Cone-Beam Computed-Tomography-Derived Augmented Fluoroscopy-Guided Biopsy for Peripheral Pulmonary Nodules in a Hybrid Operating Room: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
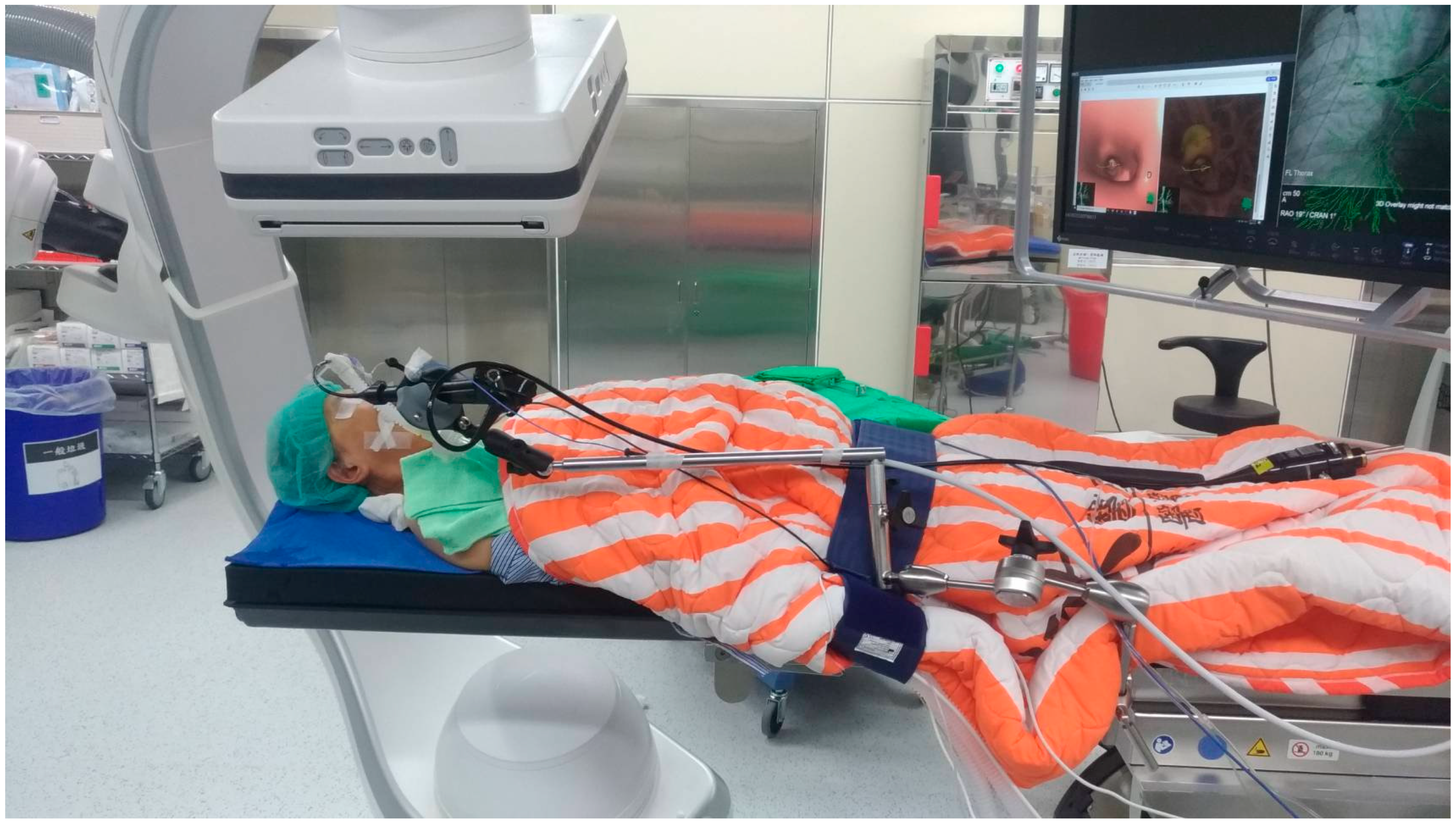
2.2. Procedures
2.3. Data Collection
3. Results
4. Discussion
4.1. Comparison with Other Modalities
4.2. Settings of Robotic CBCT in Hybrid Operating Room
4.3. Pros and Cons of General Anesthesia and Endotracheal Intubation during the Procedures
4.4. Reasons for Additional Percutaneous Biopsies
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Herth, F.J.; Eberhardt, R.; Schuhmann, M. Bronchoscopy in Lung Cancer: Navigational Modalities and their Clinical Use. Expert Rev. Respir. Med. 2016, 10, 901–906. [Google Scholar] [CrossRef]
- Levine, M.Z.; Goodman, S.; Lentz, R.J.; Maldonado, F.; Rickman, O.B.; Katsis, J. Advanced Bronchoscopic Technologies for Biopsy of the Pulmonary Nodule: A 2021 Review. Diagnostics 2021, 11, 2304. [Google Scholar] [CrossRef]
- Baaklini, W.A.; Reinoso, M.A.; Gorin, A.B.; Sharafkaneh, A.; Manian, P. Diagnostic Yield of Fiberoptic Bronchoscopy in Evaluating Solitary Pulmonary Nodules. Chest 2000, 117, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- Boskovic, T.; Stanic, J.; Pena-Karan, S.; Zarogoulidis, P.; Drevelegas, K.; Katsikogiannis, N.; Machairiotis, N.; Mpakas, A.; Tsakiridis, K.; Kesisis, G.; et al. Pneumothorax after Transthoracic Needle Biopsy of Lung Lesions under CT Guidance. J. Thorac. Dis. 2014, 6 (Suppl. 1), S99–S107. [Google Scholar] [CrossRef]
- Chockalingam, A.; Hong, K. Transthoracic Needle Aspiration: The Past, Present and Future. J. Thorac. Dis. 2015, 7 (Suppl. 4), S292–S299. [Google Scholar] [CrossRef]
- Ost, D.E.; Ernst, A.; Lei, X.; Kovitz, K.L.; Benzaquen, S.; Diaz-Mendoza, J.; Greenhill, S.; Toth, J.; Feller-Kopman, D.; Puchalski, J.; et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016, 193, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Kemp, S.V. Navigation Bronchoscopy. Respiration 2020, 99, 277–286. [Google Scholar] [CrossRef]
- Yasufuku, K.; Chiyo, M.; Sekine, Y.; Chhajed, P.N.; Shibuya, K.; Iizasa, T.; Fujisawa, T. Real-time Endobronchial Ultrasound-guided Transbronchial Needle Aspiration of Mediastinal and Hilar Lymph Nodes. Chest 2004, 126, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.K.; Fan, H.J.; Yao, Z.H.; Lin, Y.T.; Wen, Y.F.; Wu, S.G.; Ho, C.C. Cone-Beam Computed Tomography-Derived Augmented Fluoroscopy Improves the Diagnostic Yield of Endobronchial Ultrasound-Guided Transbronchial Biopsy for Peripheral Pulmonary Lesions. Diagnostics 2021, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Setser, R.; Chintalapani, G.; Bhadra, K.; Casal, R.F. Cone Beam CT Imaging for Bronchoscopy: A Technical Review. J. Thorac. Dis. 2020, 12, 7416–7428. [Google Scholar] [CrossRef] [PubMed]
