Correction of Local Brain Temperature after Severe Brain Injury Using Hypothermia and Medical Microwave Radiometry (MWR) as Companion Diagnostics
Abstract
1. Introduction
2. Correction of Brain Temperature Balance Disruption in Patients with Severe Brain Injuries
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piradov, M.A.; Suponeva, N.; Voznyuk, I.; Kondratiev, A.; Shchegolev, A.; Belkin, A.; Zaitsev, O.; Pryanikov, I.; Petrova, M.V.; Ivanova, N.E.; et al. Russian working group on problems of chronic disorders of consciousness. Chronic disorders of consciousness: Terminology and diagnostic criteria. The results of the first meeting of the Russian working group on the problems of chronic disorders of consciousness. Ann. Clin. Exp. Neurol. 2020, 14, 5–16. [Google Scholar] [CrossRef]
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Nicholas, D.; Schiffet, N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Piradov, M.V.; Suponeva, N.A.; Sergeev, D.V.; Chervyakov, A.V.; Ryabinkina, Y.V.; Kremneva, E.I.; Morozova, S.N.; Yazeva, E.A.; Legostaeva, L.A. Structural and functional bases of chronic disorders of consciousness. Ann. Clin. Exp. Neurol. 2018, 12, 6–15. [Google Scholar] [CrossRef]
- Mochalova, E.G.; Legostaeva, L.; Zimin, A.; Yusupova, D.; Sergeev, D.; Ryabinkina, Y.V.; Bodin, E.; Suponeva, N.; Piradov, M. The Russian version of the revised coma recovery scale is a standardized method for assessing patients with chronic impairment of consciousness. J. Neurol. Psychiatry 2018, 3, 25–31. [Google Scholar] [CrossRef]
- Kondziellaa, D.; Benderd, A.; Diserensf, K.; Erpg, W.; Estraneoi, A.; Formisanok, R.; Laureysg, S.; Naccachel, L.; Ozturkn, S.; Rohautl, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef]
- Michael, H. Marino, John Whyte Treatment Trials in Disorders of Consciousness: Challenges and Future Directions. Brain Sci. 2022, 12, 569. [Google Scholar] [CrossRef]
