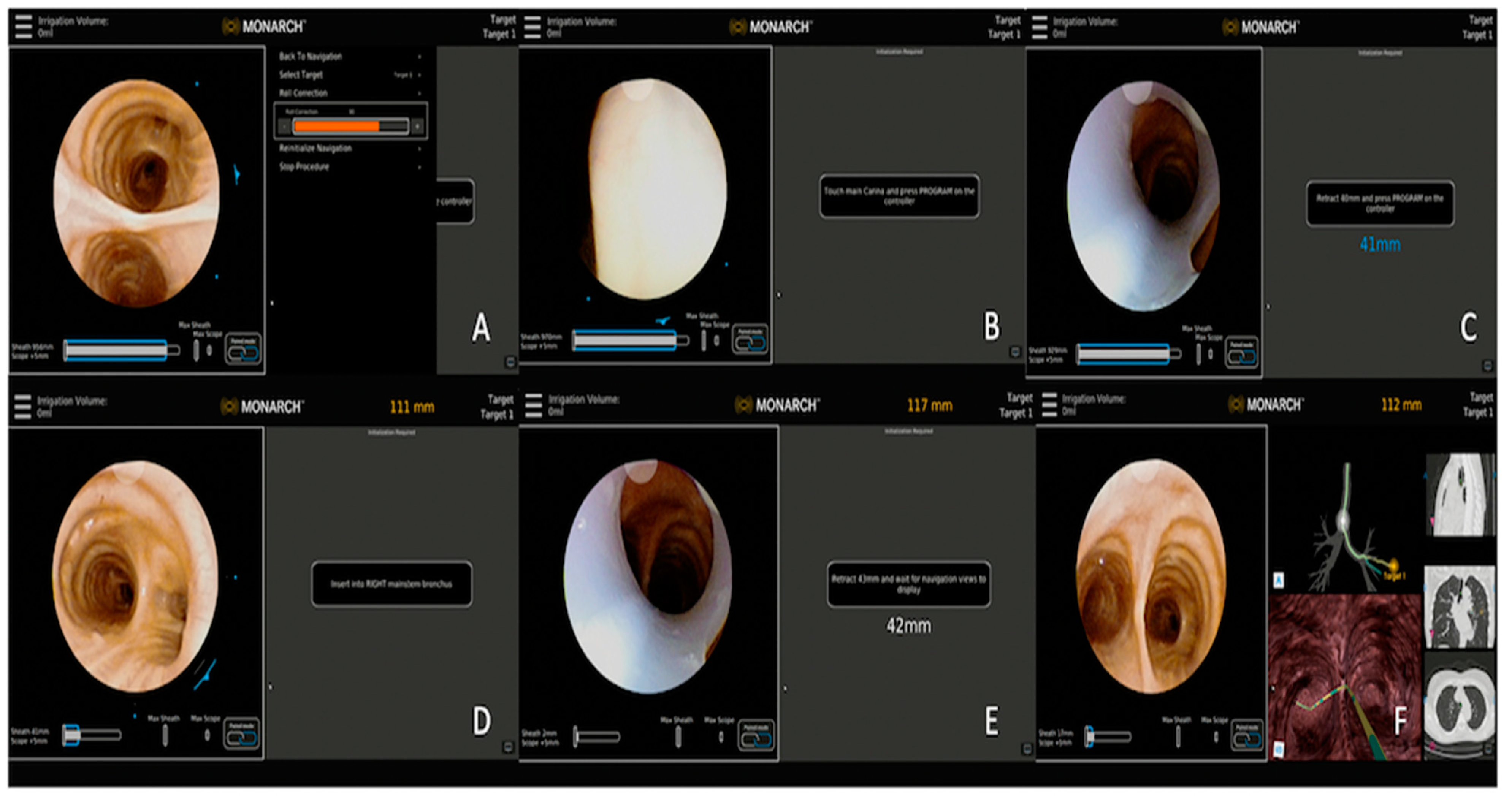
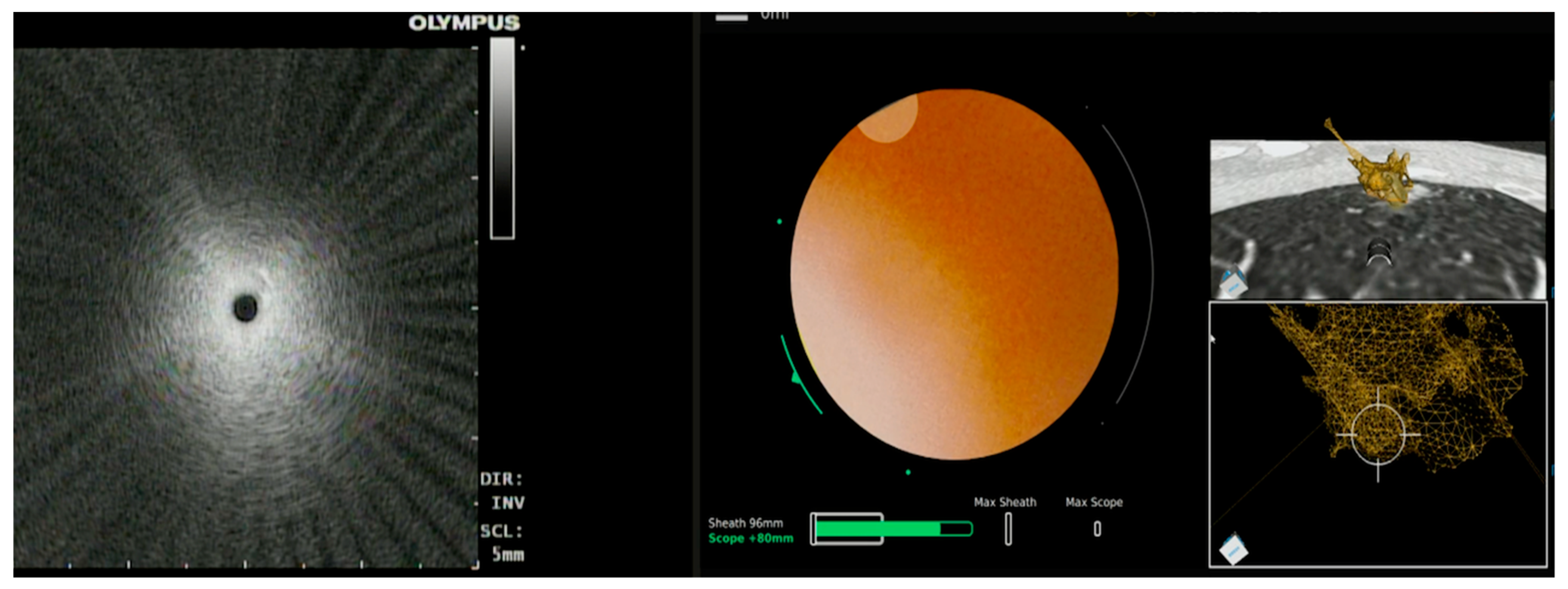

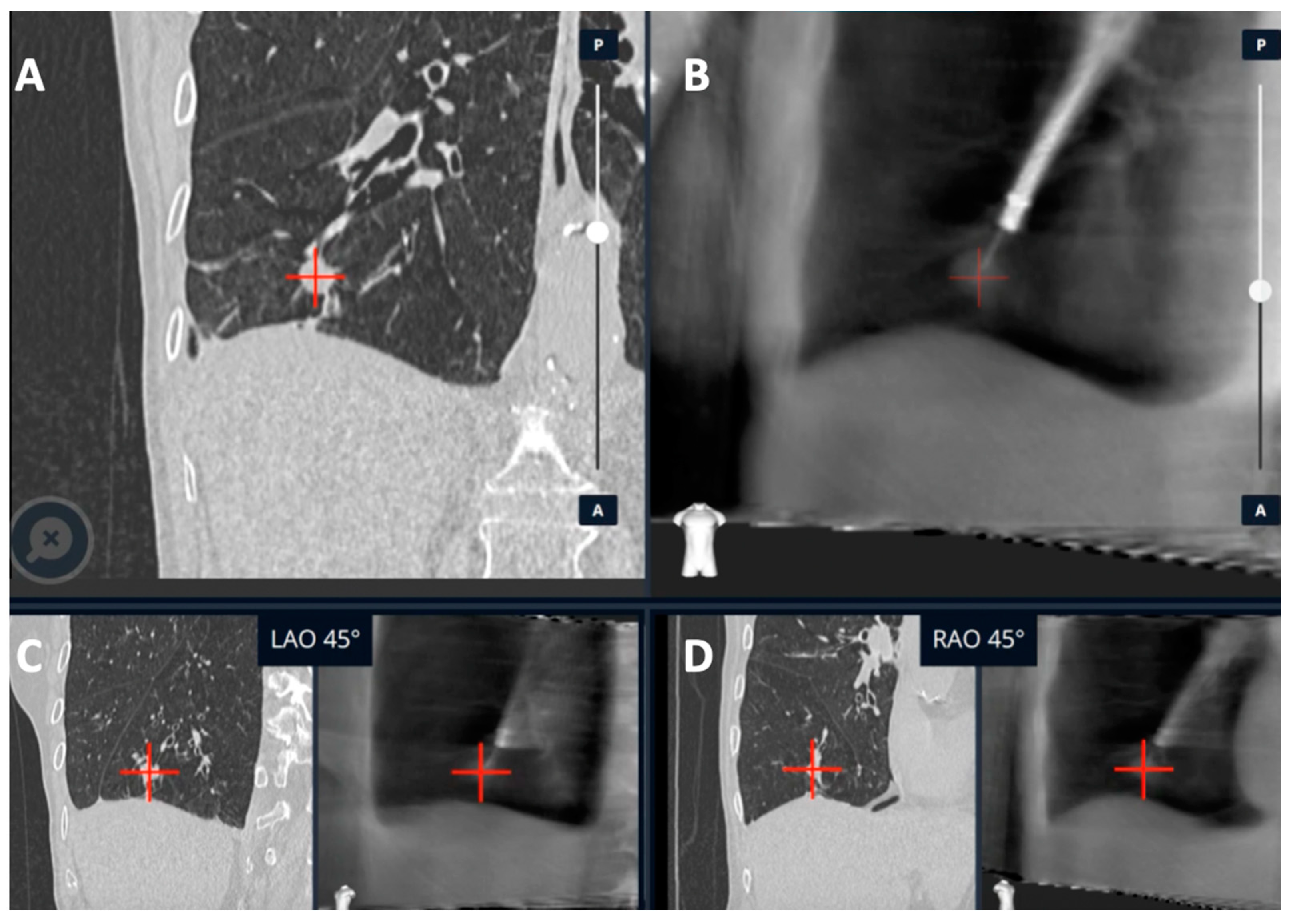
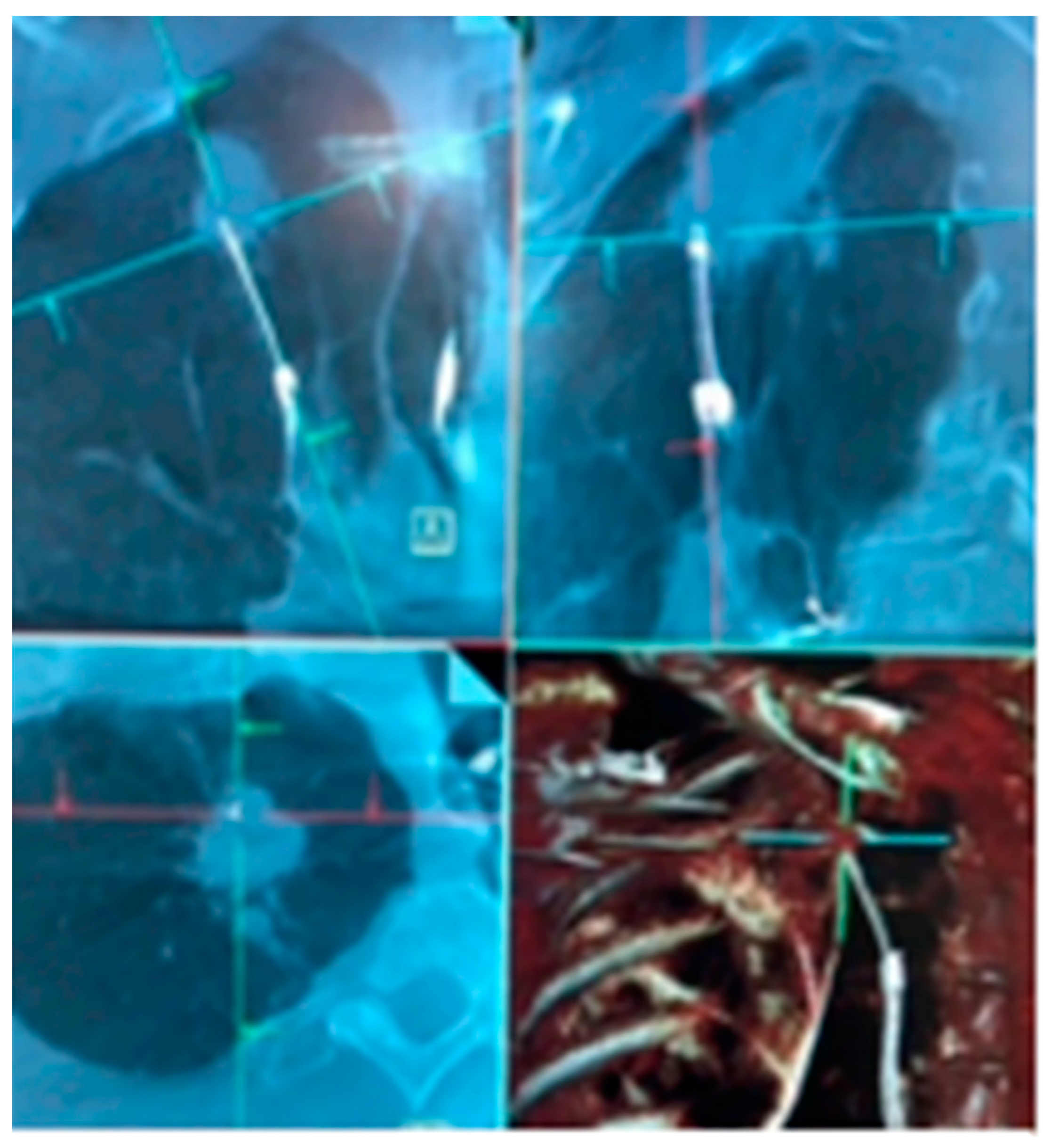
| Publication | Device | Description | Sample Size | Diagnostic Yield | Adverse Events |
|---|---|---|---|---|---|
| Aboudara et al. [15] | SuperDimension, Medtronic | Comparison of Standard ENB to Fluoroscopic ENB. | 90 lesions (S-ENB) vs. 59 lesions (F-ENB) | 79% (F-ENB) vs. 54% (S-ENB). p < 0.005. Mean divergence of 12 mm | Pneumothorax (1.9% vs 1.5%) |
| Avasarala et al. [17] | Illumisite™; Medtronic | Real-time guidance with digital tomosynthesis corrected navigation system | 100 lesions | 83% overall yield with 71% sensitivity for malignancy (52/73) | Pneumothorax—3%. Bleeding requiring intervention—2% |
| Cicenia et al. [13] | LungVision; Body Vision Medical LTD. | Real-time fluoroscopic images with integration of images from preop CT. | 55 patients | 93% nodule localization success; DYi: 75% yield on ROSE | No adverse events |
