Human Anthrax: Update of the Diagnosis and Treatment
Abstract
:1. Introduction
2. Epidemiology
3. Clinical Presentations
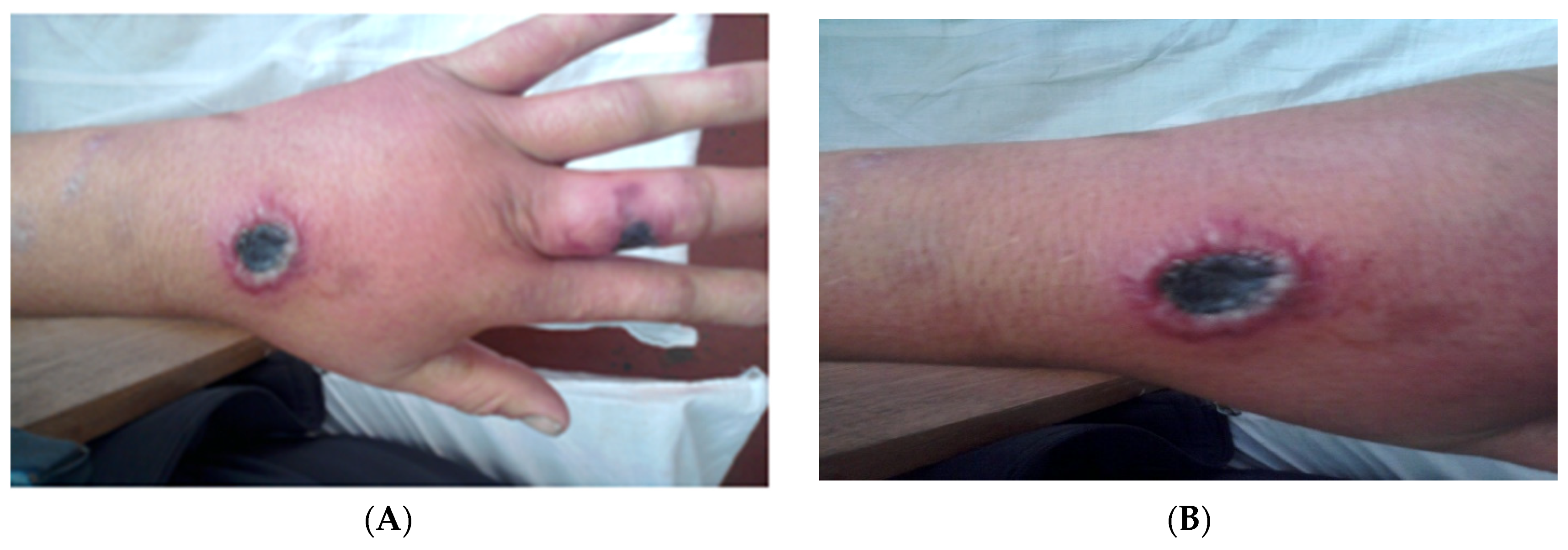
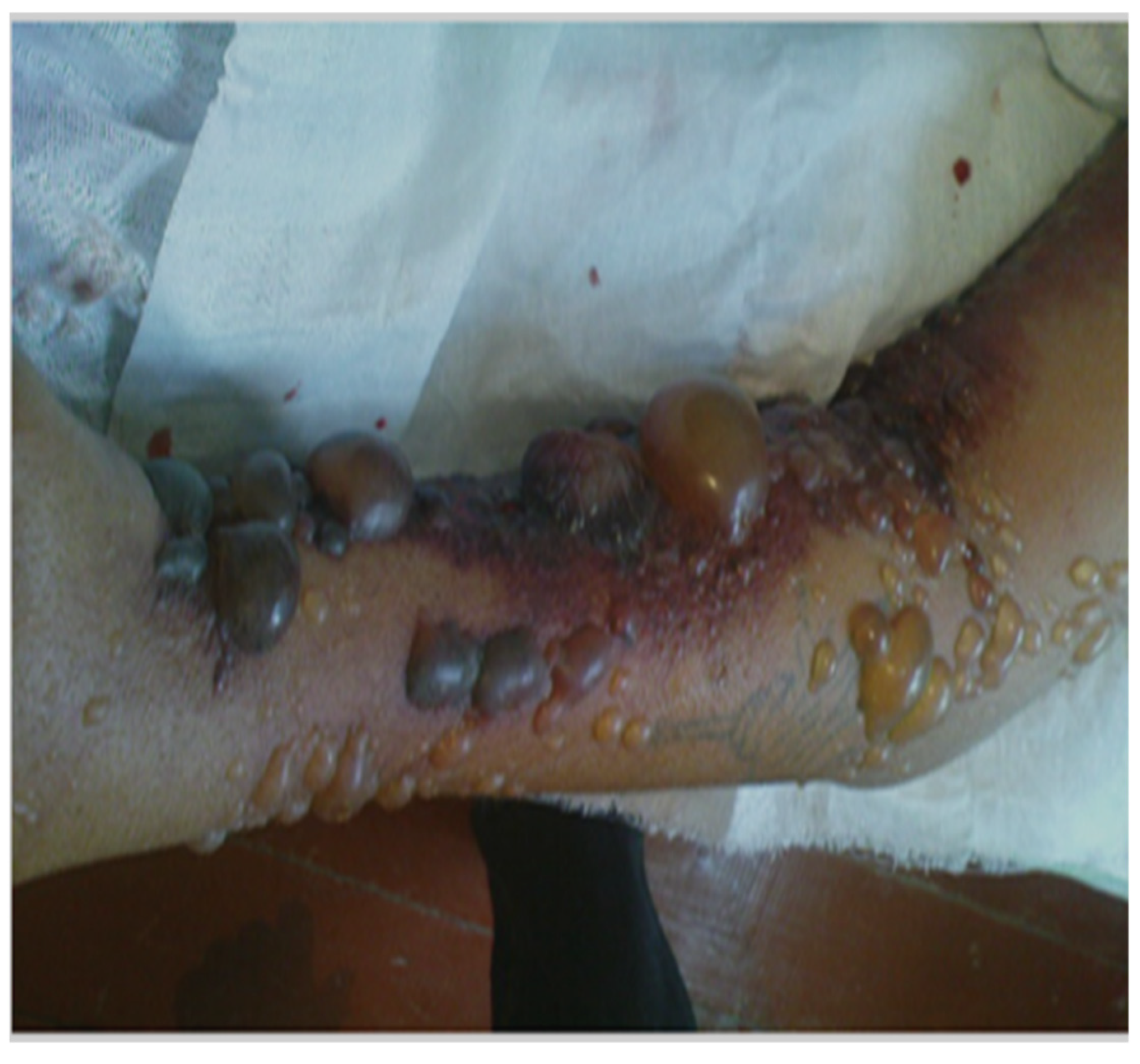
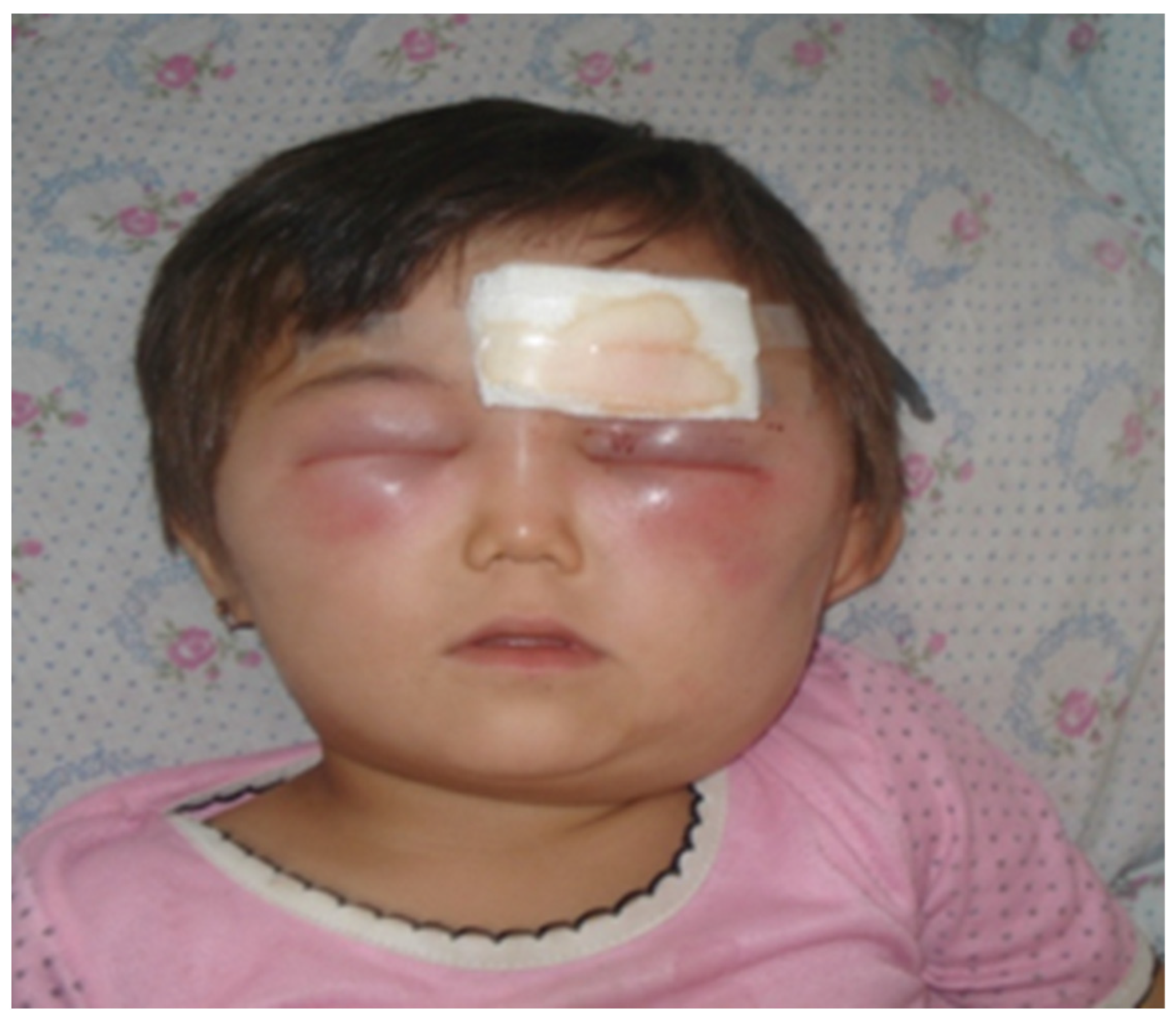
3.1. Cutaneous Anthrax
3.2. Gastrointestinal Anthrax
3.3. Inhalation Anthrax
3.4. Injectional Anthrax
4. Diagnosis
5. Management
5.1. Cutaneous Anthrax
5.2. Gastrointestinal Anthrax
5.3. Inhalation Anthrax
5.4. Anthrax Meningoencephalitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO; OIE; FAO. Anthrax in Humans and Animals, 4th ed.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Bakhteeva, I.; Timofeev, V. Some peculiarities of Anthrax epidemiology in herbivorous and carnivorous animals. Life 2022, 10, 870. [Google Scholar] [CrossRef]
- Pittiglio, C.; Shadomy, S.; El Idrissi, A.; Soumare, B.; Lubroth, J.; Makonnen, Y. Seasonality and Ecological Suitability Modelling for Anthrax (Bacillus anthracis) in Western Africa. Animals 2022, 12, 1146. [Google Scholar] [CrossRef]
- Eremenko, E.; Pechkovskii, G.; Pisarenko, S.; Ryazanova, A.; Kovalev, D.; Semenova, O.; Aksenova, L.; Timchenko, L.; Golovinskaya, T.; Bobrisheva, O.; et al. Phylogenetics of Bacillus anthracis isolates from Russia and bordering countries. Infect. Genet. Evol. 2021, 92, 104890. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, L.Y.; Zhang, X.S.; Han, Z.H.; Hu, W.B.; Qian, Q.; Haque, U.; Soares Magalhaes, R.J.; Li, S.L.; Tong, S.L.; et al. Spatiotemporal clustering analysis and risk assessments of human cutaneous Anthrax in China, 2005–2012. PLoS ONE 2015, 10, e0133736. [Google Scholar] [CrossRef]
- Jernigan, D.B.; Raghunathan, P.L.; Bell, B.P.; Brechner, R.; Bresnitz, E.A.; Butler, J.C.; Cetron, M.; Cohen, M.; Doyle, T.; Fischer, M.; et al. Investigation of bioterrorism-related Anthrax, United States, 2001: Epidemiologic findings. Emerg. Infect. Dis. 2002, 8, 1019–1028. [Google Scholar] [CrossRef]
- Booth, M.; Donaldson, L.; Cui, X.; Sun, J.; Cole, S.; Dailsey, S.; Hart, A.; Johns, N.; McConnell, P.; McLennan, T.; et al. Confirmed Bacillus anthracis infection among persons who inject drugs, Scotland, 2009–2010. Emerg. Infect. Dis. 2014, 20, 1452–1463. [Google Scholar] [CrossRef]
- Doganay, M. Anthrax. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1123–1128. [Google Scholar]
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef]
