Spontaneously Ruptured Hepatocellular Carcinoma: Computed Tomography-Based Assessment
Abstract
1. Introduction
1.1. Risk Factors for SRHCC
1.2. Clinical Presentation of SRHCC
1.3. Diagnosis of SRHCC
1.3.1. Ultrasonography (US)
1.3.2. Computed Tomography (CT)
1.3.3. Magnetic Resonance Imaging (MRI)
1.4. Treatment of SRHCC
1.5. Prognosis of SRHCC
2. CT Findings
2.1. Differential Diagnosis of HCC
- Regenerative nodules are isodense to liver parenchyma with absent wash-in during the arterial phase. The presence of surrounding fibrosis can be evaluated during the portal phase [83].
- Arterioportal shunts (APSs) are abnormal communications between a hepatic arterial branch and the corresponding portal vein, sinusoid, or peri-biliary venule, causing focal arterialization of liver parenchyma. APSs are observed in the arterial phase as peripheral wedge lesions that become isodense to liver parenchyma during the portal and late phases [84].
- Hepatic hemangiomas show peripheral, globular, and centripetal enhancement during the dynamic phases of contrast-enhanced CT [85].
- Pseudomasses are commonly observed in chronic portal vein thrombosis due to changes in the liver parenchyma from abnormal blood flow. Pseudomasses have a hypertrophic central area that shows hyperdensity in the arterial phase and appears isodense to liver parenchyma during the delayed phase. Instead, the peripheral region is usually atrophic and shows itself as constantly hypodense [86].
- Large regenerative nodules (LRNs), also known as focal nodular hyperplasia-like lesions, are clustered regenerative nodules showing rapid, intense, and persistent wash-in during the arterial phase with absent wash-out in the portal and delayed phases [87].
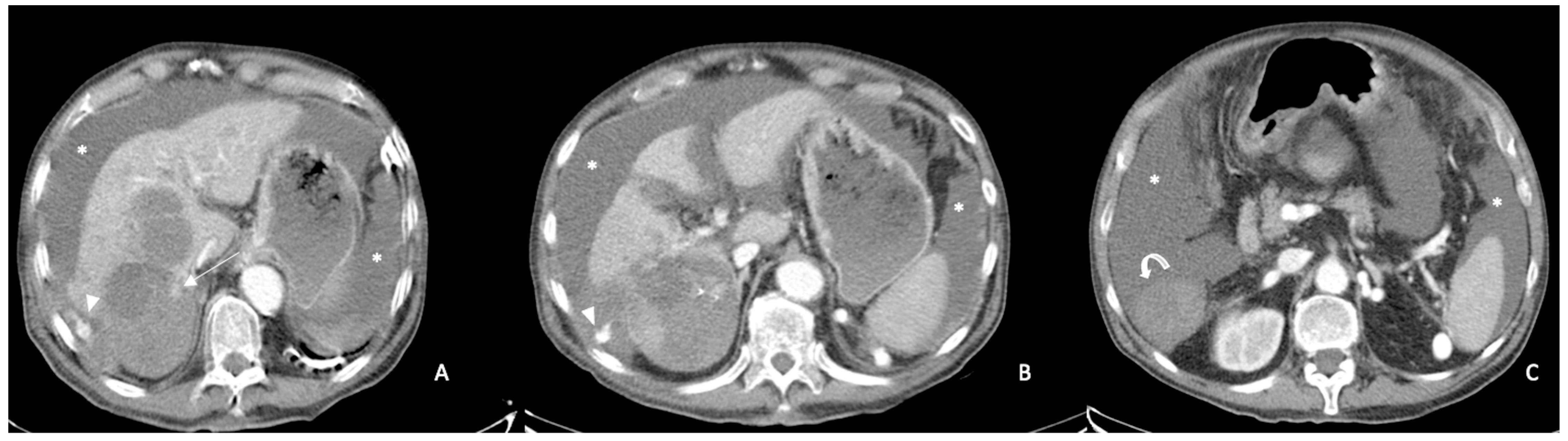
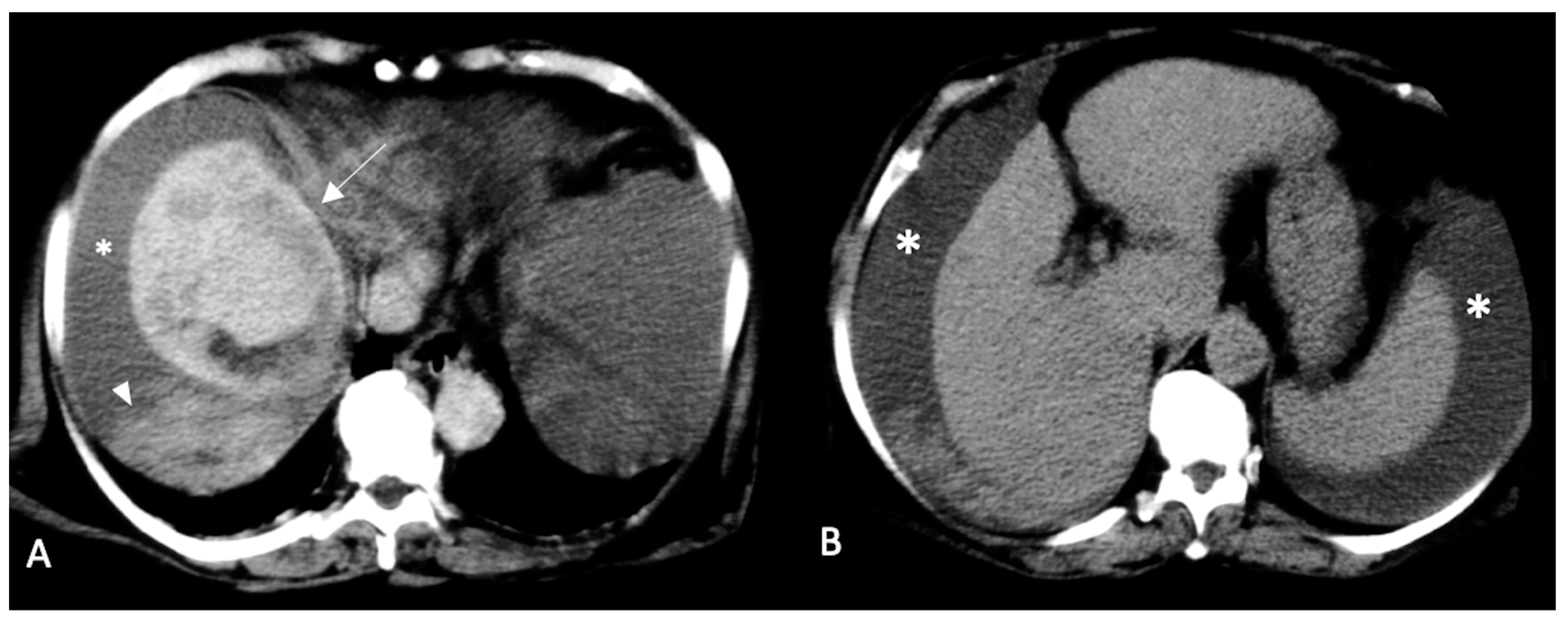
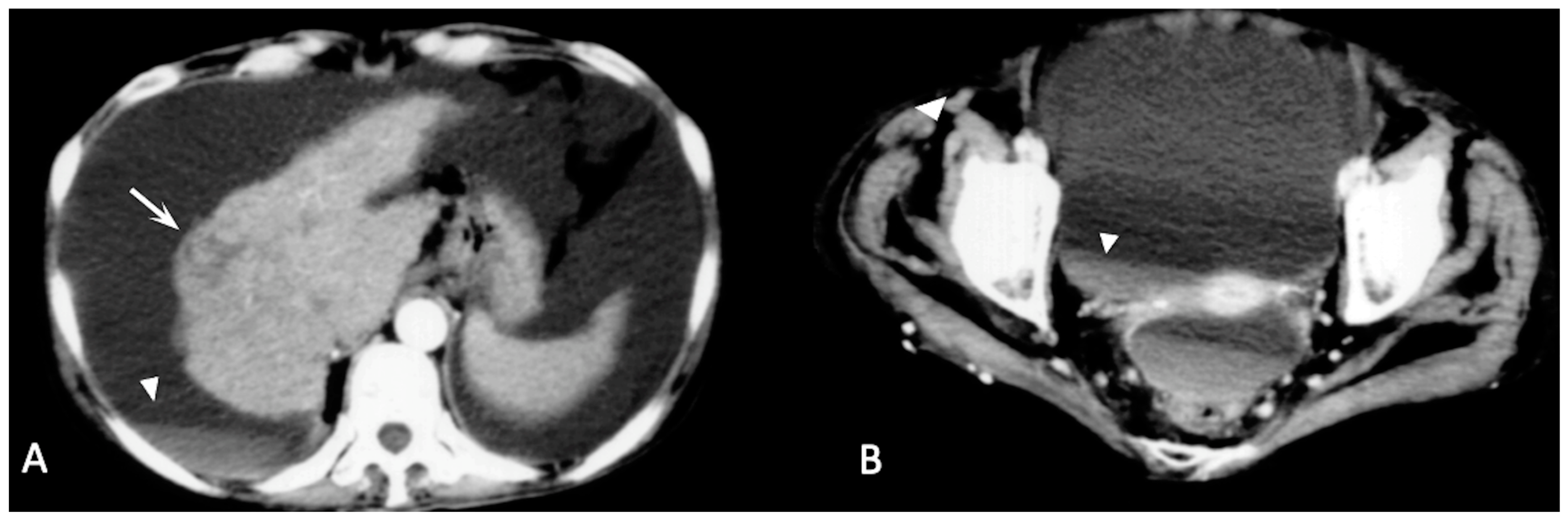
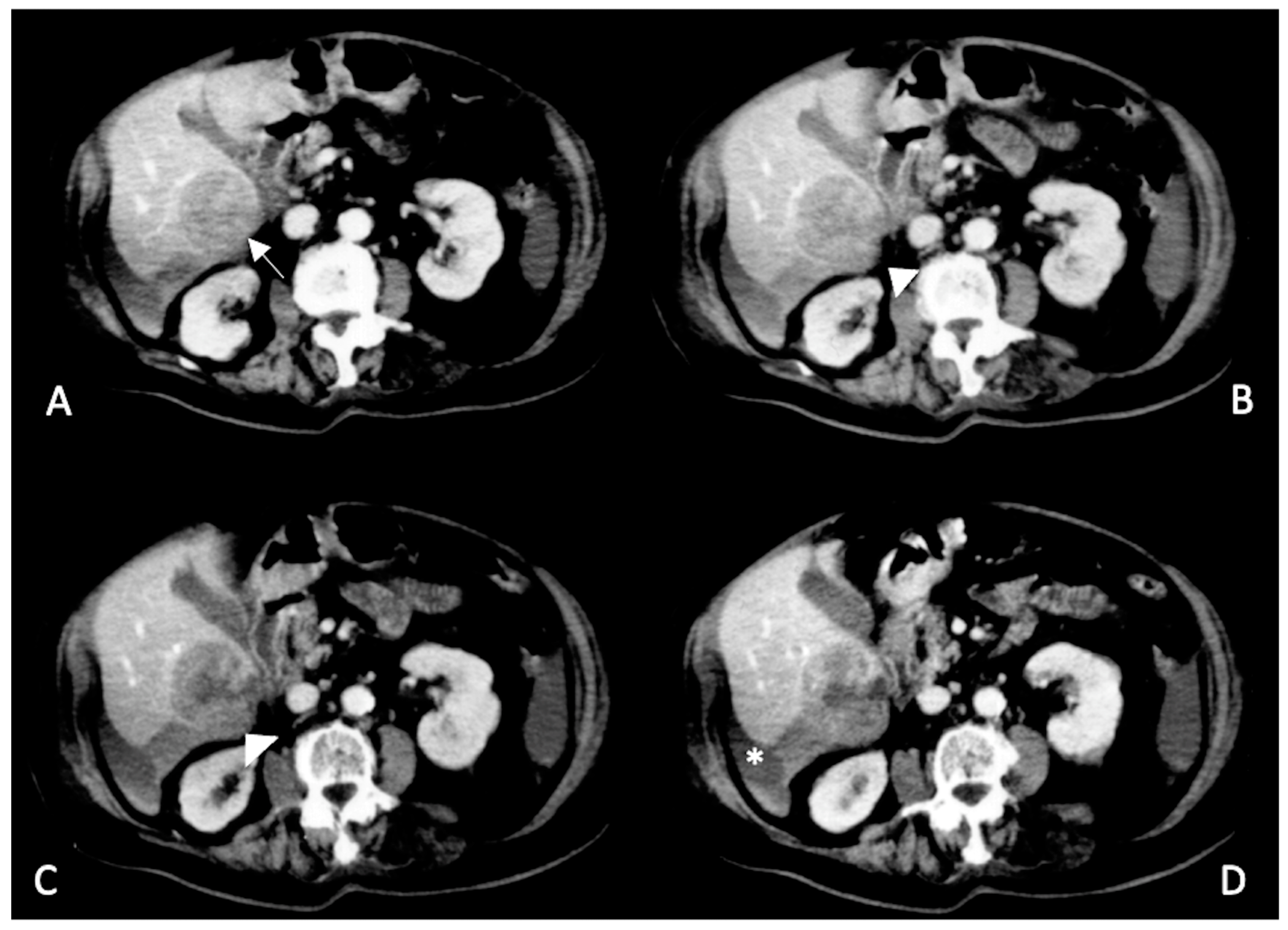
2.2. Spontaneously Ruptured HCC (SRHCC)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tartaglia, N.; Di Lascia, A.; Cianci, P.; Fersini, A.; Pacilli, M.; Pavone, G.; Ambrosi, A. Hemoperitoneum Caused by Spontaneous Rupture of Hepatocellular Carcinoma in Noncirrhotic Liver. A Case Report and Systematic Review. Open Med. 2020, 15, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Chakedis, J.; Sun, S.H.; Spolverato, G.; Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Spartalis, E.; Pawlik, T.M. Management, Outcomes, and Prognostic Factors of Ruptured Hepatocellular Carcinoma: A Systematic Review. J. Surg. Oncol. 2018, 117, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Bassi, N.; Caratozzolo, E.; Bonariol, L.; Ruffolo, C.; Bridda, A.; Padoan, L.; Antoniutti, M.; Massani, M. Management of Ruptured Hepatocellular Carcinoma: Implications for Therapy. World J. Gastroenterol. 2010, 16, 1221–1225. [Google Scholar] [CrossRef]
- Lai, E.C.H.; Lau, W.Y. Spontaneous Rupture of Hepatocellular Carcinoma: A Systematic Review. Arch. Surg. 2006, 141, 191–198. [Google Scholar] [CrossRef]