- van Erp, W.S.; Lavrijsen, J.C.; Vos, P.E.; Bor, H.; Laureys, S.; Koopmans, R.T. The Vegetative State: Prevalence, Misdiagnosis, and Treatment Limitations. J. Am. Med. Dir. Assoc. 2015, 16, 85.e9–85.e14. [Google Scholar] [CrossRef]
- Chudy, D.; Deletis, V.; Almahariq, F.; Marčinković, P.; Škrlin, J.; Paradžik, V. Deep brain stimulation for the early treatment of the minimally conscious state and vegetative state: Experience in 14 patients. J. Neurosurg. 2018, 128, 1189–1198. [Google Scholar] [CrossRef]
- Thibaut, A.; Schiff, N.; Giacino, J.; Laureys, S.; Gosseries, O. Therapeutic interventions in patients with disorders of consciousness. Lancet Neurol. 2019, 18, 600–614. [Google Scholar] [CrossRef]
- Goryanin, I.; Karbainov, S.; Shevelev, O.; Tarakanov, A.; Redpath, K.; Vesnin, S.; Ivanov, Y. Passive microwave radiometry in biomedical studies. Drug Discov. Today 2020, 25, 757–763. [Google Scholar] [CrossRef]
- Shevelev, O.A.; Grechko, A.V.; Petrova, M.V. Therapeutic Hypothermia. Monograph; RUDN University: Moscow, Russia, 2019; 265p, ISBN 978-5-209-09541-5. [Google Scholar]
- Shevelev, O.; Petrova, M.; Smolensky, A.; Osmonov, B.; Toimatov, S.; Kharybina, T.; Karbainov, S.; Ovchinnikov, L.; Vesnin, S.; Tarakanov, A.; et al. Using medical microwave radiometry for brain temperature measurements. Drug Discov. Today 2021, 27, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, O.A.; Petrova, M.; Yuriev, M.Y.; Mengistu, E.; Kostenkova, I.; Khodorovich, N.; Zhdanova, M.; Goryanin, S.V.I. The method of microwave radiothermometry in the study of circadian rhythms of brain temperature. Bull. Exp. Biol. Med. 2022, 173, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Anokhin, P.K. Biology and Neurophysiology of the Conditioned Reflex; Meditsina: Moscow, Russia, 1968. [Google Scholar]
