Real-Time Elastography versus Shear Wave Elastography on Evaluating the Timely Radiofrequency Ablation Effect of Rabbit Liver: A Preliminary Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Animals
2.2. Methods
2.2.1. Preparation before Operation
2.2.2. Ablation Progress
2.2.3. Ultrasound Examination
RTE
SWE
CEUS Examination
2.2.4. Animal Euthanasia
2.2.5. Gross Specimen
2.2.6. Statistical Analysis
3. Results
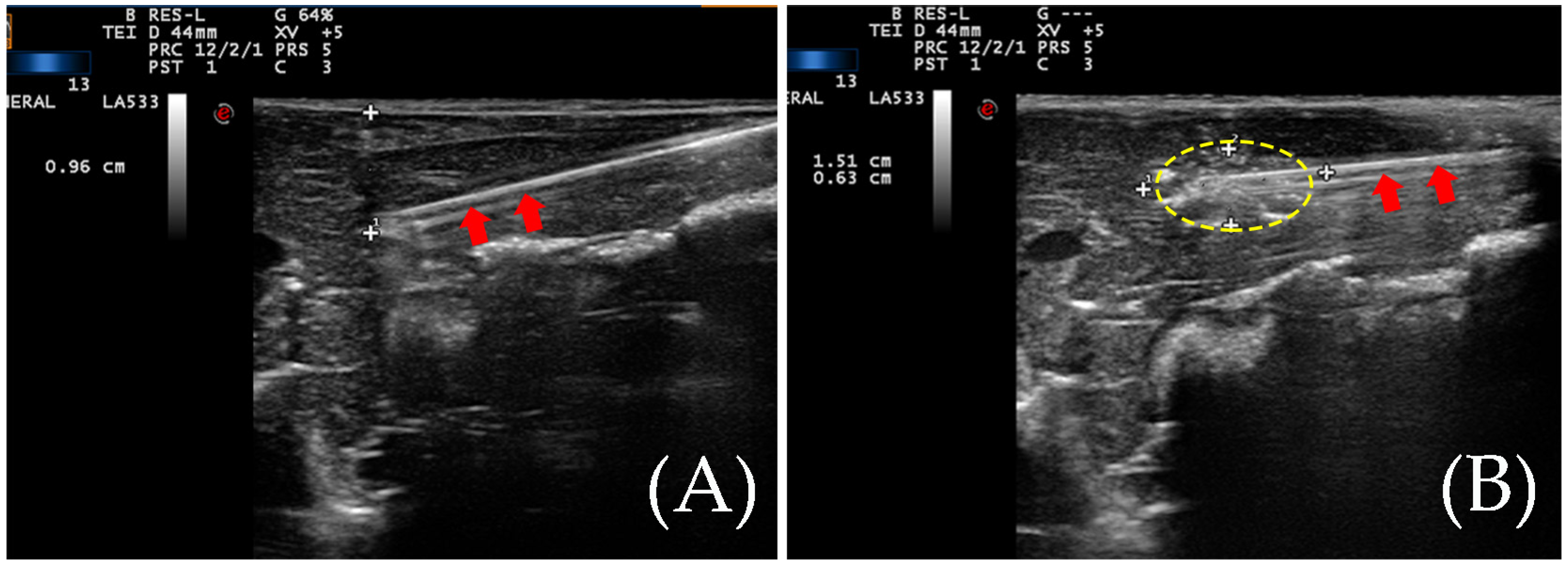
3.1. The Situation of the Ablation Zone
3.2. RTE for the Detection of the Ablation Zone
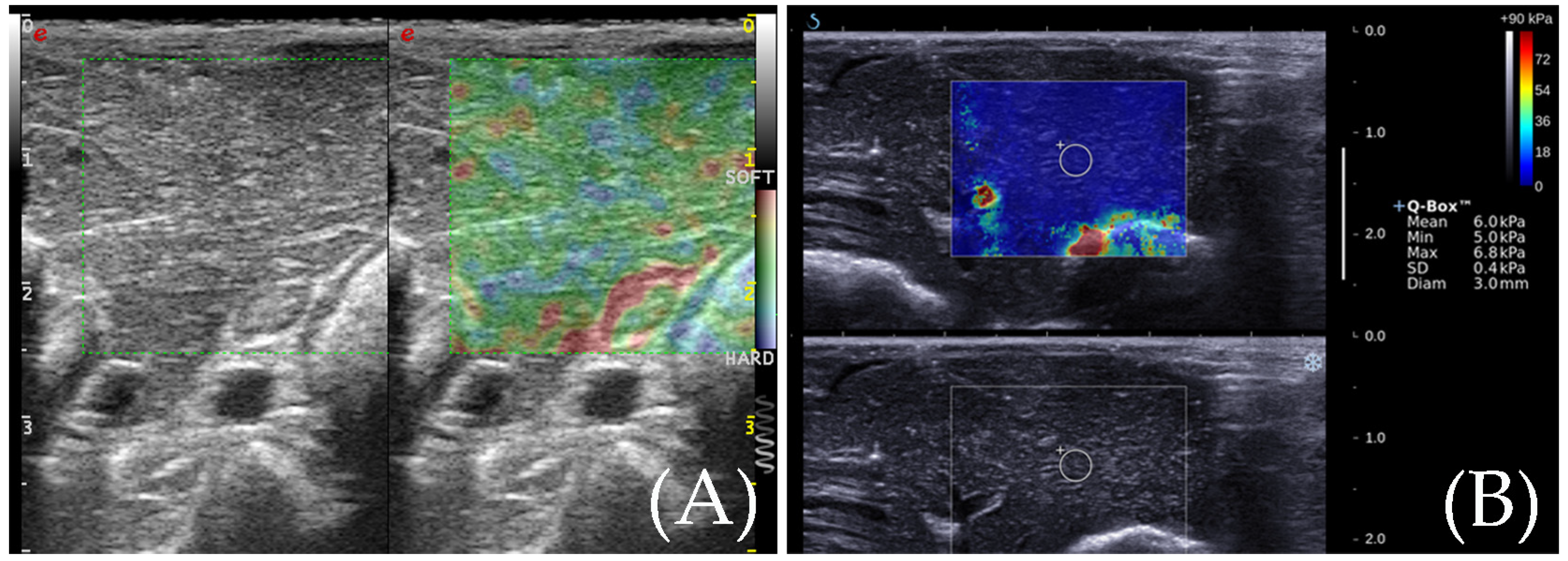
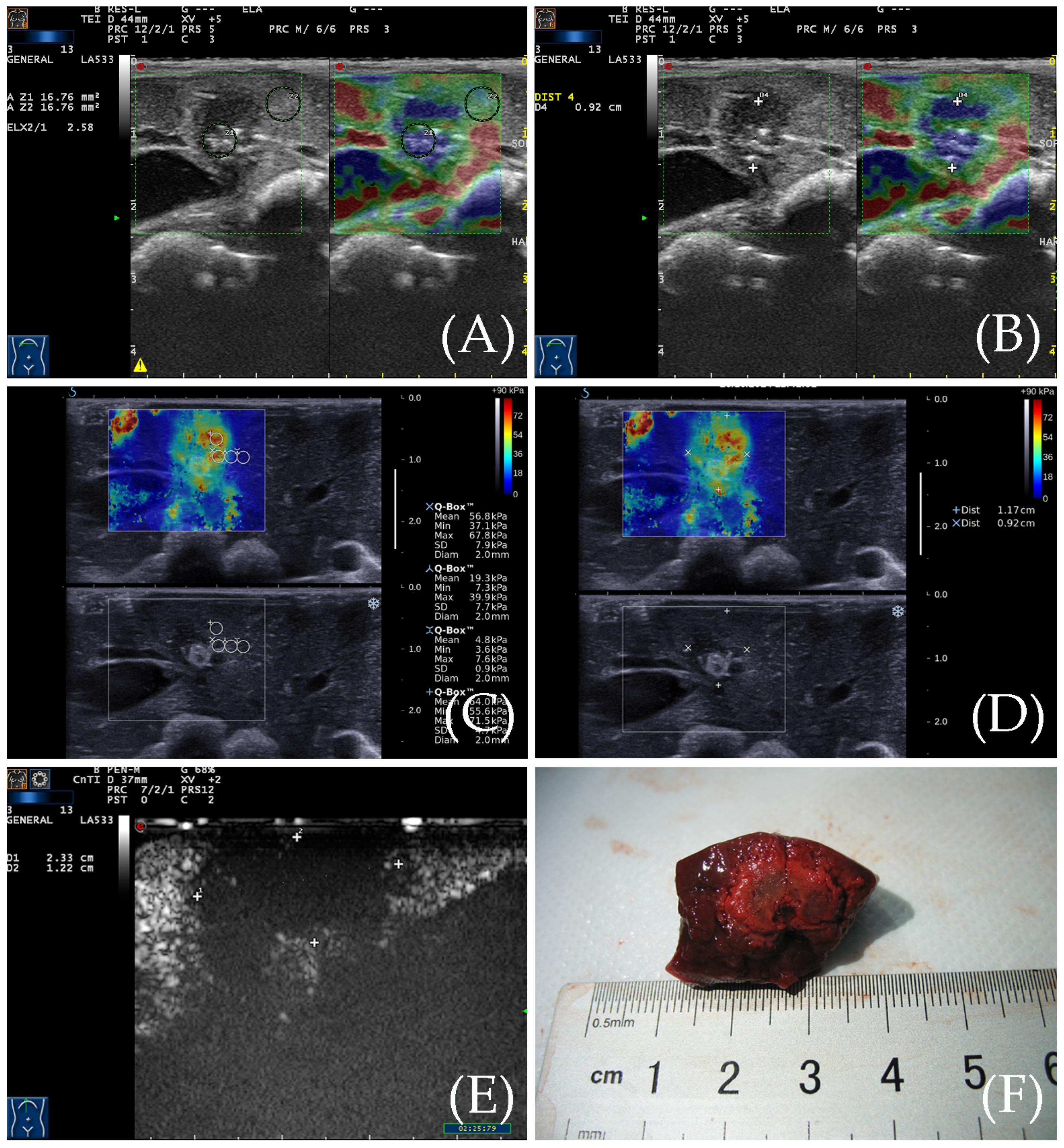
3.3. SWE for the Detection of the Ablation Zone
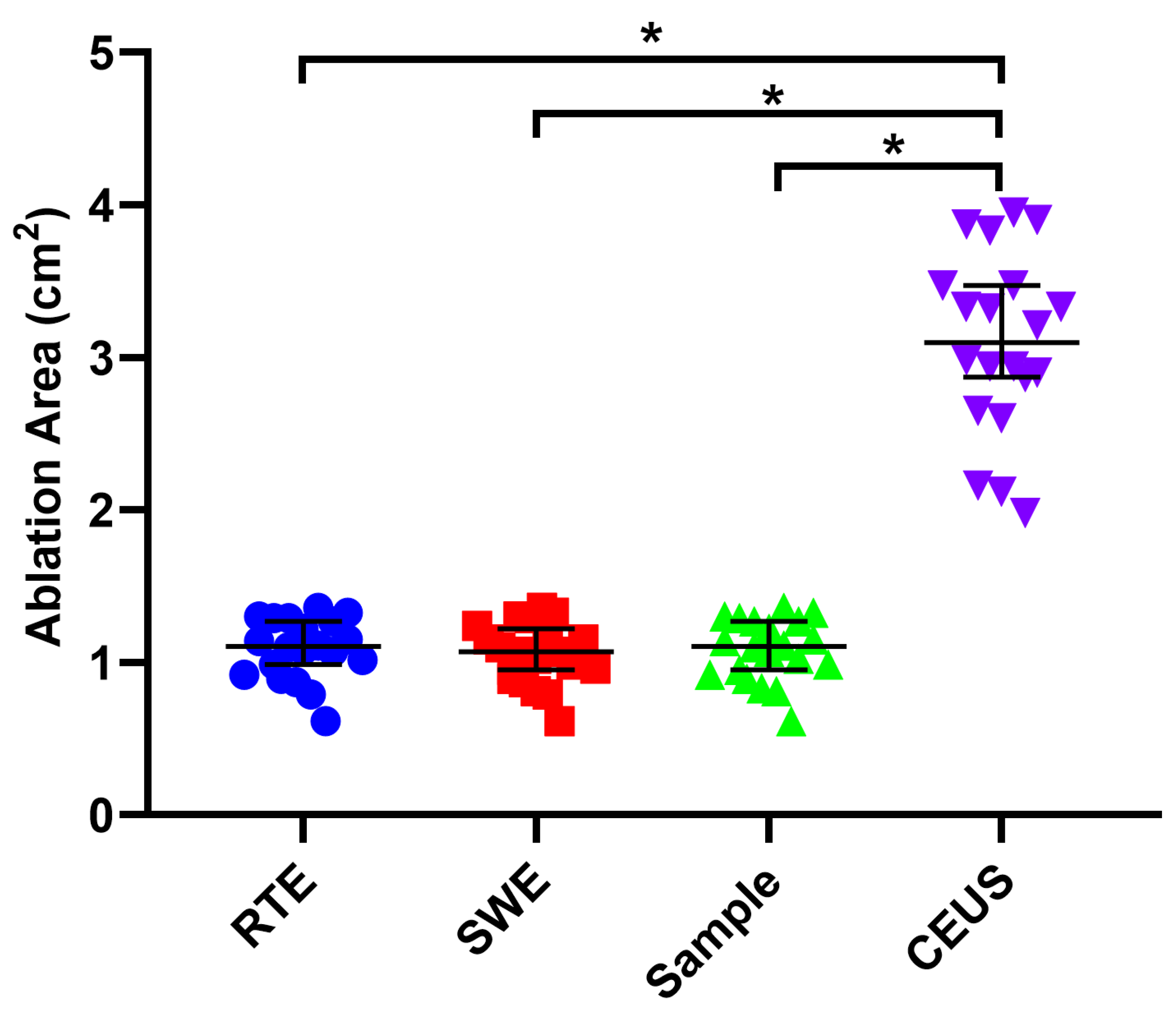
3.4. Contrast-Enhanced Ultrasound Examination
3.5. Gross Specimen Observation and Comparison of the Three Imaging Methods
3.6. Comparison of Two Elastic Imaging Methods for the Detection of the Edge of the Ablation Lesions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Morita, M.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Ida, H.; Ueshima, K.; Nishida, N.; Kudo, M. Can the Entire Ablative Hyperechoic Zone be Regarded as a Necrotic Lesion after Radiofrequency Ablation of the Liver? Ultrasound Med. Biol. 2021, 47, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Savsani, E.; Chong, W.; Eisenbrey, J.; Lyshchik, A. Meta-analysis and systematic review of contrast-enhanced ultrasound in evaluating the treatment response after locoregional therapy of hepatocellular carcinoma. Abdom. Imaging 2021, 46, 5162–5179. [Google Scholar] [CrossRef] [PubMed]
- DeWall, R.J.; Varghese, T.; Madsen, E.L. Shear Wave Velocity Imaging Using Transient Electrode Perturbation: Phantom and ex vivo Validation. IEEE Trans. Med. Imaging 2010, 30, 666–678. [Google Scholar] [CrossRef] [PubMed]
