The Use of Robotic-Assisted Bronchoscopy in the Diagnostic Evaluation of Peripheral Pulmonary Lesions: A Paradigm Shift
Abstract
1. Introduction
2. Materials and Methods
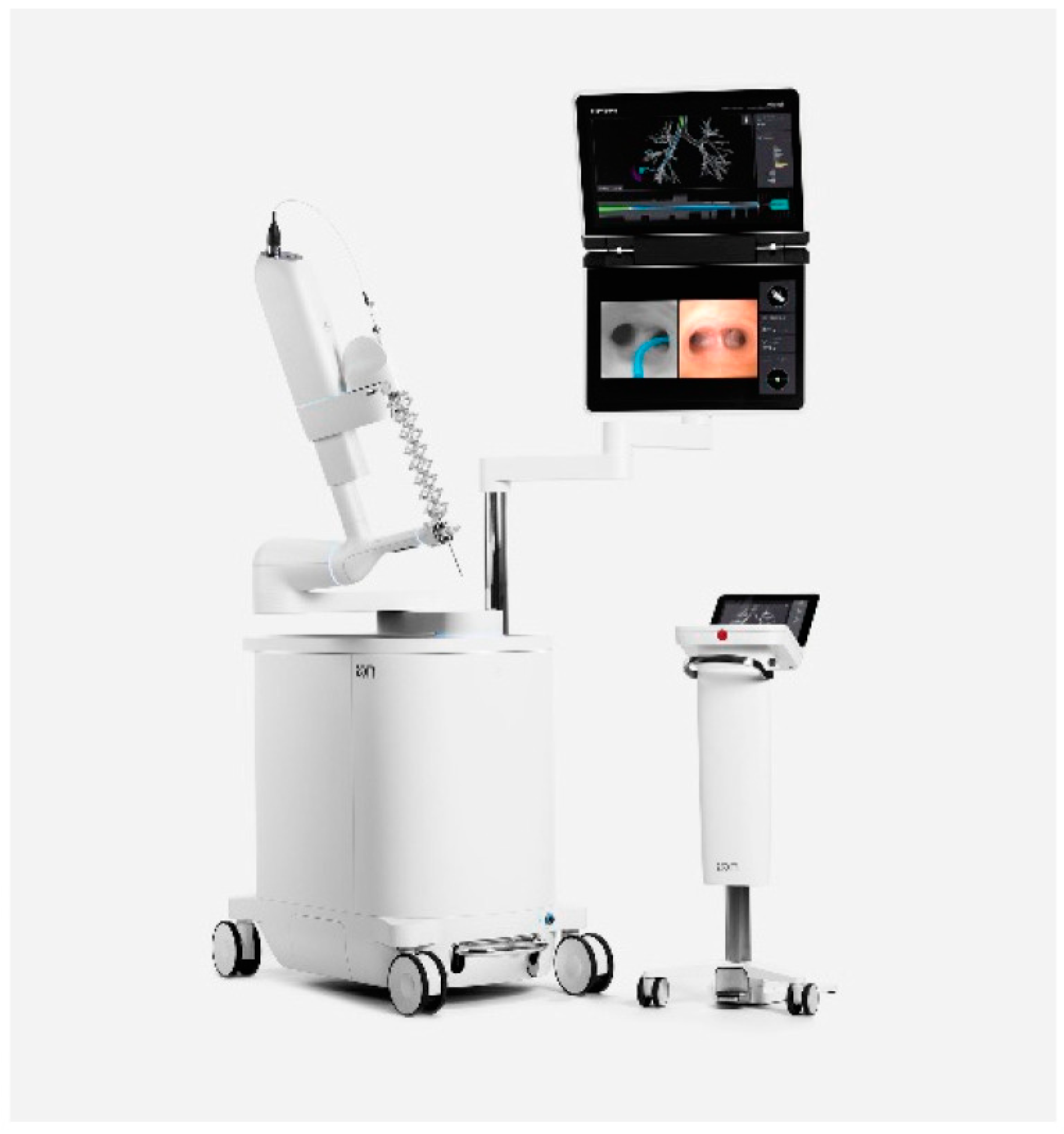
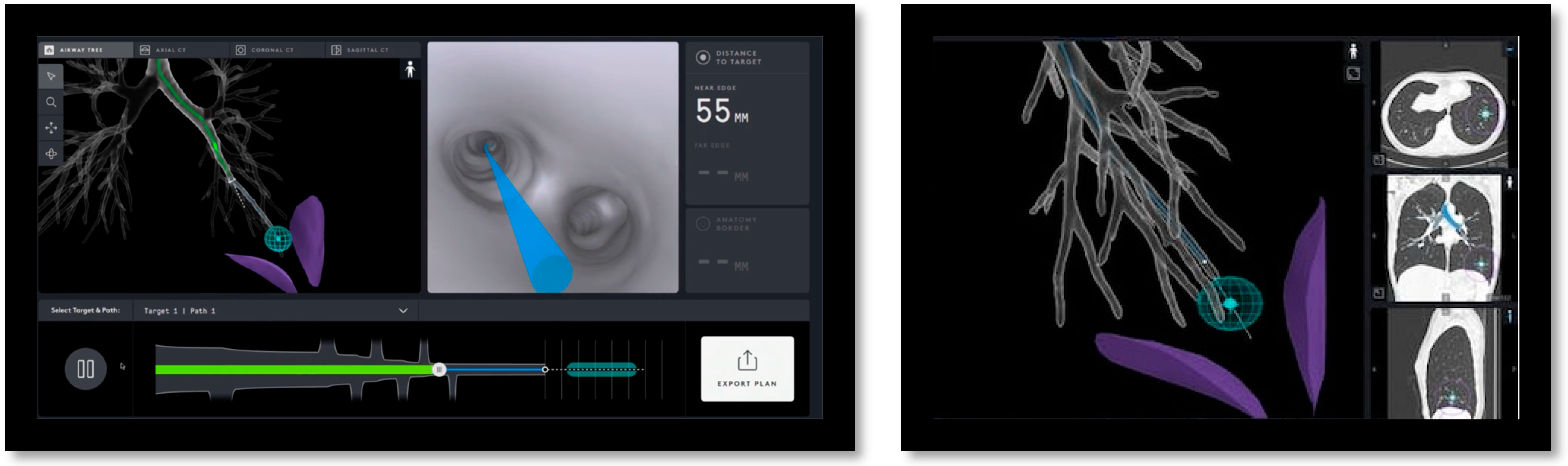
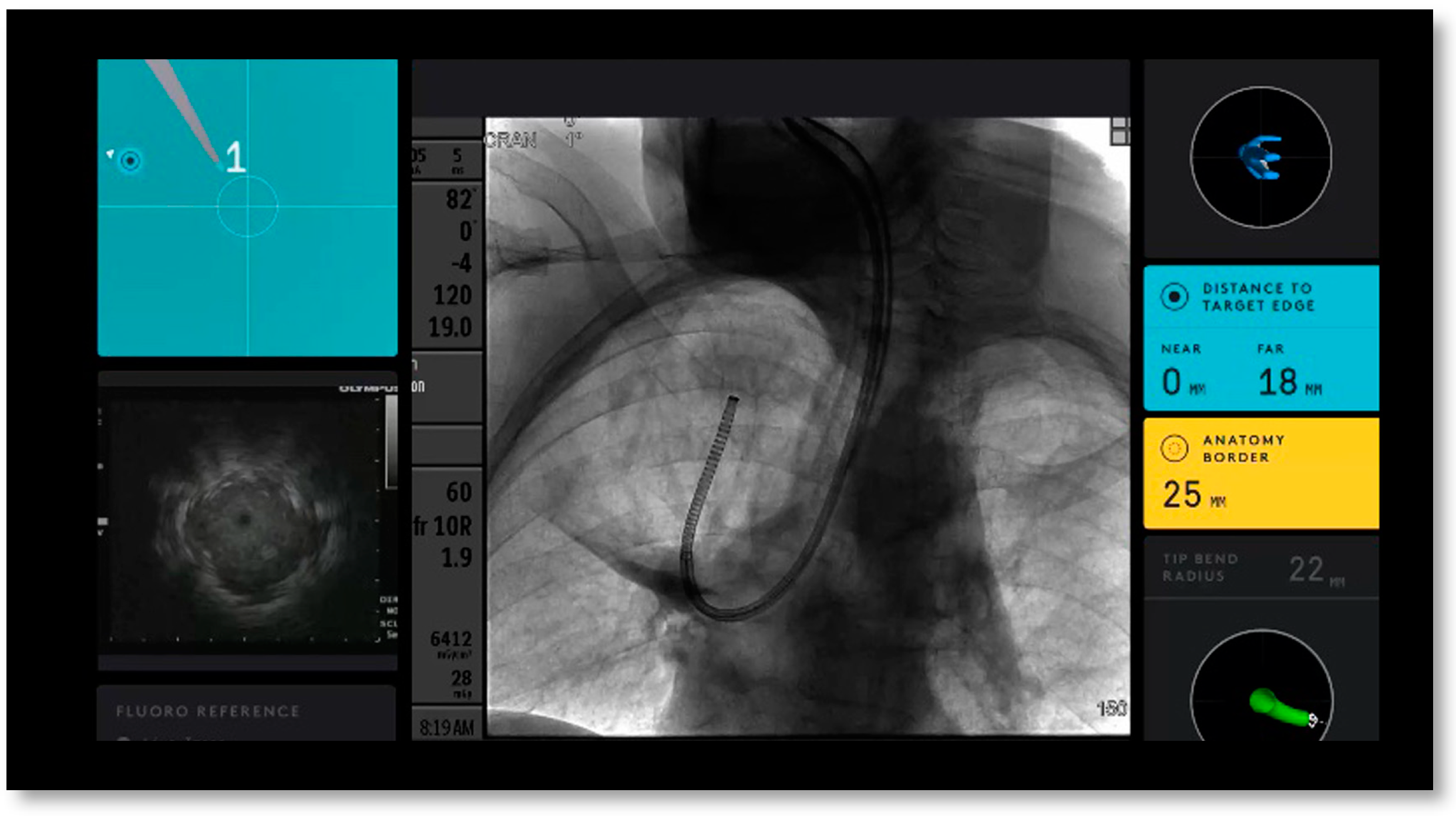
2.1. Procedure
2.2. Statistical Analysis
3. Results
3.1. Target Nodule Characteristics
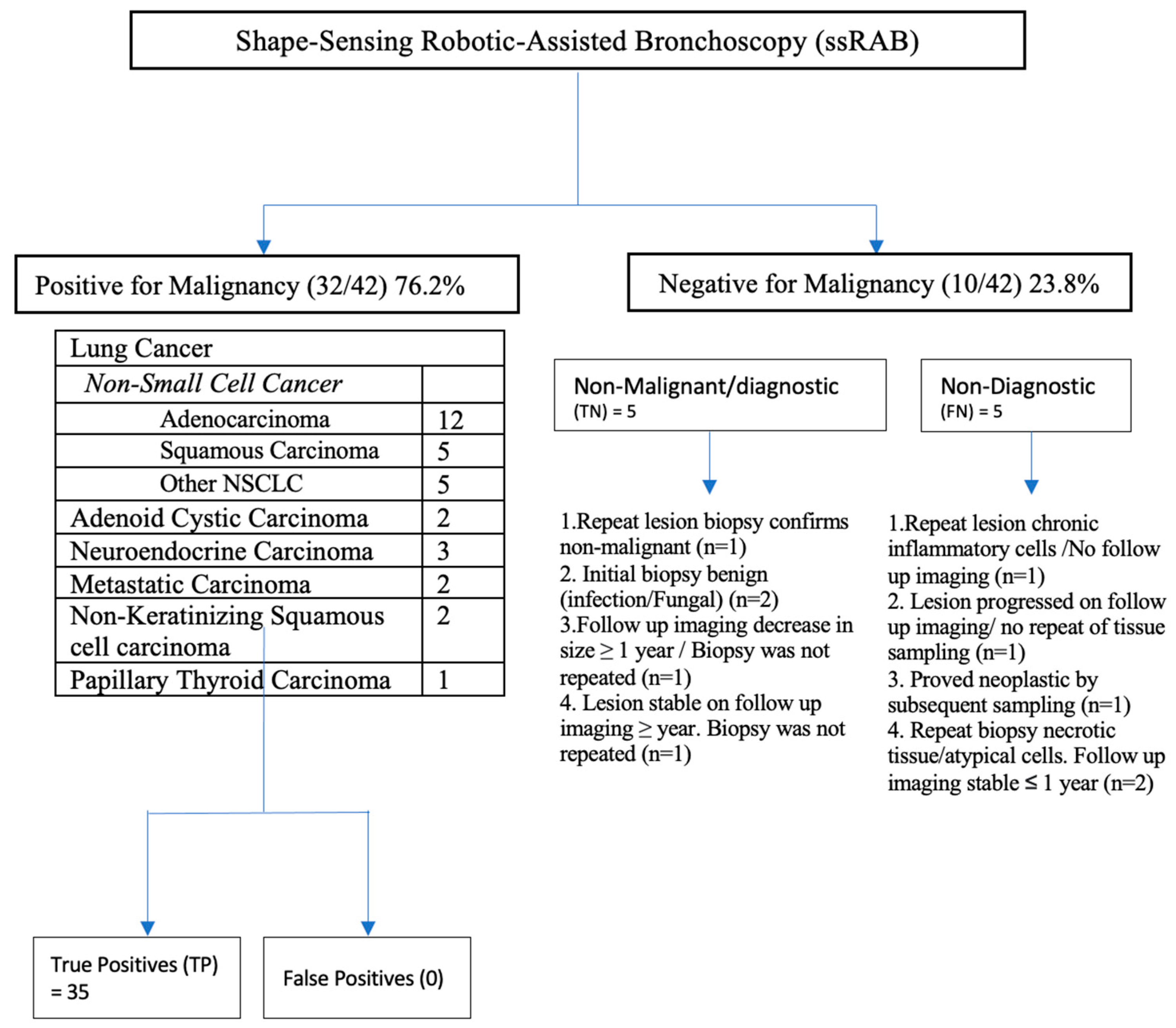
3.2. Primary End Point: Diagnostic Yield
3.3. Secondary End Point: Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Ekeke, C.N.; Vercauteren, M.; Istvaniczdravkovic, S.; Semaan, R.; Dhupar, R. Lung Nodule Evaluation Using Robotic-Assisted Bronchoscopy at a Veteran’s Affairs Hospital. J. Clin. Med. 2021, 10, 3671. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Landeras, L.; Chung, J.H. Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: Common Questions and Challenging Scenarios. Radiographics 2018, 38, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Ost, D.E.; Ernst, A.; Lei, X.; Kovitz, K.L.; Benzaquen, S.; Diaz-Mendoza, J.; Greenhill, S.; Toth, J.; Feller-Kopman, D.; Puchalski, J.; et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016, 193, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Bevill, B.T.; Huang, J.; Brooks, M.; Choi, Y.; Kennedy, G.; Lofaro, L.; Chen, A.; Rivera, M.P.; Tanner, N.T.; et al. An Evaluation of Diagnostic Yield From Bronchoscopy: The Impact of Clinical/Radiographic Factors, Procedure Type, and Degree of Suspicion for Cancer. Chest 2020, 157, 1656–1664. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, J.H.; Lee, C.T.; Jheon, S.H.; Sung, S.W.; Chung, J.-H.; Lee, K.W. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am. J. Roentgenol. 2008, 190, 234–239. [Google Scholar] [CrossRef]