- Cicenia, J.; Bhadra, K.; Sethi, S.; Nader, D.A.; Whitten, P.; Hogarth, D.K. Augmented Fluoroscopy: A New and Novel Navigation Platform for Peripheral Bronchoscopy. J. Bronchol. Interv. Pulmonol. 2021, 28, 116–123. [Google Scholar] [CrossRef]
- Verhoeven, R.L.J.; van der Sterren, W.; Kong, W.; Langereis, S.; van der Tol, P.; van der Heijden, E.H.F.M. Cone-Beam CT and Augmented Fluoroscopy–guided Navigation Bronchoscopy: Radiation Exposure and Diagnostic Accuracy Learning Curves. J. Bronchol. Interv. Pulmonol. 2021, 28, 262–271. [Google Scholar] [CrossRef]
- Yang, S.M.; Yu, K.L.; Lin, J.H.; Lin, K.H.; Liu, Y.L.; Sun, S.E.; Meng, L.H.; Ko, H.J. Cumulative Experience of Preoperative Real-Time Augmented Fluoroscopy-Guided Endobronchial Dye Marking for Small Pulmonary Nodules: An Analysis of 30 Initial Patients. J. Formos. Med. Assoc. 2019, 118, 1232–1238. [Google Scholar] [CrossRef]
- Yu, K.L.; Yang, S.M.; Ko, H.J.; Tsai, H.Y.; Ko, J.C.; Lin, C.K.; Ho, C.C.; Shih, J.Y. Efficacy and Safety of Cone-Beam Computed Tomography-Derived Augmented Fluoroscopy Combined with Endobronchial Ultrasound in Peripheral Pulmonary Lesions. Respiration 2021, 100, 538–546. [Google Scholar] [CrossRef]
- Ng, C.S.; Yu, S.C.; Lau, R.W.; Yim, A.P. Hybrid DynaCT-Guided Electromagnetic Navigational Bronchoscopic Biopsy. Eur. J. Cardiothorac. Surg. 2016, 49 (Suppl. 1), i87–i88. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.W.Y.; Lau, R.W.H.; Chu, C.M.; Ng, C.S.H. Expanding the Scope of Electromagnetic Navigation Bronchoscopy-Guided Transbronchial Biopsy and Ablation with Mobile 3D C-arm Machine Cios Spin®-Feasibility and Challenges. Transl. Lung Cancer Res. 2021, 10, 4043–4046. [Google Scholar] [CrossRef]
- Yang, S.M.; Ko, W.C.; Lin, M.W.; Hsu, H.H.; Chan, C.Y.; Wu, I.H.; Chang, Y.C.; Chen, J.S. Image-Guided Thoracoscopic Surgery with Dye Localization in A Hybrid Operating Room. J. Thorac. Dis. 2016, 8 (Suppl. 9), S681–S689. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.M.; Yu, K.L.; Chen, L.C.; Chung, W.Y.; Ko, H.J.; Chen, C.M. Augmented Fluoroscopic Bronchoscopy 2.0: Image Fusion for Endobronchial Roadmapping. J. Bronchol. Interv. Pulmonol. 2021, 28, 303–306. [Google Scholar] [CrossRef]