- Xia, F.; Ndhlovu, E.; Zhang, M.; Chen, X.; Zhang, B.; Zhu, P. Ruptured Hepatocellular Carcinoma: Current Status of Research. Front. Oncol. 2022, 12, 510. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part I. Development, Growth, and Spread: Key Pathologic and Imaging Aspects. Radiology 2014, 272, 635–654. [Google Scholar] [CrossRef]
- McGlynn, K.A.; London, W.T. Epidemiology and Natural History of Hepatocellular Carcinoma. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 3–23. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Yan, J.-J.; Huang, L.; Wu, M.-C.; Yan, Y.-Q. Predictors and Clinical Outcomes for Spontaneous Rupture of Hepatocellular Carcinoma. World J. Gastroenterol. 2012, 18, 7302–7307. [Google Scholar] [CrossRef]
- Sahu, S.K.; Chawla, Y.K.; Dhiman, R.K.; Singh, V.; Duseja, A.; Taneja, S.; Kalra, N.; Gorsi, U. Rupture of Hepatocellular Carcinoma: A Review of Literature. J. Clin. Exp. Hepatol. 2019, 9, 245–256. [Google Scholar] [CrossRef]
- Obeidat, A.E.; Wong, L.L. Spontaneous Rupture of Hepatocellular Carcinoma: New Insights. J. Clin. Exp. Hepatol. 2022, 12, 483–491. [Google Scholar] [CrossRef]
- Jain, M.K.; Rich, N.E.; Ahn, C.; Turner, B.J.; Sanders, J.M.; Adamson, B.; Quirk, L.; Perryman, P.; Santini, N.O.; Singal, A.G. Evaluation of a Multifaceted Intervention to Reduce Health Disparities in Hepatitis C Screening: A Pre-Post Analysis. Hepatology 2019, 70, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Puigvehí, M.; Moctezuma-Velázquez, C.; Villanueva, A.; Llovet, J.M. The Oncogenic Role of Hepatitis Delta Virus in Hepatocellular Carcinoma. JHEP Rep. 2019, 1, 120–130.e2. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lin, C.-C.; Mo, L.-R.; Chang, C.-Y.; Perng, D.-S.; Hsu, C.-C.; Lo, G.-H.; Chen, Y.-S.; Yen, Y.-C.; Hu, J.-T.; et al. Heavy Alcohol Consumption Increases the Incidence of Hepatocellular Carcinoma in Hepatitis B Virus-Related Cirrhosis. J. Hepatol. 2013, 58, 730–735. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Card, T.R.; Aithal, G.P.; Fleming, K.M. Risk of Hepatocellular Carcinoma among Individuals with Different Aetiologies of Cirrhosis: A Population-Based Cohort Study. Aliment. Pharmacol. Ther. 2017, 45, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Ott, P.; Andersen, P.K.; Sørensen, H.T.; Vilstrup, H. Risk for Hepatocellular Carcinoma in Patients with Alcoholic Cirrhosis: A Danish Nationwide Cohort Study. Ann. Intern. Med. 2012, 156, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. JHC 2021, 8, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837. [Google Scholar] [CrossRef]
- Stine, J.G.; Wentworth, B.J.; Zimmet, A.; Rinella, M.E.; Loomba, R.; Caldwell, S.H.; Argo, C.K. Systematic Review with Meta-Analysis: Risk of Hepatocellular Carcinoma in Non-Alcoholic Steatohepatitis without Cirrhosis Compared to Other Liver Diseases. Aliment. Pharmacol. Ther. 2018, 48, 696–703. [Google Scholar] [CrossRef]