- DeWall, R.J.; Varghese, T.; Brace, C.L. Quantifying Local Stiffness Variations in Radiofrequency Ablations with Dynamic Indentation. IEEE Trans. Biomed. Eng. 2011, 59, 728–735. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M.; Yoon, J.-H.; Kim, Y.J.; Yu, S.J.; Han, J.K. Liver Stiffness Measured by Two-Dimensional Shear-Wave Elastography: Prognostic Value after Radiofrequency Ablation for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 65–75. [Google Scholar] [CrossRef]
- Furuichi, Y.; Abe, M.; Yoshimasu, Y.; Takeuchi, H.; Itoi, T. Liver and spleen stiffness on ultrasound elastography are predictors of the occurrence of esophagogastric varices after balloon-occluded retrograde transvenous obliteration. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 713–722. [Google Scholar] [CrossRef]
- Cantisani, V.; De Silvestri, A.; Scotti, V.; Fresilli, D.; Tarsitano, M.G.; Polti, G.; Guiban, O.; Polito, E.; Pacini, P.; Durante, C.; et al. US-Elastography with Different Techniques for Thyroid Nodule Characterization: Systematic Review and Meta-analysis. Front. Oncol. 2022, 12, 845549. [Google Scholar] [CrossRef]
- Pillai, A.; Voruganti, T.; Barr, R.; Langdon, J. Diagnostic Accuracy of Shear-Wave Elastography for Breast Lesion Characterization in Women: A Systematic Review and Meta-Analysis. J. Am. Coll. Radiol. 2022, 19, 625–634. [Google Scholar] [CrossRef]
- Pareek, G.; Wilkinson, E.R.; Bharat, S.; Varghese, T.; Laeseke, P.F.; Lee, F.T., Jr.; Warner, T.F.; Zagzebski, J.A.; Zagzebski, J.A. Elastographic measurements of in-vivo radiofrequency ablation lesions of the kidney. J. Endourol. 2006, 20, 959–964. [Google Scholar] [CrossRef]