- Doganay, M.; Demiraslan, H. Human anthrax as a re-emerging disease. Recent Pat. Antiinfect. Drug Discov. 2015, 10, 10–29. [Google Scholar] [CrossRef]
- Badri, R.; Uwishema, O.; Wellington, J.; Thambi, V.D.; Pradhan, A.U.; Adanur, I.; Onyeaka, C.V.P.; Onyeaka, H. Anthrax outbreak amidst the COVID-19 pandemic in Africa: Challenges and possible solutions. Ann. Med. Surg. 2022, 81, 104418. [Google Scholar] [CrossRef]
- Doganay, M.; Metan, G. Human anthrax in Turkey from 1990 to 2007. Vector-Borne Zoonotic Dis. 2009, 9, 131–140. [Google Scholar] [CrossRef]
- Galante, D.; Manzulli, V.; Serrecchia, L.; Taranto, P.D.; Hugh-Jones, M.; Hossain, M.J.; Rondinone, V.; Cipolletta, D.; Pace, L.; Iatarola, M.; et al. Investigation on Anthrax in Bangladesh during the outbreaks of 2011 and definition of the epidemiological correlations. Pathogens 2021, 10, 481. [Google Scholar] [CrossRef]
- Alam, M.E.; Kamal, M.M.; Rahman, M.; Kabir, A.; Islam, M.S.; Hassan, J. Review of anthrax: A disease of farm animals. J. Adv. Vet. Anim. Res. 2022, 9, 323–334. [Google Scholar] [CrossRef]
- Kracalik, I.T.; Malania, L.; Tsertsvadze, N.; Manvelyan, J.; Bakanidze, L.; Imnadze, P.; Tsanava, S.; Blackburn, J.K. Evidence of local persistence of human Anthrax in the country of Georgia associated with environmental and anthropogenic factors. PLoS Negl. Trop. Dis. 2013, 7, e2388. [Google Scholar] [CrossRef] [Green Version]
- Kutmanova, A.; Doganay, M.; Zholdoshev, S. Human anthrax in Kyrgyz Republic: Epidemiology and clinical features. J. Infect. Public Health 2020, 13, 1161–1165. [Google Scholar] [CrossRef]
- Hodnik, J.J.; Acinger-Rogić, Ž.; Alishani, M.; Autio, T.; Balseiro, A.; Berezowski, J. Overview of cattle diseases listed under category C, D or E in the Animal Health Law for which control programmes are in place within Europe. Front. Vet. Sci. 2021, 8, 688078. [Google Scholar] [CrossRef]
- Hendricks, K.; Person, M.K.; Bradley, J.S.; Mongkolrattanothai, T.; Hupert, N.; Eichacker, P.; Friedlander, A.M.; Bower, W.A. Clinical features of patients hospitalized for all routes of Anthrax, 1880–2018: A Systematic Review. Clin. Infect. Dis. 2022, 75 (Suppl. 3), S341–S353. [Google Scholar] [CrossRef]
- Parlak, E.; Parlak, M. Human cutaneous anthrax, the east anatolian region of Turkey 2008–2014. Vector-Borne Zoonotic Dis. 2016, 16, 42–47. [Google Scholar] [CrossRef]