- Sudakov, K.V. The theory of functional systems: General postulates and principles of dynamic organization. Integr. Psychol. Behav. Sci. 1997, 32, 392–414. [Google Scholar] [CrossRef] [PubMed]
- Luria, A.R. Human Brain and Mental Processes; Harper & Row: New York, NY, USA, 1970; pp. 16–18. [Google Scholar]
- Zozulya, S.A.; Shevelev, O.A.; Tikhonov, D.V.; Simonov, A.N.; Kaleda, V.G.; Klyushnik, T.P.; Petrova, M.V.; Mengistu, E.M. Brain heat balance and markers of inflammatory response in patients with schizophrenia. Bull. Exp. Biol. Med. 2022, 173, 522–526. [Google Scholar] [CrossRef]
- Shevelev, O.A.; Butrov, A.V.; Cheboksary, D.V.; Khodorovich, N.A.; Lapaev, N.N.; Pokatilova, N.C. Pathogenetic Role of Cerebral hyperthermia in brain lesions clinical medicine. Russ. J. 2017. [Google Scholar] [CrossRef]
- Gavin, D.; Perkins; Gräsner, J.-T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; Van de Voorde, P.; Madar, J.; Zideman, D.; et al. European Resuscitation Council Guidelines 2021: Executive summary. Resuscitation 2021, 161, 1–60. [Google Scholar] [CrossRef]
- Yin, L.; Jiang, H.; Zhao, W.; Li, H. Inducing therapeutic hypothermia via selective brain cooling: A finite element modeling analysis. Med. Biol. Eng. Comput. 2019, 57, 1313–1322. [Google Scholar] [CrossRef]
- Schwartz, A.; Donald, F.; Gilbert, S.; Sander, C.; Edwards Niloo MMongero, L. Delayed Selective Cerebral Hypothermia Decreases Infarct Volume After Reperfused Stroke in Baboons. J. Neurosurg. Anesthesiol. 2011, 23, 124–130. [Google Scholar] [CrossRef]
- Sun, Y.-J.; Zhang, Z.-Y.; Fan, B.; Li, G.-Y. Neuroprotection by Therapeutic Hypothermia. Front. Neurosci. 2019, 13, 586. [Google Scholar] [CrossRef]
- KSchmitt, K.R.L.; Tong, G.; Berger, F. Mechanisms of hypothermia-induced cell protection in the brain. Mol. Cell. Pediatr. 2014, 1, 7. [Google Scholar] [CrossRef]
- Shevelev, O.A.; Petrova, M.V.; Yuriev, M.Y.; Mengistu, E.M. Circadian temperature rhythms of the healthy and damaged brain. J. Neurosci. Neurol. Disord. 2022, 6, 032–033. [Google Scholar] [CrossRef]