- Huang, M.D.; Weng, H.H.; Hsu, S.L.; Hsu, L.-S.; Lin, W.-M.; Chen, C.-W.; Tsai, Y.-H. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: A single-center experience. Cancer Imaging 2019, 19, 51. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Chae, K.J.; Hong, H.; Yoon, S.H.; Hahn, S.; Jin, G.Y.; Park, C.M.; Goo, J.M. Non-diagnostic Results of Percutaneous Transthoracic Needle Biopsy: A Meta-analysis. Sci. Rep. 2019, 9, 12428. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022, 161, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Murgu, S.D. Robotic assisted-bronchoscopy: Technical tips and lessons learned from the initial experience with sampling peripheral lung lesions. BMC Pulm. Med. 2019, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Asano, F.; Matsuno, Y.; Shinagawa, N.; Yamazaki, K.; Suzuki, T.; Ishida, T.; Moriya, H. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006, 130, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Asano, F.; Shinagawa, N.; Ishida, T.; Shindoh, J.; Anzai, M.; Tsuzuku, A.; Oizumi, S.; Morita, S. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am. J. Respir. Crit. Care Med. 2013, 188, 327–333. [Google Scholar] [CrossRef]
- Pritchett, M.A.; Bhadra, K.; Calcutt, M.; Folch, E. Virtual or reality: Divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. Thorac. Dis. 2020, 12, 1595–1611, Erratum in J. Thorac. Dis. 2020, 12, 4593–4595. [Google Scholar] [CrossRef]