| Pritchett [18] | LungVision; Body Vision Medical LTD. | Real-time fluoroscopic guidance for navigation and biopsy with intra-op co-relation using CBCT | 51 patients | Localization success: 96%, DYi 78%, and DA: 88%. Average divergence of 14.5 mm | No adverse events |
| Hedstrom et al. [19] | Monarch™ robotic platform with lung vision | Robotic platform for navigation with CABT from Lung vision for intra-procedural real-time guidance | 45 patients | DYi: 84% DA: 91% | Pneumothorax: 8% (4/45) |
| Kalchiem-Dekel et al. [20] | Ion™ robotic platform with CIOS | Robotic platform for navigation with 3D multiplanar fluoroscopy for intra-procedural real-time guidance | 10 lesions | Tool in lesion: 90%. Tool correction in 30% lesions with real-time imaging. DY not reported | - |
| Reisenauer et al. [11] | Ion™ robotic platform with CIOS | Robotic platform for navigation with 3D multiplanar fluoroscopy for intra-procedural real-time guidance | 30 lesions | DYi: 93%. Average divergence: 10 mm in upper lobe 20 mm in lower lobe | No adverse events |
| Pritchett et al. [16] | SuperDimension, Medtronic with CBCT | ENB system for navigation with intra-procedural CBCT. No rEBUS for any cases | 93 lesions | DY: 83% DA: 93% | Pneumothorax: 4% |
| Kheir et al. [24] | SuperDimension, Medtronic with CBCT | Standard ENB vs. ENB-CBCT | 31 patients (ENB) vs. 31 patients (ENB-CBCT) | DY: 74% (ENB-CBCT) vs. 51% (ENB) | Total adverse events (6.5%)—no difference between groups |
| Benn et al. [29] | Ion™ robotic platform with CBCT | Robotic platform for navigation with intra-operative CBCT for biopsy tool guidance | 59 lesions | DY: 83% DA: 86% | Pneumothorax: 3.8% |
