The Effects of Positive End Expiratory Pressure and Lung Volume on Diaphragm Thickness and Thickening
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Protocol
2.3. Measurements
2.4. Ultrasonographic Measurements
2.5. Statistical Analysis
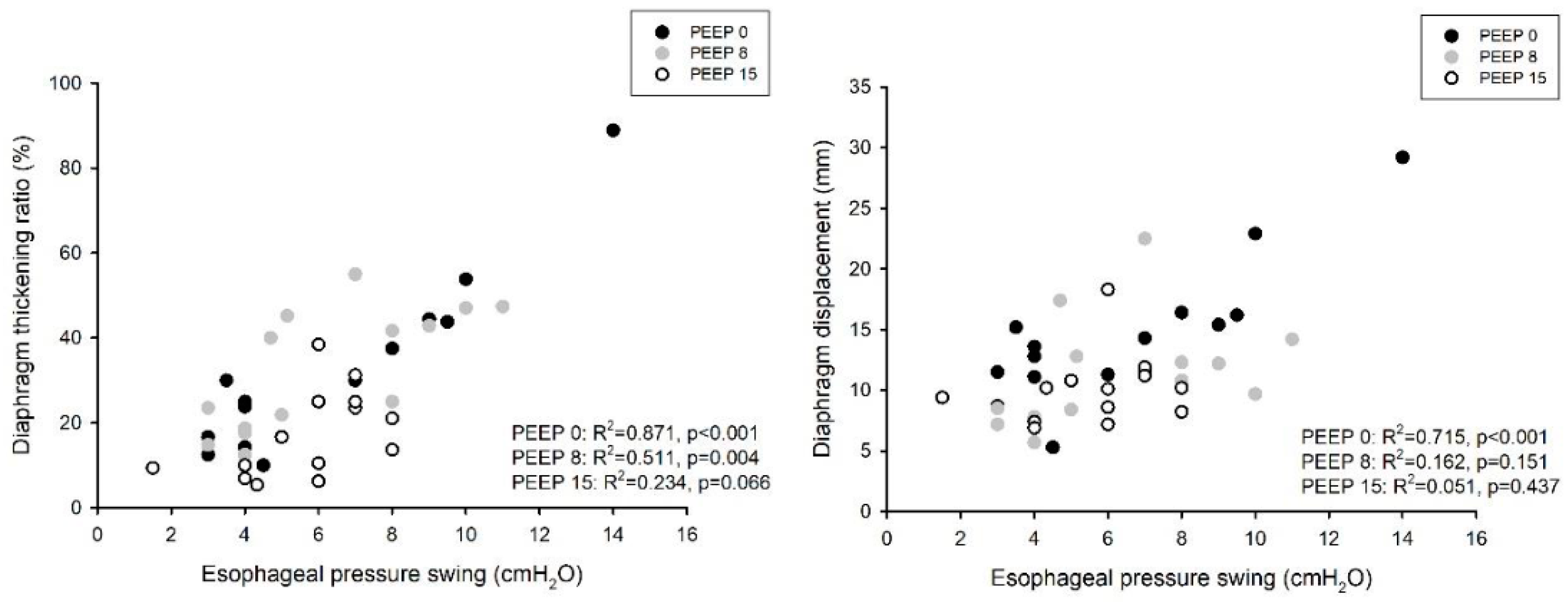
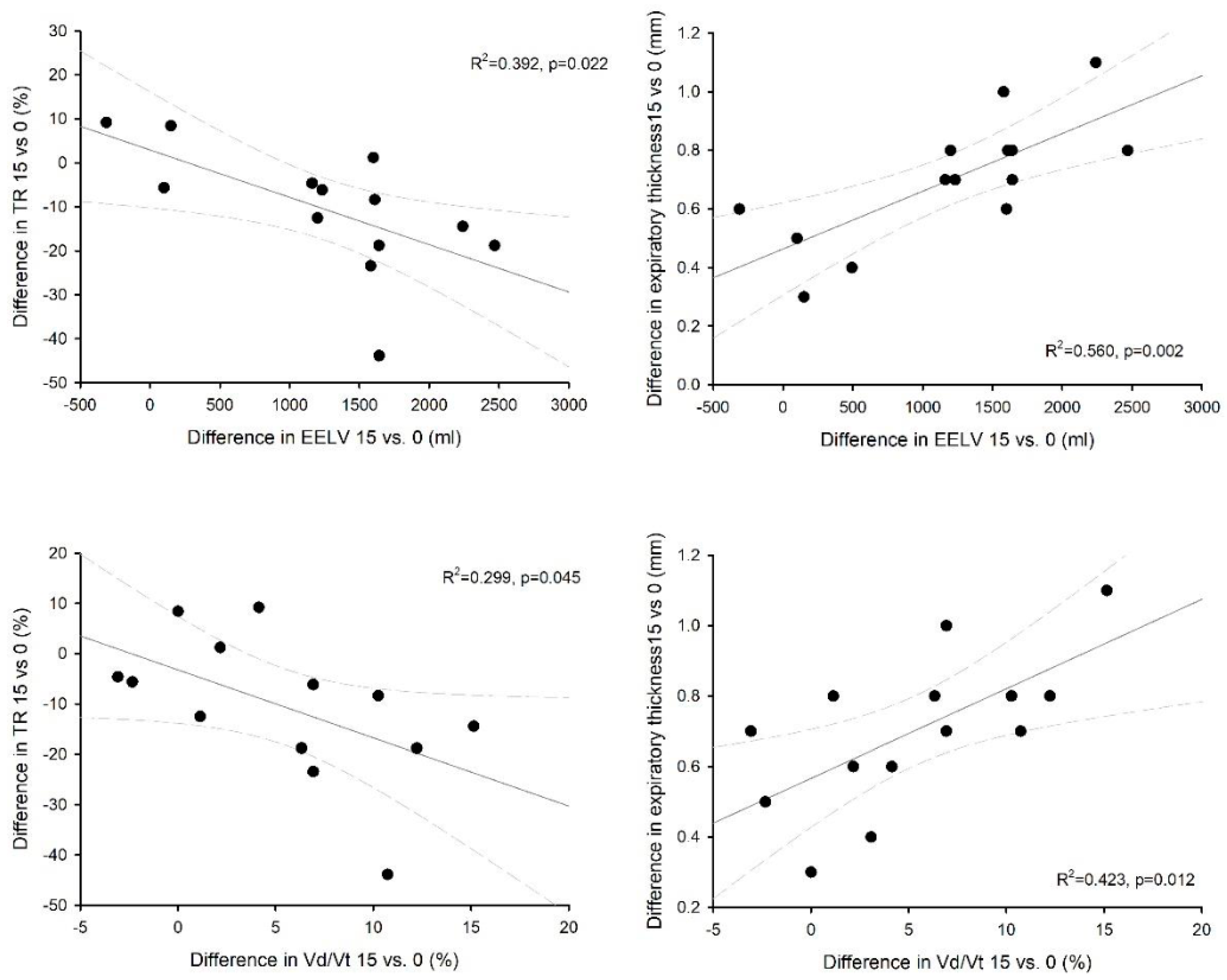
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haaksma, M.E.; Smit, J.M.; Boussuges, A.; Demoule, A.; Dres, M.; Ferrari, G.; Formenti, P.; Goligher, E.C.; Heunks, L.; Lim, E.H.T.; et al. EXpert Consensus On Diaphragm UltraSonography in the Critically Ill (EXODUS): A Delphi Consensus Statement on the Measurement of Diaphragm Ultrasound-Derived Parameters in a Critical Care Setting. Crit. Care 2022, 26, 99. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.; Greco, M.; Bocchino, S.; Cabrini, L.; Beccaria, P.F.; Zangrillo, A. Assessment of Diaphragmatic Dysfunction in the Critically Ill Patient with Ultrasound: A Systematic Review. Intensive Care Med. 2017, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Vorona, S.; Sklar, M.C.; Rittayamai, N.; Lanys, A.; et al. Mechanical Ventilation-Induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Fan, E.; Herridge, M.S.; Murray, A.; Vorona, S.; Brace, D.; Rittayamai, N.; Lanys, A.; Tomlinson, G.; Singh, J.M.; et al. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am. J. Respir. Crit. Care Med. 2015, 192, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Sklar, M.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Rittayamai, N.; Harhay, M.O.; Reid, W.D.; Tomlinson, G.; et al. Association of Low Baseline Diaphragm Muscle Mass With Prolonged Mechanical Ventilation and Mortality Among Critically Ill Adults. JAMA Netw. Open 2020, 3, e1921520. [Google Scholar] [CrossRef]
- Vivier, E.; Mekontso Dessap, A.; Dimassi, S.; Vargas, F.; Lyazidi, A.; Thille, A.W.; Brochard, L. Diaphragm Ultrasonography to Estimate the Work of Breathing during Non-Invasive Ventilation. Intensive Care Med. 2012, 38, 796–803. [Google Scholar] [CrossRef]