- Desai, A.; Sandhu, S.; Lai, J.-P.; Sandhu, D.S. Hepatocellular Carcinoma in Non-Cirrhotic Liver: A Comprehensive Review. World J. Hepatol. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Saitta, C.; Pollicino, T.; Raimondo, G. Obesity and Liver Cancer. Ann. Hepatol. 2019, 18, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- An, N. Oral Contraceptives Use and Liver Cancer Risk. Medicine 2015, 94, e1619. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Youn, G.S.; Shin, M.J.; Suk, K.T. Role of Gut Microbiota in Hepatocarcinogenesis. Microorganisms 2019, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-Strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase Levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. JGLD 2018, 27, 41–49. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut Microbiome Analysis as a Tool towards Targeted Non-Invasive Biomarkers for Early Hepatocellular Carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Carotenuto, P.; Fassan, M.; Pandolfo, R.; Lampis, A.; Vicentini, C.; Cascione, L.; Paulus-Hock, V.; Boulter, L.; Guest, R.; Quagliata, L.; et al. Wnt Signalling Modulates Transcribed-Ultraconserved Regions in Hepatobiliary Cancers. Gut 2017, 66, 1268–1277. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt–β-Catenin Signalling in Liver Development, Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef]
- Thompson, M.D.; Monga, S.P.S. WNT/β-Catenin Signaling in Liver Health and Disease. Hepatology 2007, 45, 1298–1305. [Google Scholar] [CrossRef]
- Monga, S.P. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, D.; Li, X.; Xu, C.; Zhu, Q. Predictors of Spontaneous Rupture of Hepatocellular Carcinoma and Clinical Outcomes Following Hepatectomy. Front. Oncol. 2022, 12, 820867. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, C.; Song, D.; Wang, S.; Wang, Y.; Jia, K. Risk factors of 126 spontaneous rupture of hepatocellular carcinoma patients and prognosis of transcatheter arterial embolization. Chin. J. Dig. 2021, 253–259. [Google Scholar]
- Wang, P.; Moses, A.S.; Li, C.; Chen, S.; Qi, X.; Xu, K.; Shao, H.-B.; Han, X.-J. Prognosis Factors of Predicting Survival in Spontaneously Ruptured Hepatocellular Carcinoma. Hepatol. Int. 2022, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takeda, R.; Mukaihara, S.; Hayakawa, K.; Shibata, T.; Itoh, K.; Nishida, N.; Nakao, K.; Fukuda, Y.; Chiba, T.; et al. Treatment of Ruptured Hepatocellular Carcinoma. Int. J. Clin. Oncol. 2001, 6, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, L.; Liu, C.-F.; Cao, J.; Yan, J.-J.; Xu, F.; Wu, M.-C.; Yan, Y.-Q. Risk Factors and Surgical Outcomes for Spontaneous Rupture of BCLC Stages A and B Hepatocellular Carcinoma: A Case-Control Study. World J. Gastroenterol. 2014, 20, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- Nuño-Guzmán, C.M.; Marín-Contreras, M.E. Ruptured Hepatocellular Carcinoma and Non-Alcoholic Fatty Liver Disease, a Potentially Life-Threatening Complication in a Population at Increased Risk. Ann. Hepatol. 2020, 19, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Sirlin, C.B.; Fowler, K.J. Imaging Diagnosis of Hepatocellular Carcinoma: LI-RADS. Chin. Clin. Oncol. 2021, 10, 3. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea 2018 Korean Liver Cancer Association–National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J. Radiol. 2019, 20, 1042. [CrossRef]