- Anayama, T.; Yamamoto, M.; Hirohashi, K.; Miyazaki, R.; Okada, H.; Doi, A.; Orihashi, K. The Accuracy of Cone-Beam Computed Tomography and Augmented Fluoroscopy-Guided Bronchoscopic Marking of Multiple Small-Sized Pulmonary Nodules in a Hybrid Operating Room: A Retrospective Cohort Study. Quant. Imaging Med. Surg. 2021, 11, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Schampaert, S.; de Groot, J.A.H.; Schirmer, C.C.; van der Bom, I. Cone-Beam CT with Augmented Fluoroscopy Combined with Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J. Bronchol. Interv. Pulmonol. 2018, 25, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Ekeke, C.N.; Vercauteren, M.; Istvaniczdravkovic, S.; Semaan, R.; Dhupar, R. Lung Nodule Evaluation using Robotic-Assisted Bronchoscopy at a Veteran’s Affairs Hospital. J. Clin. Med. 2021, 10, 3671. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, N.; Miyazawa, T.; Okimasa, S.; Maeda, A.; Oiwa, H.; Miyazu, Y.; Murayama, M. Endobronchial uhrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004, 126, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, R.L.J.; Fütterer, J.J.; Hoefsloot, W.; van der Heijden, E.H.F.M. Cone-Beam CT Image Guidance with and without Electromagnetic Navigation Bronchoscopy for Biopsy of Peripheral Pulmonary Lesions. J. Bronchol. Interv. Pulmonol. 2021, 28, 60–69. [Google Scholar] [CrossRef]

| Variables | Values (%) |
|---|---|
| Sex (female) | 22 (53.7) |
| Age (y) | 66 (58–76) * |
| BMI (kg/m2) | 23.7 (20.4–26.2) * |
| ASA physical status classification | |
| Class I and II | 20 (48.8) |
| Class III | 20 (48.8) |
| Class IV | 1 (2.4) |
| Smoking status | |
| Never smoker | 27 (65.9) |
| Ex-smoker | 6 (14.6) |
| Active smoker | 8 (19.5) |
| Variables | Values (%) |
|---|---|
| Lesion size (mm) (n = 31 a) | 35.0 (17.0–44.0) * |
| <10 mm | 1 (3.2) |
| 10–20 mm | 8 (25.8) |
| 20–30 mm | 1 (3.2) |
| 30–40 mm | 8 (25.8) |
| 40–50 mm | 11 (35.5) |
| >50 mm | 2 (6.5) |
| Lesion distance from the pleura (mm) (n = 31 a) | 12.0 (0.0–22.0) * |
| <20 mm | 22 (71.0) |
| 20–40 mm | 7 (22.6) |
| >40 mm | 2 (6.4) |
| Nodule appearance | |
| Pure GGN | 5 (12.2) |
| Part-solid GGN | 14 (34.1) |
| Solid | 22 (53.7) |
| Bronchial sign | |
| Positive | 33 (80.5) |
| Negative | 8 (19.5) |
| Location | |
| Right upper lobe | 14 (35.0) |
| Right middle lobe | 2 (5.0) |
| Right lower lobe | 9 (22.5) |
| Left upper lobe | 9 (22.5) |
| Left lower lobe | 7 (17.5) |
| Variables | Values a |
|---|---|
| Procedure time (min) | 97.0 (64.0–122.0) |
| Length of time under anesthesia (min) | 146.0 (124.0–193) |