- Boyarintsev, V.V.; Zhuravlev, S.V.; Ardashev, V.N.; Shevelev, O.A.; Stulin, I.D.; Sharinova, I.A.; Kalenova, I.E. Features of cerebral blood flow in normal and pathological conditions background of craniocerebral hypothermia. Aerosp. Environ. Med. 2019, 53, 59–64. [Google Scholar] [CrossRef]
- Shevelev, O.A.; Petrova, M.; Grechko, A.; Saidov, S.; Smolenskii, A.; Kondratiev, A.; Tsentsiper, A.; Arzhadeev, A.P.; Gutsalyuk, S.A.; Usmanov, A.G.; et al. Hypothermia of the Brain in the Treatment of Cerebral Lesions. Theory and Practice, Rusines; Rusayns: Moscow, Russia, 2020; p. 230. ISBN 978-5-4365-5880-6. [Google Scholar]
- Kurisu, K.; Kim, J.Y.; You, J.; Midori, A. Therapeutic Hypothermia and Neuroprotection in Acute Neurological Disease. Curr. Med. Chem. 2019, 26, 5430–5455. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Bramlett, H.M. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: Experimental and clinical experience. Brain Circ. 2017, 3, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Rzechorzek, N.M.; Connick, P.; Livesey, M.R.; Borooah, S.; Patani, R.; Burr, K.; Story, D.; Wyllie, D.J.A.; Hardingham, G.E.; Chandran, S. Hypothermic Preconditioning Reverses Tau Ontogenesis in Human Cortical Neurons and is Mimicked by Protein Phosphatase 2A Inhibition. EBioMedicine 2015, 12, 141–154. [Google Scholar] [CrossRef]
- Jackson, T.C.; Kochanek, P.M. A New Vision for Therapeutic Hypothermia in the Era of Targeted Temperature Management: A Speculative Synthesis. Ther. Hypothermia Temp. Manag. 2019, 9, 13–47. [Google Scholar] [CrossRef]
- Yenari, M.A.; Liu, J.; Zheng, Z.; Vexler, Z.S.; Lee, J.E.; Giffard, R.G. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. N. Y. Acad. Sci. 2005, 1053, 74–83. [Google Scholar] [CrossRef]