- Zhang, M.; Castaneda, B.; Christensen, J.; Saad, W.; Bylund, K.; Hoyt, K.; Strang, J.G.; Rubens, D.J.; Parker, K.J. Real-time sonoelastography of hepatic thermal lesions in a swine model. Med. Phys. 2008, 35, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Praktiknjo, M.; Krabbe, V.; Pohlmann, A.; Sampels, M.; Jansen, C.; Meyer, C.; Strassburg, C.P.; Trebicka, J.; Carmona, M.A.G. Evolution of nodule stiffness might predict response to local ablative therapy: A series of patients with hepatocellular carcinoma. PLoS ONE 2018, 13, e0192897. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, P.; Zhang, T.; Zheng, J.; Li, S.; Zeng, J.; Kudo, M.; Zheng, R. Comparison of Two-Dimensional Shear Wave Elastography and Real-Time Tissue Elastography for Assessing Liver Fibrosis in Chronic Hepatitis B. Dig. Dis. 2016, 34, 640–649. [Google Scholar] [CrossRef]
- Solbiati, L.; Ierace, T.; Goldberg, S.N.; Sironi, S.; Livraghi, T.; Fiocca, R.; Servadio, G.; Rizzatto, G.; Mueller, P.R.; Del Maschio, A.; et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: Treatment and follow-up in 16 patients. Radiology 1997, 202, 195–203. [Google Scholar] [CrossRef]
- Huang, J.; Liu, B.; Lin, M.; Zhang, X.; Zheng, Y.; Xie, X.; Xu, M.; Xie, X. Ultrasound-guided percutaneous radiofrequency ablation in treatment of neuroendocrine tumor liver metastases: A single-center experience. Int. J. Hyperth. 2022, 39, 497–503. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Walovitch, R.C.; Straub, J.A.; Shore, M.T.; Gazelle, G.S. Radio-frequency-induced Coagulation Necrosis in Rabbits: Immediate Detection at US with a Synthetic Microsphere Contrast Agent. Radiology 1999, 213, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, X.