- Doganay, M.; Almac, A.; Hanagasi, R. Primary throat anthrax. A report of six cases. Scand. J. Infect. Dis. 1986, 18, 415–419. [Google Scholar] [CrossRef]
- Sirisanthana, T.; Brown, A.E. Anthrax of the gastrointestinal tract. Emerg. Infect. Dis. 2002, 8, 649–651. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Ghossain, A.; Sharara, A.I.; Hatem, J.M.; Kanj, S.S. Endemic gastrointestinal anthrax in 1960s Lebanon: Clinical manifestations and surgical findings. Emerg. Infect. Dis. 2003, 9, 520–525. [Google Scholar] [CrossRef]
- Meric, M.; Willke, A.; Muezzinoglu, B.; Karadenizli, A.; Hosten, T. A case of pneumonia caused by Bacillus anthracis secondary to gastrointestinal anthrax. Int. J. Infect. Dis. 2009, 13, e456–e458. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.J.; Friedlander, A.M. Bacillus anthracis. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 2550–2569. [Google Scholar]
- Shifman, O.; Aminov, T.; Aftalion, M.; Gur, D.; Cohen, H.; Bar-David, E.; Cohen, O.; Mamroud, E.; Levy, H.; Aloni-Grinstein, R.; et al. Evaluation of the European Committee on Antimicrobial Susceptibility Testing Guidelines for rapid antimicrobial susceptibility testing of Bacillus anthracis, Yersinia pestis and Francisella tularensis positive blood cultures. Microorganisms 2021, 9, 1055. [Google Scholar] [CrossRef]
- Tomaso, H.; Bartling, C.; Al Dahouk, S.; Hagen, R.M.; Scholz, H.C.; Beyer, W.; Neubauer, H. Growth characteristics of Bacillus anthracis compared to other Bacillus spp. on the selective nutrient media Anthrax Blood Agar and Cereus Ident Agar. Syst. Appl. Microbiol. 2006, 29, 24–28. [Google Scholar] [CrossRef]
- Abshire, T.G.; Brown, J.E.; Ezzell, J.W. Production and validation of the use of gamma phage for identification of Bacillus anthracis. J. Clin. Microbiol. 2005, 43, 4780–4788. [Google Scholar] [CrossRef] [Green Version]
- EUCAST. Rapid AST Directly from Blood Culture Bottles. Available online: https://www.eucast.org/rapid_ast_in_blood_cultures/ (accessed on 31 January 2023).
- Zasada, A.A.; Zacharczuk, K.; Formi’nska, K.; Wiatrzyk, A.; Ziółkowski, R.; Malinowska, E. Isothermal DNA amplification combined with lateral flow dipsticks for detection of biothreat agents. Anal. Biochem. 2018, 560, 60–66. [Google Scholar] [CrossRef]