- Babkina, A.S.; Baeva, A.A.; Bashirova, A.R.; Blagonravov, M.L.; Golubev, A.M.; Grebenchikov, O.A.; Grechko, A.V.; Ershov, A.V.; Zakharchenko, V.E.; Kuzovlev, A.N.; et al. Biological markers of damage and regeneration of the central nervous system. 2021; 432, 43–45. [Google Scholar]
- Tong, G.; Endersfelder, S.; Rosenthal, L.-M.; Wollersheim, S.; Sauer, I.M.; Bührer, C.; Berger, F.; Schmitt, K.R.L. Effects of moderate and deep hypothermia on RNA-binding proteins RBM3 and CIRP expressions in murine hippocampal brain slices. Brain Res. 2013, 1504, 74–84. [Google Scholar] [CrossRef]
- Xia, W.; Su, L.; Jiao, J. Cold-induced protein RBM3 orchestrates neurogenesis via modulating Yap mRNA stability in cold stress. J. Cell Biol. 2018, 217, 3464–3479. [Google Scholar] [CrossRef]
- Liu, J.; Xue, J.; Zhang, H.; Li, S.; Liu, Y.; Xu, D.; Zou, M.; Zhang, Z.; Diao, J. Cloning, expression, and purification of cold inducible RNA-binding protein and its neuroprotective mechanism of action. Brain Res. 2015, 1597, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.Z.; Zhang, W.; Chen, X.; Wang, J.; Chen, J.; Luo, W. Involvement of Cold Inducible RNA-Binding Protein in Severe Hypoxia-Induced Growth Arrest of Neural Stem Cells In Vitro. Mol. Neurobiol. 2017, 54, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Laios, E.; Rebeyka, I.M.; Prody, C.A. Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol. Cell Biochem. 1997, 173, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Neutelings, T.; Lambert, C.A.; Nusgens, B.V.; Colige, A.C. Colige Effects of Mild Cold Shock (25 °C) Followed by Warming Up at 37 °C on the Cellular Stress Response. PLoS ONE 2013, 8, e69687. [Google Scholar] [CrossRef]
- Shintani, Y.; Terao, Y.; Ohta, H. Molecular Mechanisms Underlying Hypothermia-Induced Neuroprotection. Stroke Restarch Treat. 2011, 2011, 809874. [Google Scholar] [CrossRef]