- Catalano, O.; Lobianco, R.; Sandomenico, F.; Siani, A. Splenic Trauma: Evaluation with Contrast-Specific Sonography and a Second-Generation Contrast Medium: Preliminary Experience. J. Ultrasound. Med. 2003, 22, 467–477. [Google Scholar] [CrossRef]
- Tang, A.; Cruite, I.; Mitchell, D.G.; Sirlin, C.B. Hepatocellular Carcinoma Imaging Systems: Why They Exist, How They Have Evolved, and How They Differ. Abdom. Radiol. 2018, 43, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.J.; Potretzke, T.A.; Hope, T.A.; Costa, E.A.; Wilson, S.R. LI-RADS M (LR-M): Definite or Probable Malignancy, Not Specific for Hepatocellular Carcinoma. Abdom. Radiol. 2018, 43, 149–157. [Google Scholar] [CrossRef]
- Kim, M.Y.; Joo, I.; Kang, H.J.; Bae, J.S.; Jeon, S.K.; Lee, J.M. LI-RADS M (LR-M) Criteria and Reporting Algorithm of V2018: Diagnostic Values in the Assessment of Primary Liver Cancers on Gadoxetic Acid-Enhanced MRI. Abdom. Radiol. 2020, 45, 2440–2448. [Google Scholar] [CrossRef]
- Elmohr, M.; Elsayes, K.M.; Chernyak, V. LI-RADS: Review and Updates. Clin. Liver Dis. 2021, 17, 108–112. [Google Scholar] [CrossRef]
- Chernyak, V.; Sirlin, C.B. LI-RADS: Future Directions. Clin. Liver Dis. 2021, 17, 149–153. [Google Scholar] [CrossRef]
- Caraiani, C.; Boca, B.; Bura, V.; Sparchez, Z.; Dong, Y.; Dietrich, C. CT/MRI LI-RADS V2018 vs. CEUS LI-RADS V2017—Can Things Be Put Together? Biology 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.; Menias, C.; Rucker, C.; Bhalla, S.; Peterson, C.M.; Wang, L.; Gratz, B. Blood in the Belly: CT Findings of Hemoperitoneum. RadioGraphics 2007, 27, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Current Role of Ultrasound in the Diagnosis of Hepatocellular Carcinoma. J. Med. Ultrason. 2020, 47, 239–255. [Google Scholar] [CrossRef]
- Kamat, P.P.; Gupta, S.; Ensor, J.E.; Murthy, R.; Ahrar, K.; Madoff, D.C.; Wallace, M.J.; Hicks, M.E. Hepatic Arterial Embolization and Chemoembolization in the Management of Patients with Large-Volume Liver Metastases. Cardiovasc. Interv. Radiol. 2008, 31, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lyshchik, A.; Kono, Y.; Dietrich, C.F.; Jang, H.-J.; Kim, T.K.; Piscaglia, F.; Vezeridis, A.; Willmann, J.K.; Wilson, S.R. Contrast-Enhanced Ultrasound of the Liver: Technical and Lexicon Recommendations from the ACR CEUS LI-RADS Working Group. Abdom. Radiol. 2018, 43, 861–879. [Google Scholar] [CrossRef]
- Durot, I.; Wilson, S.R.; Willmann, J.K. Contrast-Enhanced Ultrasound of Malignant Liver Lesions. Abdom. Radiol. 2018, 43, 819–847. [Google Scholar] [CrossRef] [PubMed]