- Bentahir, M.; Ambroise, J.; Delcorps, C.; Pilo, P.; Gala, J.L. Sensitive and specific recombinase polymerase amplification assays for fast screening, detection, and identification of Bacillus anthracis in a field setting. Appl. Environ. Microbiol. 2018, 84, e00506–e00518. [Google Scholar] [CrossRef] [Green Version]
- Tamborrini, M.; Holzer, M.; Seeberger, P.H.; Schürch, N.; Pluschke, G. Anthrax spore detection by a luminex assay based on monoclonal antibodies that recognize anthrose-containing oligosaccharides. Clin. Vaccine Immunol. 2010, 17, 1446–1451. [Google Scholar] [CrossRef] [Green Version]
- Zasada, A.A. Detection and Identification of Bacillus anthracis: From Conventional to Molecular Microbiology Methods. Microorganisms 2020, 16, 125. [Google Scholar] [CrossRef] [Green Version]
- Cohen, N.; Zahavy, E.; Zichel, R.; Fisher, M. An internal standard approach for homogeneous TR-FRET immunoassays facilitates the detection of bacteria, biomarkers, and toxins in complex matrices. Anal. Bioanal. Chem. 2016, 408, 5179–5188. [Google Scholar] [CrossRef]
- Zasada, A.A.; Formi´nska, K.; Zacharczuk, K.; Jacob, D.; Grunow, R. Comparison of eleven commercially available rapid tests for detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis. Lett. Appl. Microbiol. 2015, 60, 409–413. [Google Scholar] [CrossRef]
- Kim, J.; Gedi, V.; Lee, S.C.; Cho, J.H.; Moon, J.Y.; Yoon, M.Y. Advances in anthrax detection: Overview of bioprobes and biosensors. Appl. Biochem. Biotechnol. 2015, 176, 957–977. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, M.; Andrade, A.F.B.; Gonzalez-Rodriguez, J. Selective and sensitive electrochemical DNA biosensor for the detection of Bacillus anthracis. Int. J. Electrochem. Sci. 2016, 11, 763–776. [Google Scholar]
- Ziółkowski, R.; Oszwałdowski, S.; Zacharczuk, K.; Zasada, A.A.; Malinowska, E. Electrochemical detection of Bacillus anthracis protective antigen gene using DNA biosensor based on stem−loop probe. J. Electrochem. Soc. 2018, 165, B187–B195. [Google Scholar] [CrossRef]
- Zhang, B.; Dallo, S.; Peterson, R.; Hussain, S.; Weitao, T.; Ye, J.Y. Detection of anthrax lef with DNA-based photonic crystal sensors. J. Biomed. Opt. 2011, 16, 127006. [Google Scholar] [CrossRef] [Green Version]