- Terao, Y.; Miyamoto, S.; Hirai, K.; Kamiguchi, H.; Ohta, H.; Shimojo, M.; Kiyota, Y.; Asahi, S.; Sakura, Y.; Shintani, Y. Hypothermia enhances heat-shock protein 70 production in ischemic brains. Neuroreport 2009, 20, 745–749. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, Z.; Tsai, L.K.; Leeds, P.; Fessler, E.B.; Wang, J.; Chuang, D.M. FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol. Psychiatry 2015, 20, 215–223. [Google Scholar] [CrossRef]
- Jackson, T.C.; Manole, M.D.; Kotermanski, S.E.; Jackson, E.K.; Clark, R.S.; Kochanek, P.M. Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience 2015, 305, 268–278. [Google Scholar] [CrossRef]
- Kuroda, M.; Muramatsu, R.; Maedera, N.; Koyama, Y.; Hamaguchi, M.; Fujimura, H.; Yoshida, M.; Konishi, M.; Itoh, N.; Mochizuki, H.; et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J. Clin. Investig. 2017, 127, 3496–3509. [Google Scholar] [CrossRef]
- Chen, J.; Hu, J.; Liu, H.; Xiong, Y.; Zou, Y.; Huang, W.; Shao, M.; Wu, J.; Yu, L.; Wang, X.; et al. FGF21 Protects the Blood-Brain Barrier by Upregulating PPARγ via FGFR1 /β-klotho after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2091–2103. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, N.; Wang, Q.; Yu, Z.; Lin, L.; Yuan, J.; Guo, S.; Ahn, B.J.; Wang, X.J.; Li, X.; et al. Endocrine Regulator rFGF21 (Recombinant Human Fibroblast Growth Factor 21) Improves Neurological Outcomes Following Focal Ischemic Stroke of Type 2 Diabetes Mellitus Male Mice. Stroke 2018, 49, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Braidy, N.; Aminzadeh, M. Protective effects of fibroblast growth factor 21 against amyloid-beta1-42-induced toxicity in SH-SY5Y cells. Neurotox. Res. 2018, 34, 574–583. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Diano, S.; Horvath, T.L. Mitochondrial uncoupling proteins in the CNS: In support of function and survival. Nat. Rev. Neurosci. 2005, 6, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Andrews, Z.B.; Horvath, T.L. Exercise-Induced Synaptogenesis in the Hippocampus Is Dependent on UCP2-Regulated Mitochondrial Adaptation. J. Neurosci. 2008, 28, 10766–10771. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef]