- Umbrello, M.; Formenti, P.; Longhi, D.; Galimberti, A.; Piva, I.; Pezzi, A.; Mistraletti, G.; Marini, J.J.; Iapichino, G. Diaphragm Ultrasound as Indicator of Respiratory Effort in Critically Ill Patients Undergoing Assisted Mechanical Ventilation: A Pilot Clinical Study. Crit. Care 2015, 19, 161. [Google Scholar] [CrossRef]
- Umbrello, M.; Formenti, P.; Lusardi, A.C.; Guanziroli, M.; Caccioppola, A.; Coppola, S.; Chiumello, D. Oesophageal Pressure and Respiratory Muscle Ultrasonographic Measurements Indicate Inspiratory Effort during Pressure Support Ventilation. Br. J. Anaesth. 2020, 125, e148–e157. [Google Scholar] [CrossRef]
- Cardenas, L.Z.; Santana, P.V.; Caruso, P.; Ribeiro de Carvalho, C.R.; Pereira de Albuquerque, A.L. Diaphragmatic Ultrasound Correlates with Inspiratory Muscle Strength and Pulmonary Function in Healthy Subjects. Ultrasound Med. Biol. 2018, 44, 786–793. [Google Scholar] [CrossRef]
- Tuinman, P.R.; Jonkman, A.H.; Dres, M.; Shi, Z.-H.; Goligher, E.C.; Goffi, A.; de Korte, C.; Demoule, A.; Heunks, L. Respiratory Muscle Ultrasonography: Methodology, Basic and Advanced Principles and Clinical Applications in ICU and ED Patients-a Narrative Review. Intensive Care Med. 2020, 46, 594–605. [Google Scholar] [CrossRef]
- Matamis, D.; Soilemezi, E.; Tsagourias, M.; Akoumianaki, E.; Dimassi, S.; Boroli, F.; Richard, J.-C.M.; Brochard, L. Sonographic Evaluation of the Diaphragm in Critically Ill Patients. Technique and Clinical Applications. Intensive Care Med. 2013, 39, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.-P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS Statement on Respiratory Muscle Testing at Rest and during Exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef] [PubMed]
- Oppersma, E.; Hatam, N.; Doorduin, J.; van der Hoeven, J.G.; Marx, G.; Goetzenich, A.; Fritsch, S.; Heunks, L.M.A.; Bruells, C.S. Functional Assessment of the Diaphragm by Speckle Tracking Ultrasound during Inspiratory Loading. J. Appl. Physiol. 2017, 123, 1063–1070. [Google Scholar] [CrossRef]