-Y.; Liu, G.-J.; Xu, H.-X.; Xu, Z.-F.; Huang, G.-L.; Chen, P.-F.; Luo, J.; Lü, M.-D. The application value of contrast-enhanced ultrasound in the differential diagnosis of pancreatic solid-cystic lesions. Eur. J. Radiol. 2012, 81, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, G.; Ye, J.; Xu, M.; Cong, L.; He, X.; Huang, T.; Kuang, M.; Xie, X. 3-D Contrast-Enhanced Ultrasound Fusion Imaging: A New Technique to Evaluate the Ablative Margin of Radiofrequency Ablation for Hepatocellular Carcinoma. Ultrasound Med. Biol. 2019, 45, 1933–1943. [Google Scholar] [CrossRef]
- Faccia, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers 2022, 14, 481. [Google Scholar] [CrossRef]
- Nishigaki, Y.; Hayashi, H.; Tomita, E.; Suzuki, Y.; Watanabe, N.; Watanabe, S.; Watanabe, C.; Takagi, Y.; Kato, T.; Naiki, T. Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol. Res. 2014, 45, 432–440. [Google Scholar] [CrossRef]
- Xu, E.; Long, Y.; Li, K.; Zeng, Q.; Tan, L.; Luo, L.; Huang, Q.; Zheng, R. Comparison of CT/MRI-CEUS and US-CEUS fusion imaging techniques in the assessment of the thermal ablation of liver tumors. Int. J. Hyperth. 2018, 35, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Morrow, K.W.; Raman, S.S.; McWilliams, J.P.; Sayre, J.W.; Lu, D.S. CT-monitored minimal ablative margin control in single-session microwave ablation of liver tumors: An effective strategy for local tumor control. Eur. Radiol. 2022, 32, 6327–6335. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Tian, W.; Xu, M.; Lin, M.; Zhuang, B.; Huang, T.; Ye, J.; Lv, M.; Xie, X. Performance of Shear Wave Elastography in Delineating the Radiofrequency Ablation Boundary: An in Vivo experiment. Ultrasound Med. Biol. 2019, 45, 1324–1330. [Google Scholar] [CrossRef]