- Asadi, Y.; Gorjipour, F.; Behrouzifar, S.; Vakili, A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018, 43, 1549–1560. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhao, H.; Sung, J.H.; Sun, G.H.; Steinberg, G.K. Hypothermia blocks ischemic changes in ubiquitin distribution and levels following stroke. Neuroreport 2006, 17, 1691–1695. [Google Scholar] [CrossRef]
- Yamashita, K.; Eguchi, Y.; Kajiwara, K.; Ito, H. Mild hypothermia ameliorates ubiquitin synthesis and prevents delayed neuronal death in the gerbil hippocampus. Stroke 1991, 22, 1574–1581. [Google Scholar] [CrossRef]

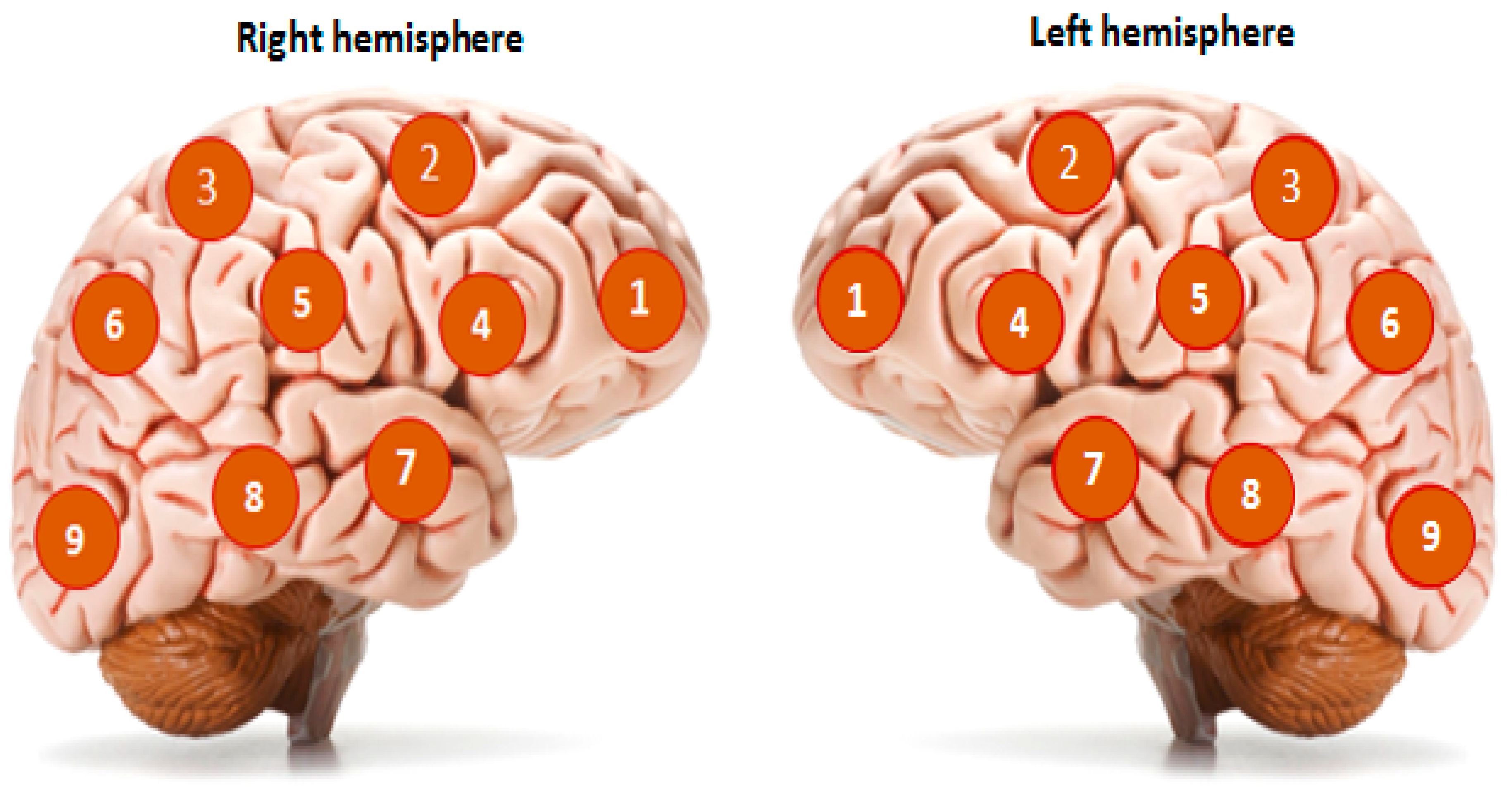

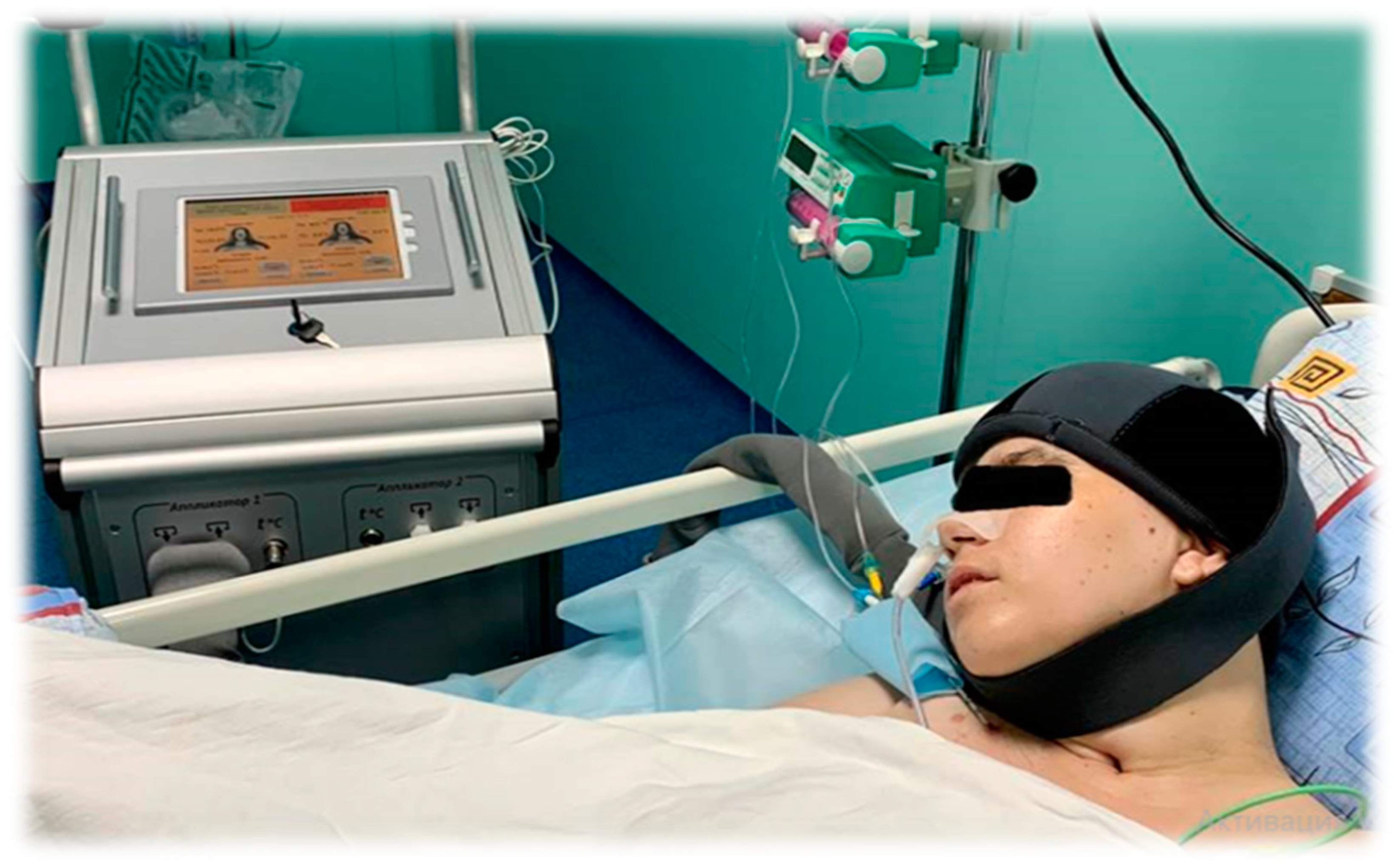


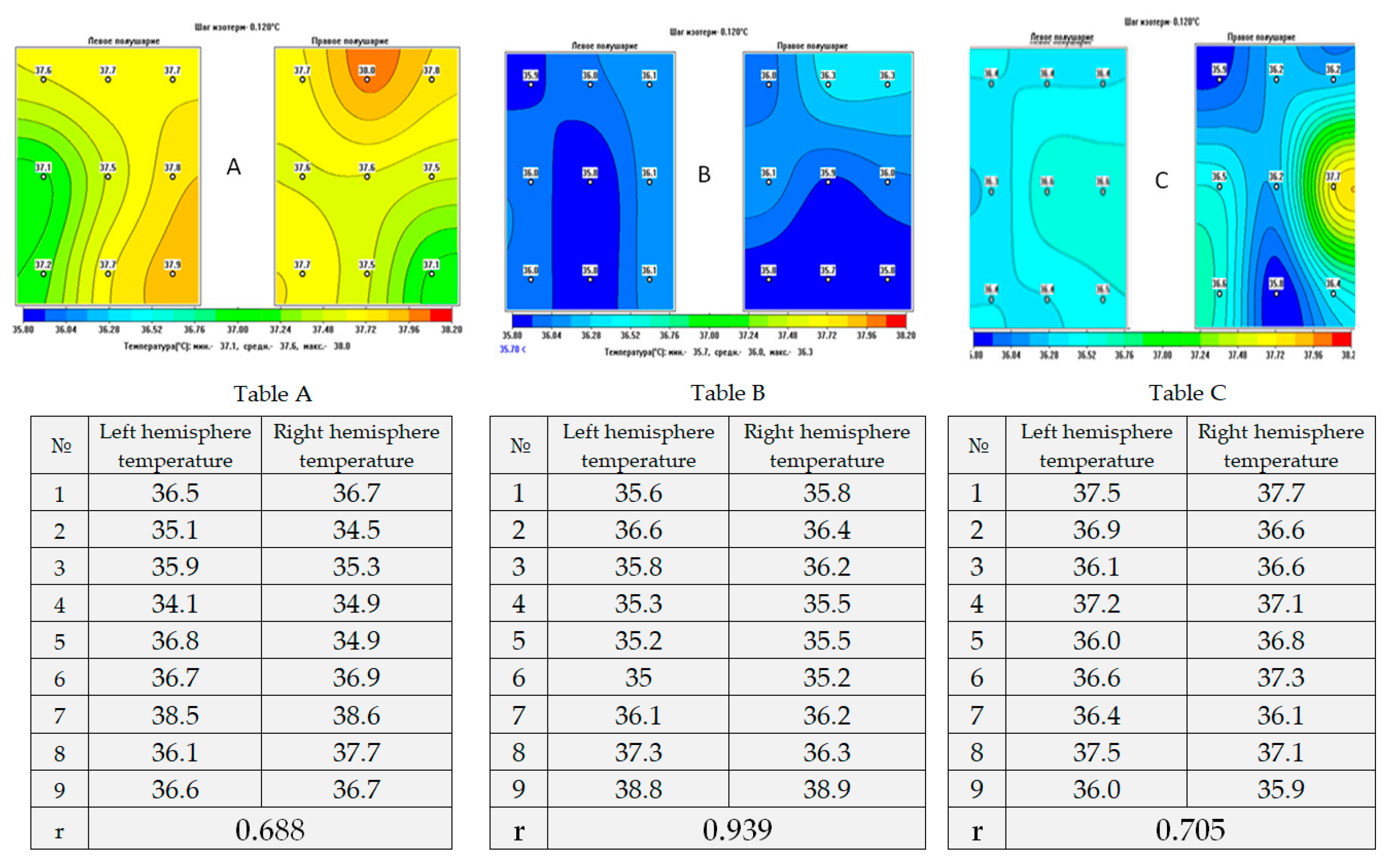
| Functions According to the CRS-R Scale | Main Group | |||
|---|---|---|---|---|
| Subgroup O1 | Subgroup O2 | |||
| 1st Day | 14th Day | 1st Day | 14th Day | |
| Hearing ability | 0.7 ± 0.10 | 1.5 ± 0.18 *** | 2.2 ± 0.23 | 3.3 ± 0.12 *** |
| Visual function | 0.8 ± 0.11 | 1.9 ± 0.23 *** | 2.6 ± 0.31 | 4.1 ± 0.22 *** |
| Mobility | 1.3 ± 0.13 | 2.1 ± 0.24 ** | 3.1 ± 0.31 | 4.8 ± 0.19 *** |
| Speech function | 0.4 ± 0.09 | 0.9 ± 0.13 *** | 0.8 ± 0.15 | 1.8 ± 0.17 *** |
| Communication | 0.1 ± 0.04 | 0.6 ± 0.11 *** | 0.6 ± 0.15 | 1.5 ± 0.11 *** |
| Wakefulness | 1.3 ± 0.11 | 1.8 ± 0.14 *** | 2.1 ± 0.16 | 2.8 ± 0.12 *** |
| Result | 4.5 ± 0.33 | 8.7 ± 0.91 *** | 11.3 ± 1.0 | 18.2 ± 0.70 *** |
| Functions According to the CRS-R Scale | Control Group | |||
|---|---|---|---|---|
| Subgroup C1 | Subgroup C2 | |||
| 1st Day | 14th Day | 1st Day | 14th Day | |
| Hearing ability | 0.7 ± 0.11 | 1.3 ± 0.11 ** | 1.6 ± 0.16 | 1.5 ± 0.19 |
| Visual function | 0.8 ± 0.10 | 1.3 ± 0.10 *** | 1.8 ± 0.16 | 2.1 ± 0.21 |