- Kumar, A.; Caceres, J.D.; Vaithilingam, S.; Sandhu, G.; Meena, N.K. Robotic Bronchoscopy for Peripheral Pulmonary Lesion Biopsy: Evidence-Based Review of the Two Platforms. Diagnostics 2021, 11, 1479. [Google Scholar] [CrossRef]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef]
- Folch, E.E.; Mahajan, A.K.; Oberg, C.L.; Maldonado, F.; Toloza, E.; Krimsky, W.S.; Oh, S.; Bowling, M.R.; Benzaquen, S.; Kinsey, C.M.; et al. Standardized Definitions of Bleeding After Transbronchial Lung Biopsy: A Delphi Consensus Statement From the Nashville Working Group. Chest 2020, 158, 393–400. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef]
- Gould, M.K.; Tang, T.; Liu, I.L.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; Di Fiore, J.L.; Suh, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Usman, Y.; Abdo, T.; Chaudry, F.; Keddissi, J.I.; Youness, H.A. Diagnosis and management of peripheral lung nodule. Ann. Transl. Med. 2019, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Baaklini, W.A.; Reinoso, M.A.; Gorin, A.B.; Sharafkaneh, A.; Manian, P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000, 117, 1049–1054. [Google Scholar] [CrossRef]
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P.; Murgu, S. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: Results from the initial multicenter experience. BMC Pulm. Med. 2019, 19, 243. [Google Scholar] [CrossRef]
- Wohlschläger, J.; Darwiche, K.; Ting, S.; Hager, T.; Freitag, L.; Schmid, K.; Kühl, H.; Theegarten, D. “Rapid on-site evaluation” (ROSE) in der zytologischen Diagnostik von Lungen- und Mediastinalerkrankungen [Rapid on-site evaluation (ROSE) in cytological diagnostics of pulmonary and mediastinal diseases]. Pathologe 2012, 33, 308–315. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; Paioli, D.; Scudeller, L.; Casadei, G.P.; Parri, S.F.; Livi, V.; Bondi, A.; Boaron, M.; et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: A randomized trial. Chest 2011, 139, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Oezkan, F.; Eisenmann, S.; Darwiche, K.; Gassa, A.; Carbone, D.P.; Merritt, R.E.; Kneuertz, P.J. Linear Endobronchial Ultrasound in the Era of Personalized Lung Cancer Diagnostics-A Technical Review. J. Clin. Med. 2021, 10, 5646. [Google Scholar] [CrossRef]
| Demographics | N = 42 Subjects |
|---|---|
| Female, No. (%) | 23/42 (54.7) |
| Age, mean (SD), y n = 42 | 68.89 (11.8) |
| BMI, mean (SD), Kg/m2 n = 42 | 27.14(5.6) |
| Chronic lung disease (COPD/ILD/Asthma) No. (%) | 26/42 (61.9) |
| History of cancer No. (%) | 19/42 (45.2) |
| History of lung cancer No. (%) | 3/42 (7) |
| Smoking history No. (%) | 31/42 (73.8) |
| Lesion Properties | |
| Median Nodule size (mm) n = 42 (IQR) | 12 (10–18) |
| FDG avid No. (%) | 27/32 84.4% |
| Median SUV if FDG avid (mm) n = 27 (IQR) | 4 (2.1–6.7) |
| Bronchus sign, No. (%) | 25/42 (59.2) |
| Nodule location, No. (%) | |
| Right upper lobe | 19/42 (45.2) |
| Left upper lobe | 10 /42(23.8) |
| Right middle lobe | 4/42 (9.5) |
| Right lower lobe | 6/42 (14.3) |
| Left lower lobe | 3/42 (7.1) |
| Lesion in central third of the lung | 12/42 (28.6) |
| Lesion in middle third of the lung | 20/42 (47.6) |
| Lesion in outer third of the lung | 10/42 (23.8) |
| Nodule Radiologic features No. (%) | |
| Solid | 37/42 (88) |
| Semi Solid | 2/42 (4.8) |
| Ground glass | 3/42 (7.1) |