- Tang, G.-X.; Xiao, X.-Y.; Xu, X.-L.; Yang, H.-Y.; Cai, Y.-C.; Liu, X.-D.; Tian, J.; Luo, B.-M. Diagnostic value of ultrasound elastography for differentiation of benign and malignant axillary lymph nodes: A meta-analysis. Clin. Radiol. 2020, 75, 481.e9–481.e16. [Google Scholar] [CrossRef] [PubMed]
- Rempp, H.; Hoffmann, R.; Roland, J.; Buck, A.; Kickhefel, A.; Claussen, C.D.; Pereira, P.L.; Schick, F.; Clasen, S. Threshold-based prediction of the coagulation zone in sequential temperature mapping in MR-guided radiofrequency ablation of liver tumours. Eur. Radiol. 2011, 22, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, S.; Lee, S.-K.; Cheon, B.; Hong, S.; Cho, H.; Park, J.-G.; Alfajaro, M.M.; Cho, K.-O.; Woo, D.; et al. Comparison of elastography, contrast-enhanced ultrasonography, and computed tomography for assessment of lesion margin after radiofrequency ablation in livers of healthy dogs. Am. J. Vet.-Res. 2017, 78, 295–304. [Google Scholar] [CrossRef]
- Luo, C.; Li, T.; Li, Z.; Zuo, Y.; He, G.; Lin, J.; Liu, G.; Dai, L. Evaluation of Microwave Ablation Efficacy by Strain Elastography and Shear Wave Elastography in ex Vivo Porcine Liver. Ultrasound Med. Biol. 2021, 47, 2636–2645. [Google Scholar] [CrossRef]
- Bo, X.W.; Li, X.L.; Xu, H.X.; Guo, L.H.; Li, D.D.; Liu, B.J.; Xu, X.H. 2D shear-wave ultrasound elastography (SWE) evaluation of ablation zone following radiofrequency ablation of liver lesions: Is it more accurate? Br. J. Radiol. 2016, 89, 20150852. [Google Scholar] [CrossRef]