- Abushamat, F.; Dietrich, C.F.; Clevert, D.; Piscaglia, F.; Fetzer, D.T.; Meloni, M.F.; Shiehmorteza, M.; Kono, Y. Contrast-Enhanced Ultrasound (CEUS) in the Evaluation of Hemoperitoneum in Patients With Cirrhosis. J. Ultrasound Med. 2023, 42, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: A Pictorial Review. Insights Imaging 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-G. Management of Hepatocellular Carcinoma: The Role of Contrast-Enhanced Ultrasound. WJR 2014, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Cusati, B.; Nunziata, A.; Siani, A. Active Abdominal Bleeding: Contrast-Enhanced Sonography. Abdom. Imaging 2006, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, T.; Koda, M.; Okamoto, T.; Miyoshi, K.; Matono, T.; Isomoto, H. Two Patterns of Contrast-Enhanced Ultrasonography with Sonazoid® in Spontaneous Rupture of Hepatocellular Carcinoma: A Report of Four Cases. J. Med. Ultrason. 2018, 45, 319–323. [Google Scholar] [CrossRef]
- Kim, B.; Reardon, R.; Cross, J.; Fehlberg, T.; Allanson, B.; Punch, G.J. Case Report: Haemoperitoneum Secondary to Acute Rupture of Primary Hepatic Angiosarcoma. Int. J. Surg. Case Rep. 2021, 84, 106090. [Google Scholar] [CrossRef]
- Yang, Y.; Nagano, H.; Ota, H.; Morimoto, O.; Nakamura, M.; Wada, H.; Noda, T.; Damdinsuren, B.; Marubashi, S.; Miyamoto, A.; et al. Patterns and Clinicopathologic Features of Extrahepatic Recurrence of Hepatocellular Carcinoma after Curative Resection. Surgery 2007, 141, 196–202. [Google Scholar] [CrossRef]
- Hidaka, T.; Anai, H.; Sakaguchi, H.; Sueyoshi, S.; Tanaka, T.; Yamamoto, K.; Morimoto, K.; Nishiofuku, H.; Maeda, S.; Nagata, T.; et al. Efficacy of Combined Bland Embolization and Chemoembolization for Huge (≥10 Cm) Hepatocellular Carcinoma. Minim. Invasive Ther. Allied Technol. 2021, 30, 221–228. [Google Scholar] [CrossRef]
- Kocyigit, A.; Dicle, O.; Goktay, Y.; Astarcioglu, I. The Effect of Using Different Embolic Agents on Survival in Transarterial Chemoembolization of Hepatocellular Carcinoma: Gelfoam versus Polyvinyl Alcohol. Diagn. Interv. Radiol. 2014, 20, 323–329. [Google Scholar] [CrossRef]
- Moustafa, A.S.; Abdel Aal, A.K.; Ertel, N.; Saad, N.; DuBay, D.; Saddekni, S. Chemoembolization of Hepatocellular Carcinoma with Extrahepatic Collateral Blood Supply: Anatomic and Technical Considerations. RadioGraphics 2017, 37, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Liu, K.-L.; Lin, C.-L.; Kao, C.-H. Risk of Acute Kidney Injury after Transarterial Chemoembolisation in Hepatocellular Carcinoma Patients: A Nationwide Population-Based Cohort Study. Eur. Radiol. 2017, 27, 4482–4489. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.F.; Zhang, L.W.; Bai, J.X.; Li, Y.J.; Liu, J.N.; Zhang, X.L.; Han, J.M.; Li, X.; Jiang, H.; Cao, N. Incidence, Risk Factors, and Prognosis of Acute Kidney Injury Following Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma: A Prospective Cohort Study. Indian J. Cancer 2015, 51 (Suppl. 2), e3–e8. [Google Scholar] [CrossRef]
- Yan, J.; Li, T.; Deng, M.; Fan, H. Ruptured Hepatocellular Carcinoma: What Do Interventional Radiologists Need to Know? Front. Oncol. 2022, 12, 927123. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Fan, S.T.; Lo, C.M.; Tso, W.K.; Poon, R.T.; Lam, C.M.; Wong, J. Management of Spontaneous Rupture of Hepatocellular Carcinoma: Single-Center Experience. J. Clin. Oncol. 2001, 19, 3725–3732. [Google Scholar] [CrossRef]