- Mazzaracchio, V.; Neagu, D.; Porchetta, A.; Marcoccio, E.; Pomponi, A.; Faggioni, G.; D’Amore, N.; Notargiacomo, A.; Pea, M.; Moscone, D.; et al. A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Biosens. Bioelectron. 2019, 126, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Mwilu, S.K.; Aluoch, A.O.; Miller, S.; Wong, P.; Sadik, O.A.; Fatah, A.A.; Arcilesi, R.D. Identification and quantitation of Bacillus globigii using metal enhanced electrochemical detection and capillary biosensor. Anal.Chem. 2009, 81, 7561–7570. [Google Scholar] [CrossRef]
- Waller, D.F.; Hew, B.E.; Holdaway, C.; Jen, M.; Peckham, G.D. Rapid detection of Bacillus anthracis spores using immunomagnetic separation and amperometry. Biosensors 2016, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Pauker, V.I.; Thoma, B.R.; Grass, G.; Bleichert, P.; Hanczaruk, M.; Zöller, L.; Zange, S. Improved discrimination of Bacillus anthracis from closely related species in the Bacillus cereus Sensu Lato group based on Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2018, 56, e01900–e01917. [Google Scholar] [CrossRef] [Green Version]
- Sternbach, G. The history of anthrax. J. Emerg. Med. 2003, 24, 463–467. [Google Scholar] [CrossRef]
- James, F.S. ‘A remedy for this dread disease’: Achille Sclavo, anthrax and serum therapy in early twentieth-century Britain. Br. J. Hist. Sci. 2022, 55, 207–226. [Google Scholar]
- Metan, G.; Doganay, M. The antimicrobial susceptibility of Bacillus anthracis isolated from human cases: A review of Turkish literature. Turk. Klin. J. Med. Sci. 2009, 29, 229–235. [Google Scholar]
- Doganay, M. Ingestional (Oral Route/Enteric) Anthrax: Is It a Problem in Turkey? FLORA 2009, 14, 97–104. [Google Scholar]
- Savransky, V.; Ionin, B.; Reece, J. Current Status and Trends in Prophylaxis and Management of Anthrax Disease. Pathogens 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Elbahr, U.S.; Tekin, R.; Papić, M.; Pandak, N.; Erdem, H.; Can, F.K.; Alpat, S.N.; Pekok, A.U.; Pehlivanoglu, F.; Karamese, M.; et al. Factors leading to dissemination of cutaneous anthrax: An international ID-IRI study. New Microbes New Infect. 2022, 48, 101028. [Google Scholar] [CrossRef]
- Denk, A.; Tartar, A.S.; Ozden, M.; Demir, B.; Akbulut, A. Cutaneous anthrax: Evaluation of 28 cases in the Eastern Anatolian region of Turkey. Cutan. Ocul. Toxicol. 2016, 35, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Kutmanova, A.; Zholdoshev, S.; Roguski, K.M.; Sholpanbay uulu, M.; Person, M.K.; Cook, R.; Bugrysheva, J.; Nadol, P.; Buranchieva, A.; Imanbaeva, L.; et al. Risk Factors for Severe Cutaneous Anthrax in a Retrospective Case Series and Use of a Clinical Algorithm to Identify Likely Meningitis and Evaluate Treatment Outcomes, Kyrgyz Republic, 2005–2012. Clin. Infect. Dis. 2022, 75, 478–486. [Google Scholar] [CrossRef]