| Styrvoky et al. [30] | Ion™ robotic platform with CBCT | Robotic platform for navigation with intra-operative CBCT for biopsy tool guidance | 209 lesions | DA: 91% | Pneumothorax: 1% |
| Cumbo-Nacheli et al. [31] | Monarch™ robotic platform with CBCT | Robotic platform for navigation with intra-operative CBCT for biopsy tool guidance | 20 lesions | Sensitivity for malignancy: 86% | - |
| Procedural Step | Recommendations |
|---|---|
| Pre-Oxygenation | Avoid FiO2 of 1.0 and use a lower FiO2 of 0.6–0.8. |
| Anesthesia Type | TIVA with Propofol and paralytics. |
| Intubation | Use larger endotracheal tube if able (≥8.5 mm). Use non-depolarizing muscle relaxant. |
| Post-Intubation | Perform 4 recruitment maneuvers as able. Maintain FiO2 at lowest tolerated level for saturations of above 90% with PEEP up to 10–12 cm H2O. Use a tidal volume of 8–10 cc/Kg of ideal body weight. |
| Breath Hold | Peak inspiratory breath hold. Adjust APL valve to maintain circuit pressure at desired PEEP for 5–10 s before beginning advanced imaging sweep. |
| Biopsy | Ensure ventilator settings are the same as those when performing sweep. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravikumar, N.; Ho, E.; Wagh, A.; Murgu, S. Advanced Imaging for Robotic Bronchoscopy: A Review. Diagnostics 2023, 13, 990. https://doi.org/10.3390/diagnostics13050990
Ravikumar N, Ho E, Wagh A, Murgu S. Advanced Imaging for Robotic Bronchoscopy: A Review. Diagnostics. 2023; 13(5):990. https://doi.org/10.3390/diagnostics13050990
Chicago/Turabian StyleRavikumar, Nakul, Elliot Ho, Ajay Wagh, and Septimiu Murgu. 2023. "Advanced Imaging for Robotic Bronchoscopy: A Review" Diagnostics 13, no. 5: 990. https://doi.org/10.3390/diagnostics13050990
APA StyleRavikumar, N., Ho, E., Wagh, A., & Murgu, S. (2023). Advanced Imaging for Robotic Bronchoscopy: A Review. Diagnostics, 13(5), 990. https://doi.org/10.3390/diagnostics13050990