| Global operation room time (min) | 152.0 (130.0–197.0) |
| Radiation reports (n = 25) | |
| Number of DynaCT scans | 2 (2–3) |
| Duration of fluoroscopy (min) | 9.4 (5.1–11.7) |
| Total dose area product (µGym2) | 6645 (5453–8326) |
| Length of postoperative stay (day) | 2.0 (1.0–4.0) |
| Number (%) | |
|---|---|
| Transbronchial biopsy | 25 (61.97) |
| Concurrent procedures | |
| Percutaneous biopsy | 6 (14.6) |
| Transbronchial needle aspiration | 10 (24.4) |
| Diagnosis | |
| Presumptive benign diagnosis | 17 (41.5) |
| Acute and chronic inflammation | 1 (2.4) |
| Chronic inflammation | 5 (12.2) |
| Chronic inflammation and interstitial fibrosis | 7 (17.1) |
| Necrosis | 1 (2.4) |
| Peribronchiolar metaplasia | 1 (2.4) |
| Benign peribronchial tissue | 2 (4.9) |
| Presumptive malignant diagnosis | 24 (58.5) |
| Non-small-cell carcinoma | 14 (34.1) |
| Small cell carcinoma/neuroendocrine tumor | 2 (4.9) |
| Metastasis | 1 (2.4) |
| Atypia | 2 (4.9) |
| Negative for malignancy | 5 (12.2) |
| Complications | |
| Pneumothorax a | 3 (7.3) |
| Conservative management | 1 (2.4) |
| Simple drainage | 2 (4.9) |
| Bleeding (hemothorax) b | 2 (4.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Yang, S.-M.; Malwade, S.; Chang, H.-C.; Chang, L.-K.; Chung, W.-Y.; Ko, J.-C.; Yu, C.-J. Cone-Beam Computed-Tomography-Derived Augmented Fluoroscopy-Guided Biopsy for Peripheral Pulmonary Nodules in a Hybrid Operating Room: A Case Series. Diagnostics 2023, 13, 1055. https://doi.org/10.3390/diagnostics13061055
Chen L-C, Yang S-M, Malwade S, Chang H-C, Chang L-K, Chung W-Y, Ko J-C, Yu C-J. Cone-Beam Computed-Tomography-Derived Augmented Fluoroscopy-Guided Biopsy for Peripheral Pulmonary Nodules in a Hybrid Operating Room: A Case Series. Diagnostics. 2023; 13(6):1055. https://doi.org/10.3390/diagnostics13061055
Chicago/Turabian StyleChen, Lun-Che, Shun-Mao Yang, Shwetambara Malwade, Hao-Chun Chang, Ling-Kai Chang, Wen-Yuan Chung, Jen-Chung Ko, and Chong-Jen Yu. 2023. "Cone-Beam Computed-Tomography-Derived Augmented Fluoroscopy-Guided Biopsy for Peripheral Pulmonary Nodules in a Hybrid Operating Room: A Case Series" Diagnostics 13, no. 6: 1055. https://doi.org/10.3390/diagnostics13061055
APA StyleChen, L.-C., Yang, S.-M., Malwade, S., Chang, H.-C., Chang, L.-K., Chung, W.-Y., Ko, J.-C., & Yu, C.-J. (2023). Cone-Beam Computed-Tomography-Derived Augmented Fluoroscopy-Guided Biopsy for Peripheral Pulmonary Nodules in a Hybrid Operating Room: A Case Series. Diagnostics, 13(6), 1055. https://doi.org/10.3390/diagnostics13061055