- Hendricks, K.A.; Wright, M.E.; Shadomy, S.V.; Bradley, J.S.; Morrow, M.G.; Pavia, A.T.; Rubinstein, E.; Holty, J.E.; Messonnier, N.E.; Smith, T.L.; et al. Centers for Disease Control and Prevention Expert Panel Meetings on Prevention and Treatment of Anthrax in Adults. Emerg. Infect. Dis. 2014, 20, e130687. [Google Scholar] [CrossRef]
- Holty, J.E.; Bravata, D.M.; Liu, H.; Olshen, R.A.; McDonald, K.M.; Owens, D.K. Systematic review: A century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 2006, 144, 270–280. [Google Scholar] [CrossRef]
- Walsh, J.J.; Pesik, N.; Quinn, C.P.; Urdaneta, V.; Dykewicz, C.A.; Boyer, A.E.; Guarner, J.; Wilkins, P.; Norville, K.J.; Barr, J.R.; et al. A case of naturally acquired inhalation anthrax: Clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 2007, 44, 968–971. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Tenover, F.C.; Stephens, D.S. Management of anthrax meningitis. Lancet Infect. Dis. 2005, 5, 287–295. [Google Scholar] [CrossRef]
- Slay, R.M.; Cook, R.; Hendricks, K.; Boucher, D.; Merchlinsky, M. Pre- and Postlicensure Animal Efficacy Studies Comparing Anthrax Antitoxins. Clin. Infect. Dis. 2022, 75 (Suppl. 3), S441–S450. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Moon, J.J. Particulate delivery systems for vaccination against bioterrorism agents and emerging infectious pathogens. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1403. [Google Scholar] [CrossRef] [Green Version]
- Brenneman, K.E.; Doganay, M.; Akmal, A.; Goldman, S.; Galloway, D.R.; Mateczun, A.J.; Baillie, L.W. The early humoral immune response to Bacillus anthracis toxins in patients infected with cutaneous anthrax. FEMS Immunol. Med. Microbiol. 2011, 62, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Galloway, D.R.; Baillie, L. DNA vaccines against anthrax. Expert Opin. Biol. Ther. 2004, 4, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Wolfe, D.N. Current State of Anthrax Vaccines and Key R&D Gaps Moving Forward. Microorganisms 2020, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, M.J.; Dyson, E.H.; Simpson, A.J.; Brenneman, K.E.; Bailey, N.; Wilkinson, L.; Hornby, R.; Mateczun, A.J.; Bell, M.G.; Baillie, L.W. Immune response to two different dosing schedules of the anthrax vaccine precipitated (AVP) vaccine. Vaccine 2007, 25, 6089–6097. [Google Scholar] [CrossRef] [PubMed]