- Kerdsuknirun, J.; Vilaichone, V.; Vilaichone, R.-K. Risk Factors and Prognosis of Spontaneously Ruptured Hepatocellular Carcinoma in Thailand. Asian Pac. J. Cancer Prev. 2018, 19, 3629–3634. [Google Scholar] [CrossRef]
- Zhong, F.; Cheng, X.-S.; He, K.; Sun, S.-B.; Zhou, J.; Chen, H.-M. Treatment Outcomes of Spontaneous Rupture of Hepatocellular Carcinoma with Hemorrhagic Shock: A Multicenter Study. Springerplus 2016, 5, 1101. [Google Scholar] [CrossRef]
- Hsueh, K.-C.; Fan, H.-L.; Chen, T.-W.; Chan, D.-C.; Yu, J.-C.; Tsou, S.-S.; Chang, T.-M.; Hsieh, C.-B. Management of Spontaneously Ruptured Hepatocellular Carcinoma and Hemoperitoneum Manifested as Acute Abdomen in the Emergency Room. World J. Surg. 2012, 36, 2670–2676. [Google Scholar] [CrossRef]
- Xu, J.; Hong, J.; Wang, Y.; Zhou, L.; Xu, B.; Si, Y.; He, Y.; Chen, Y. Prognostic Influence of Spontaneous Tumor Rupture in Patients With Hepatocellular Carcinoma After Hepatectomy: A Meta-Analysis of Observational Studies. Front. Surg. 2021, 8, 594. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Liu, Q.; Huang, X. A Meta-Analysis of TAE/TACE Versus Emergency Surgery in the Treatment of Ruptured HCC. Cardiovasc. Interv. Radiol. 2020, 43, 1263–1276. [Google Scholar] [CrossRef]
- Patidar, Y.; Khisti, R.; Yadav, A.; Mukund, A.; Sarin, S.K. Outcome of Conventional Transarterial Chemoembolization (CTACE) in the Management of Spontaneously Ruptured Hepatocellular Carcinoma. Indian J. Radiol. Imaging 2019, 29, 177–181. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.; Lan, X.; Lan, D.; Huang, Z.; Ye, S.; Ran, Y.; Bi, X.; Zhou, J.; Che, X. Research Progress of Spontaneous Ruptured Hepatocellular Carcinoma: Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 973857. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, G.H.; Choi, J.S.; Han, K.-H.; Ahn, S.H.; Kim, D.Y.; Park, J.Y.; Kim, S.U.; Kim, S.H.; Yoon, D.S.; et al. Staged Partial Hepatectomy versus Transarterial Chemoembolization for the Treatment of Spontaneous Hepatocellular Carcinoma Rupture: A Multicenter Analysis in Korea. Ann. Surg. Treat. Res. 2019, 96, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-J.; Zhu, P.; Zhang, Z.-G.; Zhang, B.-X.; Shu, C.; Mba’nbo-Koumpa, A.-A.; Zhang, Z.-W.; Huang, Z.-Y.; Zhang, W.-G.; Lau, W.Y.; et al. Spontaneous Rupture of Hepatocellular Carcinoma: Optimal Timing of Partial Hepatectomy. Eur. J. Surg. Oncol. 2019, 45, 1887–1894. [Google Scholar] [CrossRef]
- Shimada, R.; Imamura, H.; Makuuchi, M.; Soeda, J.; Kobayashi, A.; Noike, T.; Miyagawa, S.; Kawasaki, S. Staged Hepatectomy after Emergency Transcatheter Arterial Embolization for Ruptured Hepatocellular Carcinoma. Surgery 1998, 124, 526–535. [Google Scholar] [CrossRef]
- Hawatmeh, A.; Jumean, K.; Arqoub, A.A.; Shaaban, H. Spontaneous Rupture of Hepatocellular Carcinoma. Hepatoma Res. 2016, 2, 103–106. [Google Scholar] [CrossRef]
- Catalano, O.; Cusati, B.; Sandomenico, F.; Nunziata, A.; Lobianco, R.; Siani, A. Multiple-phase spiral computerized tomography of small hepatocellular carcinoma: Technique optimization and diagnostic yield. Radiol. Med. 1999, 98, 53–64. [Google Scholar]