- Poulard, T.; Bachasson, D.; Fossé, Q.; Niérat, M.-C.; Hogrel, J.-Y.; Demoule, A.; Gennisson, J.-L.; Dres, M. Poor Correlation between Diaphragm Thickening Fraction and Transdiaphragmatic Pressure in Mechanically Ventilated Patients and Healthy Subjects. Anesthesiology 2022, 136, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Yoshida, T.; Bellani, G.; Goligher, E.C.; Carteaux, G.; Rittayamai, N.; Mojoli, F.; Chiumello, D.; Piquilloud, L.; Grasso, S.; et al. Esophageal and Transpulmonary Pressure in the Clinical Setting: Meaning, Usefulness and Perspectives. Intensive Care Med. 2016, 42, 1360–1373. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.; Petrof, B.J. Ventilator-Induced Diaphragmatic Dysfunction. Am. J. Respir. Crit. Care Med. 2004, 169, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, V.; Proklou, A.; Vaporidi, K. Lung and Diaphragm Protective Ventilation: A Synthesis of Recent Data. Expert Rev. Respir. Med. 2022, 16, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Dres, M.; Demoule, A. Diaphragm Dysfunction during Weaning from Mechanical Ventilation: An Underestimated Phenomenon with Clinical Implications. Crit. Care 2018, 22, 73. [Google Scholar] [CrossRef]
- Gattinoni, L.; Collino, F.; Maiolo, G.; Rapetti, F.; Romitti, F.; Tonetti, T.; Vasques, F.; Quintel, M. Positive End-Expiratory Pressure: How to Set It at the Individual Level. Ann. Transl. Med. 2017, 5, 288. [Google Scholar] [CrossRef]
- Sahetya, S.K.; Goligher, E.C.; Brower, R.G. Fifty Years of Research in ARDS.Setting Positive End-Expiratory Pressure in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1429–1438. [Google Scholar] [CrossRef]
- Jansen, D.; Jonkman, A.H.; de Vries, H.J.; Wennen, M.; Elshof, J.; Hoofs, M.A.; van den Berg, M.; de Man, A.M.E.; Keijzer, C.; Scheffer, G.-J.; et al. Positive End-Expiratory Pressure Affects Geometry and Function of the Human Diaphragm. J. Appl. Physiol. 2021, 131, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Laghi, F.; Detsky, M.E.; Farias, P.; Murray, A.; Brace, D.; Brochard, L.J.; Bolz, S.