- CDC. Anthrax Vaccine Recommendations. Available online: https://www.cdc.gov/vaccines/vpd/anthrax/hcp/recommendations.html (accessed on 16 February 2023).

| TRANSMISSION OF THE INFECTION | COMMENTS |
|---|---|
| Contact with dying or dead animals | Slaughtering and skinning, handling and processing of dead animals. The disposal of contaminated carcasses |
| Contact with contaminated animal products | Wool coats, shaving brushes, leather (e.g., drumheads made of animal skin) and bone meal (e.g., fertilizer). |
| Ingestion of contaminated meat | Eating contaminated, uncooked, improperly cooked meat, traditional raw food or meat products. |
| Self-injection | Injection of contaminated, illegal heroin |
| Nosocomial transmission | Human to human spread is rare |
| RISK FACTORS | |
| Living in an endemic area | |
| Agricultural occupations | Herdsman, butchers, skinners, slaughterhouse workers, diary workers, veterinarians |
| Industrial occupations | Tanners, leather gift makers, furriers, shoemakers, drum makers, carpet weavers, wool spinners, bone meal processers, wool textile factory workers |
| Illegal drug use | Injection of contaminated materials |
| CLINICAL PRESENTATIONS | COMPLICATIONS * |
| Cutaneous anthrax | Sepsis Meningoencephalitis Pneumonia |
| Gastrointestinal Anthrax Oropharyngeal Intestinal | |
| Inhalation anthrax |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doganay, M.; Dinc, G.; Kutmanova, A.; Baillie, L. Human Anthrax: Update of the Diagnosis and Treatment. Diagnostics 2023, 13, 1056. https://doi.org/10.3390/diagnostics13061056
Doganay M, Dinc G, Kutmanova A, Baillie L. Human Anthrax: Update of the Diagnosis and Treatment. Diagnostics. 2023; 13(6):1056. https://doi.org/10.3390/diagnostics13061056
Chicago/Turabian StyleDoganay, Mehmet, Gokcen Dinc, Ainura Kutmanova, and Les Baillie. 2023. "Human Anthrax: Update of the Diagnosis and Treatment" Diagnostics 13, no. 6: 1056. https://doi.org/10.3390/diagnostics13061056
APA StyleDoganay, M., Dinc, G., Kutmanova, A., & Baillie, L. (2023). Human Anthrax: Update of the Diagnosis and Treatment. Diagnostics, 13(6), 1056. https://doi.org/10.3390/diagnostics13061056