| Mobility | 1.2 ± 0.15 | 1.7 ± 0.11 ** | 2.3 ± 0.18 | 2.4 ± 0.27 |
| Speech function | 0.2 ± 0.07 | 0.6 ± 0.12 ** | 0.7 ± 0.15 | 1.1 ± 0.17 * |
| Communication | 0.2 ± 0.07 | 0.5 ± 0.12 ** | 0.9 ± 0.15 | 1.0 ± 0.20 |
| Wakefulness | 1.3 ± 0.12 | 1.5 ± 0.13 | 1.8 ± 0.16 | 2.0 ± 0.13 |
| Result | 4.3 ± 0.37 | 6.8 ± 0.49 *** | 9.1 ± 0.57 | 10.1 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevelev, O.A.; Petrova, M.V.; Mengistu, E.M.; Yuriev, M.Y.; Kostenkova, I.Z.; Vesnin, S.G.; Kanarskii, M.M.; Zhdanova, M.A.; Goryanin, I. Correction of Local Brain Temperature after Severe Brain Injury Using Hypothermia and Medical Microwave Radiometry (MWR) as Companion Diagnostics. Diagnostics 2023, 13, 1159. https://doi.org/10.3390/diagnostics13061159
Shevelev OA, Petrova MV, Mengistu EM, Yuriev MY, Kostenkova IZ, Vesnin SG, Kanarskii MM, Zhdanova MA, Goryanin I. Correction of Local Brain Temperature after Severe Brain Injury Using Hypothermia and Medical Microwave Radiometry (MWR) as Companion Diagnostics. Diagnostics. 2023; 13(6):1159. https://doi.org/10.3390/diagnostics13061159
Chicago/Turabian StyleShevelev, Oleg A., Marina V. Petrova, Elias M. Mengistu, Mikhail Y. Yuriev, Inna Z. Kostenkova, Sergey G. Vesnin, Michael M. Kanarskii, Maria A. Zhdanova, and Igor Goryanin. 2023. "Correction of Local Brain Temperature after Severe Brain Injury Using Hypothermia and Medical Microwave Radiometry (MWR) as Companion Diagnostics" Diagnostics 13, no. 6: 1159. https://doi.org/10.3390/diagnostics13061159
APA StyleShevelev, O. A., Petrova, M. V., Mengistu, E. M., Yuriev, M. Y., Kostenkova, I. Z., Vesnin, S. G., Kanarskii, M. M., Zhdanova, M. A., & Goryanin, I. (2023). Correction of Local Brain Temperature after Severe Brain Injury Using Hypothermia and Medical Microwave Radiometry (MWR) as Companion Diagnostics. Diagnostics, 13(6), 1159. https://doi.org/10.3390/diagnostics13061159