-S.; Rubenfeld, G.D.; Kavanagh, B.P.; et al. Measuring Diaphragm Thickness with Ultrasound in Mechanically Ventilated Patients: Feasibility, Reproducibility and Validity. Intensive Care Med. 2015, 41, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.C.A.; Koyama, Y.; Yoshida, T.; Plens, G.M.; Gomes, S.; Lima, C.A.S.; Ramos, O.P.S.; Pereira, S.M.; Kawaguchi, N.; Yamamoto, H.; et al. High Positive End-Expiratory Pressure Renders Spontaneous Effort Noninjurious. Am. J. Respir. Crit. Care Med. 2018, 197, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Roldan, R.; Beraldo, M.A.; Torsani, V.; Gomes, S.; De Santis, R.R.; Costa, E.L.V.; Tucci, M.R.; Lima, R.G.; Kavanagh, B.P.; et al. Spontaneous Effort During Mechanical Ventilation: Maximal Injury With Less Positive End-Expiratory Pressure. Crit. Care Med. 2016, 44, e678–e688. [Google Scholar] [CrossRef]
- Pengelly, L.D.; Alderson, A.M.; Milic-Emili, J. Mechanics of the Diaphragm. J. Appl. Physiol 1971, 30, 797–805. [Google Scholar] [CrossRef]
- Firstiogusran, A.M.F.; Yoshida, T.; Hashimoto, H.; Iwata, H.; Fujino, Y. Positive End-Expiratory Pressure and Prone Position Alter the Capacity of Force Generation from Diaphragm in Acute Respiratory Distress Syndrome: An Animal Experiment. BMC Anesthesiol. 2022, 22, 373. [Google Scholar] [CrossRef]
- Ueki, J.; De Bruin, P.F.; Pride, N.B. In Vivo Assessment of Diaphragm Contraction by Ultrasound in Normal Subjects. Thorax 1995, 50, 1157–1161. [Google Scholar] [CrossRef]
- Wait, J.L.; Nahormek, P.A.; Yost, W.T.; Rochester, D.P. Diaphragmatic Thickness-Lung Volume Relationship In Vivo. J. Appl. Physiol. 1989, 67, 1560–1568. [Google Scholar] [CrossRef]
- Macklem, P.T.; Gross, D.; Grassino, G.A.; Roussos, C. Partitioning of Inspiratory Pressure Swings between Diaphragm and Intercostal/Accessory Muscles. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 200–208. [Google Scholar] [CrossRef]
- De Troyer, A.; Boriek, A.M. Mechanics of the Respiratory Muscles. Compr. Physiol. 2011, 1, 1273–1300. [Google Scholar] [CrossRef]