- Galia, M.; Taibbi, A.; Marin, D.; Furlan, A.; Burgio, M.D.; Agnello, F.; Cabibbo, G.; Beers, B.E.V.; Bartolotta, T.V.; Midiri, M.; et al. Focal Lesions in Cirrhotic Liver: What Else beyond Hepatocellular Carcinoma? Diagn. Interv. Radiol. 2014, 20, 222–228. [Google Scholar] [CrossRef]
- Marks, R.M.; Masch, W.R.; Chernyak, V. LI-RADS: Past, Present, and Future, From the AJR Special Series on Radiology Reporting and Data Systems. Am. J. Roentgenol. 2021, 216, 295–304. [Google Scholar] [CrossRef]
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis: Roberts et Al. Hepatology 2018, 67, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhao, S.; Aishanjiang, K.; Cai, H.; Wei, T.; Zhang, Y.; Liu, Z.; Zhou, J.; Han, B.; Wang, J.; et al. Deep Learning for Differential Diagnosis of Malignant Hepatic Tumors Based on Multi-Phase Contrast-Enhanced CT and Clinical Data. J. Hematol. Oncol. 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Kong, D.; He, K.; Xia, Q. Regeneration and Activation of Liver Progenitor Cells in Liver Cirrhosis. Genes Dis. 2021, 8, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Kamel, I.R.; Liapi, E.; Fishman, E.K. Incidental Nonneoplastic Hypervascular Lesions in the Noncirrhotic Liver: Diagnosis with 16-MDCT and 3D CT Angiography. Am. J. Roentgenol. 2006, 187, 682–687. [Google Scholar] [CrossRef]
- Dane, B.; Shanbhogue, K.; Menias, C.O.; Taffel, M.T. The Humbling Hemangioma: Uncommon CT and MRI Imaging Features and Mimickers of Hepatic Hemangiomas. Clin. Imaging 2021, 74, 55–63. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Y.; Shen, K.-R.; Zhu, X.-L.; Lu, C.-Y.; Li, Q.-H.; Han, S.-G.; Fu, Y.-B.; Xu, X.-F.; Yu, R.-S. Contrast-Enhanced Multiple-Phase Imaging Features of Intrahepatic Mass-Forming Cholangiocarcinoma and Hepatocellular Carcinoma with Cirrhosis: A Comparative Study. Oncol. Lett. 2017, 14, 4213–4219. [Google Scholar] [CrossRef]
- Nolan, P.E.; Catania, R.; Vendrami, C.L.; Borhani, A.A.; Miller, F.H. Large Regenerative Nodules and Focal Nodular Hyperplasia-Like Lesions. Radiol. Clin. N. Am. 2022, 60, 795–808. [Google Scholar] [CrossRef]
- Kim, H.C.; Yang, D.M.; Jin, W.; Park, S.J. The Various Manifestations of Ruptured Hepatocellular Carcinoma: CT Imaging Findings. Abdom. Imaging 2008, 33, 633–642. [Google Scholar] [CrossRef]
- Gomez, E.; Horton, K.; Fishman, E.K.; Johnson, P.T. CT of Acute Abdominopelvic Hemorrhage: Protocols, Pearls, and Pitfalls. Abdom. Radiol. 2022, 47, 475–484. [Google Scholar] [CrossRef]
- Jabi, R.; Sergi, B.; Soufi, M.; El Arabi, S.; Miry, A.; El Harroudi, T.; Bouziane, M. Acute Hemoperitoneum after Ruptured Hepatocellular Carcinoma: First Moroccan SCARE-Compliant Case Report and Literature Review. Int. J. Surg. Case Rep. 2020, 66, 390–393. [Google Scholar] [CrossRef]