| Spiculated lesion border | 10 /42 (23.8) |
| Procedure characteristics | |
| General anesthesia | 42 (100%) |
| Radial EBUS used during ENB | 42 (100%) |
| Endobronchial ultrasound (EBUS-TBNA) No. (%) | 23/40 (57.5) |
| Bronchioalveolar lavage No. (%) | |
| +Malignancy | 4/42 (9.5%) |
| +Infection Fungi | 2/42 (4.7) |
| Negative (normal cellular analysis) | 36/42 (85%) |
| Fluoroscopy used during ENB | 42 (100%) |
| ROSE used | 42 (100%) |
| Median number of passes (mm) n = 35 (IQR) | 10 (7–2) |
| 12-month yield | 88.10 |
| Sensitivity | 86.49 |
| Specificity | 100.00 |
| Positive predictive value | 100.00 |
| Negative predictive value | 50.00 |
| Factor | OR | 95% CI Lower Limit | 95% CI Higher Limit | p Value |
|---|---|---|---|---|
| age | 0.952 | 0.874 | 1.038 | 0.2646 |
| Sex F vs. M | 0.559 | 0.091 | 3.446 | 0.5307 |
| BMI | 1.017 | 0.867 | 1.192 | 0.8350 |
| Diabetes | >999.999 | <0.001 | >999.999 | 0.9618 |
| HTN | 0.447 | 0.073 | 2.759 | 0.3861 |
| Cardiovascular disease | 1.000 | 0.160 | 6.255 | 1.0000 |
| History of cancer | 1.789 | 0.290 | 11.035 | 0.5307 |
| Chronic lung disease | 0.280 | 0.030 | 2.649 | 0.2669 |
| Current or former smoking hx | 0.520 | 0.054 | 5.021 | 0.5719 |
| Py smoking hx | 0.012 | 0.965 | 1.061 | 0.6271 |
| EBUS done | 0.633 | 0.102 | 3.938 | 0.6244 |
| Multiple lesions samples | >999.999 | <0.001 | >999.999 | 0.9867 |
| Number of passes | 0.703 | 0.497 | 0.995 | 0.0465 |
| spiculated | 1.667 | 0.171 | 16.225 | 0.6600 |
| Bronchogenic sign | 0.250 | 0.026 | 2.361 | 0.2263 |
| FDG avid | <0.001 | <0.001 | >999.999 | 0.9686 |
| SUV | 2.549 | 0.950 | 6.841 | 0.0632 |
| size | 1.137 | 0.908 | 1.425 | 0.2638 |