- Cho, R.J.; Adams, A.; Ambur, S.; Lunos, S.; Shapiro, R.; Prekker, M.E. Ultrasound Assessment of Diaphragmatic Motion in Subjects With ARDS During Transpulmonary Pressure-Guided PEEP Titration. Respir. Care 2020, 65, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Xiao, H.; Bai, W.; Liang, Y.; Chen, M.; Zhang, S. Two-Dimensional Strain Ultrasound Speckle Tracking as a Novel Approach for the Evaluation of Right Hemidiaphragmatic Longitudinal Deformation. Exp. Ther. Med. 2013, 6, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.-M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal Guidelines: Management of Acute Respiratory Distress Syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef]
- Dubé, B.-P.; Dres, M. Diaphragm Dysfunction: Diagnostic Approaches and Management Strategies. J. Clin. Med. 2016, 5, 113. [Google Scholar] [CrossRef] [PubMed]

| Age (years) | 77 (63; 78) |
| Male sex, N (%) | 9 (64) |
| Height (cm) | 170.1 ± 5.7 |
| Actual body weight (kg) | 76.0 ± 18.9 |
| Ideal body weight (kg) | 63.9 ± 5.7 |
| Body mass index (kg/m2) | 25.7 ± 6.4 |
| Diagnosis: | |
| Pneumonia | 9 (64.4%) |
| Peritonitis | 3 (21.4%) |
| Pancreatitis | 1 (7.1%) |
| Trauma | 1 (7.1%) |
| SOFA score at ICU admission | 7 (4; 8) |
| SAPS II score at ICU admission | 39 (33; 54) |
| Hemoglobin concentration (g/dL) | 10.9 ± 0.9 |
| Heart rate (b/min) | 87 ± 11 |
| Mean arterial blood pressure (mmHg) | 77.5 ± 7.1 |
| FiO2 | 0.4 (0.3; 0.5) |
| PEEP (cmH2O) | 7 (6; 8) |
| Pressure support (cmH2O) | 5 (4; 6) |
| Peripheral oxygen saturation (%) | 97.9 ± 1.3 |
| Respiratory rate (1/min) | 19.7 ± 4.0 |
| PEEP 0 | PEEP 8 | PEEP 15 | p | |
|---|---|---|---|---|
| Respiratory rate (min−1) | 22.1 ± 4.8 | 23.1 ± 4.8 | 22.4 ± 4.0 | 0.5699 |
| pH | 7.47 ± 0.04 | 7.46 ± 0.05 | 7.45 ± 0.03 | 0.1384 |
| PaO2 (mmHg) | 88.5 ± 10.6 | 110.1 ± 19.7 * | 123.3 ± 36.7 *° | 0.0030 |
| PaCO2 (mmHg) | 42.0 ± 4.6 | 43.3 ± 4.2 * | 45.2 ± 4.3 *° | <0.0001 |
| EtCO2 (mmHg) | 38.3 ± 4.7 | 39.2 ± 3.1 | 38.7 ± 3.3 | 0.3850 |
| Δ A-EtCO2 (mmHg) | 3.7 ± 1.6 | 4.1 ± 2.5 * | 6.5 ± 1.9 *° | 0.0010 |
| Vd/Vt alv (%) | 9.0 ± 3.6 | 9.2 ± 5.1 | 14.3 ± 3.3 *° | 0.0026 |
| EELV (mL) | 1066 (660; 2400) | 1537 (1300; 3240) * | 3094 (2111; 3500) *° | <0.0001 |
| P0.1 (cmH2O) | 3.1 ± 1.6 | 2.36 ± 1.27 | 3.35 ± 1.97 | 0.0596 |