- Tian, W.-S.; Lin, M.-X.; Zhou, L.-Y.; Pan, F.-S.; Huang, G.-L.; Wang, W.; Lu, M.-D.; Xie, X.-Y. Maximum Value Measured by 2-D Shear Wave Elastography Helps in Differentiating Malignancy from Benign Focal Liver Lesions. Ultrasound Med. Biol. 2016, 42, 2156–2166. [Google Scholar] [CrossRef]
- Lee, D.; Park, S.; Ang, M.J.C.; Park, J.-G.; Yoon, S.; Kim, C.; Lee, S.-K.; Cho, K.-O.; Choi, J. Evaluation of liver lesions by use of shear wave elastography and computed tomography perfusion imaging after radiofrequency ablation in clinically normal dogs. Am. J. Vet.-Res. 2018, 79, 1140–1149. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Li, X.; Liao, W.; Wu, W.; Xu, M. Real-Time Elastography versus Shear Wave Elastography on Evaluating the Timely Radiofrequency Ablation Effect of Rabbit Liver: A Preliminary Experimental Study. Diagnostics 2023, 13, 1145. https://doi.org/10.3390/diagnostics13061145
Shi L, Li X, Liao W, Wu W, Xu M. Real-Time Elastography versus Shear Wave Elastography on Evaluating the Timely Radiofrequency Ablation Effect of Rabbit Liver: A Preliminary Experimental Study. Diagnostics. 2023; 13(6):1145. https://doi.org/10.3390/diagnostics13061145
Chicago/Turabian StyleShi, Li, Xiaoju Li, Wei Liao, Wenxin Wu, and Ming Xu. 2023. "Real-Time Elastography versus Shear Wave Elastography on Evaluating the Timely Radiofrequency Ablation Effect of Rabbit Liver: A Preliminary Experimental Study" Diagnostics 13, no. 6: 1145. https://doi.org/10.3390/diagnostics13061145
APA StyleShi, L., Li, X., Liao, W., Wu, W., & Xu, M. (2023). Real-Time Elastography versus Shear Wave Elastography on Evaluating the Timely Radiofrequency Ablation Effect of Rabbit Liver: A Preliminary Experimental Study. Diagnostics, 13(6), 1145. https://doi.org/10.3390/diagnostics13061145