- Aseni, P.; Di Domenico, S.L.; Barbosa, F.; Rampoldi, A.; Berry, C. Hemoperitoneum in Cirrhotic Patients in the Absence of Abdominal Trauma. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Fakhran, S.; Federle, M.P. Spontaneous Abdominal Hemorrhage: Causes, CT Findings, and Clinical Implications. AJR Am. J. Roentgenol. 2009, 193, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.N.; Kim, I.Y.; Bae, W.K.; Lee, B.H. Computed Tomographic Findings of Ruptured Hepatic Malignancy. Gastrointest. Radiol. 1991, 16, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.M.; Anderson, S.W.; Soto, J.A.; Kertesz, J.L.; Ozonoff, A.; Rhea, J.T. Active Extravasation of the Abdomen and Pelvis in Trauma Using 64MDCT. Emerg. Radiol. 2009, 16, 375–382. [Google Scholar] [CrossRef]
- Catalano, O.; Lobianco, R.; Esposito, M.; Sandomenico, F.; Siani, A. Contrast media extravasation in upper abdominal injuries: Detection with spiral computerized tomography. Radiol. Med. 1999, 97, 138–143. [Google Scholar]
- Ilyas, M.; Bashir, M.; Robbani, I.; Rasool, S.R.; Shera, F.A.; Hamid, I. Sentinel Clot Sign in Hemoperitoneum. Abdom. Radiol. 2019, 44, 1955–1956. [Google Scholar] [CrossRef]
- Orwig, D.; Federle, M.P. Localized Clotted Blood as Evidence of Visceral Trauma on CT: The Sentinel Clot Sign. AJR Am. J. Roentgenol. 1989, 153, 747–749. [Google Scholar] [CrossRef]
- Singhal, M.; Sinha, U.; Kalra, N.; Duseja, A.; Khandelwal, N. Enucleation Sign: A Computed Tomographic Appearance of Ruptured Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2016, 6, 335–336. [Google Scholar] [CrossRef]
- Casillas, V.J.; Amendola, M.A.; Gascue, A.; Pinnar, N.; Levi, J.U.; Perez, J.M. Imaging of Nontraumatic Hemorrhagic Hepatic Lesions. RadioGraphics 2000, 20, 367–378. [Google Scholar] [CrossRef]
- Tsitouridis, I.; Michaelides, M.; Christopoulou, A.; Sidiropoulos, D.; Kyriakou, V.; Diamantopoulou, A.; Bintoudi, A. Early Stage of Intraperitoneal Rupture of Hepatocellular Carcinoma: CT and MRI Evaluation. Ann. Gastroenterol. 2007, 20, 282–285. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandomenico, F.; Arpaia, V.; De Rosa, F.; Catalano, O.; Buonaiuto, R.A.; Notarangelo, M.; Iovino, M.; Giovine, S.; Brunetti, A.; Scaglione, M. Spontaneously Ruptured Hepatocellular Carcinoma: Computed Tomography-Based Assessment. Diagnostics 2023, 13, 1021. https://doi.org/10.3390/diagnostics13061021
Sandomenico F, Arpaia V, De Rosa F, Catalano O, Buonaiuto RA, Notarangelo M, Iovino M, Giovine S, Brunetti A, Scaglione M. Spontaneously Ruptured Hepatocellular Carcinoma: Computed Tomography-Based Assessment. Diagnostics. 2023; 13(6):1021. https://doi.org/10.3390/diagnostics13061021
Chicago/Turabian StyleSandomenico, Fabio, Valerio Arpaia, Ferdinando De Rosa, Orlando Catalano, Roberto Antonino Buonaiuto, Marianna Notarangelo, Maria Iovino, Sabrina Giovine, Arturo Brunetti, and Mariano Scaglione. 2023. "Spontaneously Ruptured Hepatocellular Carcinoma: Computed Tomography-Based Assessment" Diagnostics 13, no. 6: 1021. https://doi.org/10.3390/diagnostics13061021
APA StyleSandomenico, F., Arpaia, V., De Rosa, F., Catalano, O., Buonaiuto, R. A., Notarangelo, M., Iovino, M., Giovine, S., Brunetti, A., & Scaglione, M. (2023). Spontaneously Ruptured Hepatocellular Carcinoma: Computed Tomography-Based Assessment. Diagnostics, 13(6), 1021. https://doi.org/10.3390/diagnostics13061021