| NIF (cmH2O) | 26.7 ± 9.3 | 31.1 ± 11.4 | 27.6 ± 8.0 | 0.7775 |
| Tidal Breathing | PEEP 0 | PEEP 8 | PEEP 15 | p |
|---|---|---|---|---|
| Tidal volume (mL) | 384 ± 79 | 367 ± 96 | 302 ± 81 *° | 0.0073 |
| Displacement (mm) | 14.6 ± 5.8 | 11.2 ± 4.5 * | 10.1 ± 2.8 * | 0.0378 |
| Thickness EI (mm) | 2.21 ± 0.83 | 2.62 ± 0.82 * | 2.77 ± 0.75 *° | 0.0010 |
| Thickness EE (mm) | 1.70 ± 0.72 | 1.98 ± 0.60 * | 2.41 ± 0.75 *° | 0.0001 |
| Thickening fraction (%) | 32.6 ± 20.8 | 32.4 ± 14.4 | 17.4 ± 10.2 * | 0.0152 |
| ΔPes (cmH2O) | 6.4 ± 3.3 | 6.1 ± 2.7 | 5.7 ± 1.8 | 0.6281 |
| Maximal breathing | PEEP 0 | PEEP 8 | PEEP 15 | p |
| Tidal volume (ml) | 905 ± 278 # | 813 ± 227 # | 667 ± 202 *# | 0.0240 |
| Displacement (mm) | 20.4 ± 12.9 | 28.9 ± 19.6 # | 23.3 ± 14.8 # | 0.0795 |
| Thickness EI (mm) | 2.63 ± 0.94 # | 3.01 ± 1.09 *# | 3.48 ± 1.23 *°# | 0.0152 |
| Thickness EE (mm) | 1.79 ± 0.79 | 2.01 ± 0.57 * | 2.42 ± 0.58 *° | 0.0010 |
| Thickening fraction (%) | 53.8 ± 28.4 # | 63.5 ± 26.1 # | 42.2 ± 28.2 # | 0.2684 |
| ΔPes (cmH2O) | 10.9 ± 4.8 # | 11.2 ± 5.3 # | 11.4 ± 4.3 # | 0.9538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formenti, P.; Miori, S.; Galimberti, A.; Umbrello, M. The Effects of Positive End Expiratory Pressure and Lung Volume on Diaphragm Thickness and Thickening. Diagnostics 2023, 13, 1157. https://doi.org/10.3390/diagnostics13061157
Formenti P, Miori S, Galimberti A, Umbrello M. The Effects of Positive End Expiratory Pressure and Lung Volume on Diaphragm Thickness and Thickening. Diagnostics. 2023; 13(6):1157. https://doi.org/10.3390/diagnostics13061157
Chicago/Turabian StyleFormenti, Paolo, Sara Miori, Andrea Galimberti, and Michele Umbrello. 2023. "The Effects of Positive End Expiratory Pressure and Lung Volume on Diaphragm Thickness and Thickening" Diagnostics 13, no. 6: 1157. https://doi.org/10.3390/diagnostics13061157
APA StyleFormenti, P., Miori, S., Galimberti, A., & Umbrello, M. (2023). The Effects of Positive End Expiratory Pressure and Lung Volume on Diaphragm Thickness and Thickening. Diagnostics, 13(6), 1157. https://doi.org/10.3390/diagnostics13061157