| Location RUL | reference | |||
| LLL | 0.235 | 0.014 | 3.917 | 0.4210 |
| LUL | 1.059 | 0.084 | 13.329 | 0.4923 |
| RLL | 0.588 | 0.044 | 7.914 | 0.9494 |
| RML | 0.353 | 0.024 | 5.231 | 0.6578 |
| Type solid | reference | |||
| Ground glass | 0.313 | 0.024 | 4.119 | 0.9657 |
| Semi-solid | >999.999 | <0.001 | >999.999 | 0.9705 |
| Centrality: outer | Reference | |||
| Central | >999.999 | <0.001 | >999.999 | 0.9525 |
| middle | 1.000 | 0.150 | 6.671 | 0.9525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad Altaq, H.; Parmar, M.; Syed Hussain, T.; Salim, D.J.; Chaudry, F.A. The Use of Robotic-Assisted Bronchoscopy in the Diagnostic Evaluation of Peripheral Pulmonary Lesions: A Paradigm Shift. Diagnostics 2023, 13, 1049. https://doi.org/10.3390/diagnostics13061049
Hammad Altaq H, Parmar M, Syed Hussain T, Salim DJ, Chaudry FA. The Use of Robotic-Assisted Bronchoscopy in the Diagnostic Evaluation of Peripheral Pulmonary Lesions: A Paradigm Shift. Diagnostics. 2023; 13(6):1049. https://doi.org/10.3390/diagnostics13061049
Chicago/Turabian StyleHammad Altaq, Hiba, Miloni Parmar, Talal Syed Hussain, Daouk J. Salim, and Fawad A. Chaudry. 2023. "The Use of Robotic-Assisted Bronchoscopy in the Diagnostic Evaluation of Peripheral Pulmonary Lesions: A Paradigm Shift" Diagnostics 13, no. 6: 1049. https://doi.org/10.3390/diagnostics13061049
APA StyleHammad Altaq, H., Parmar, M., Syed Hussain, T., Salim, D. J., & Chaudry, F. A. (2023). The Use of Robotic-Assisted Bronchoscopy in the Diagnostic Evaluation of Peripheral Pulmonary Lesions: A Paradigm Shift. Diagnostics, 13(6), 1049. https://doi.org/10.3390/diagnostics13061049






