Identifying ITGB2 as a Potential Prognostic Biomarker in Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Microarray Data
2.2. DEGs (Differentially Expressed Genes) Identification
2.3. Pathway Enrichment and Functional Analysis of the DEGs
2.4. Hub Genes Identification
2.5. Hub Genes Prognosis Analysis
2.6. Key Genes Immune Infiltration Analysis
2.7. Immunofluorescence
2.8. Western Blot
2.9. Immunohistochemistry Analysis
2.10. QRT-PCR (Quantitative, Real Time-Polymerase Chain Reaction)
2.11. Statistics Analysis
3. Results
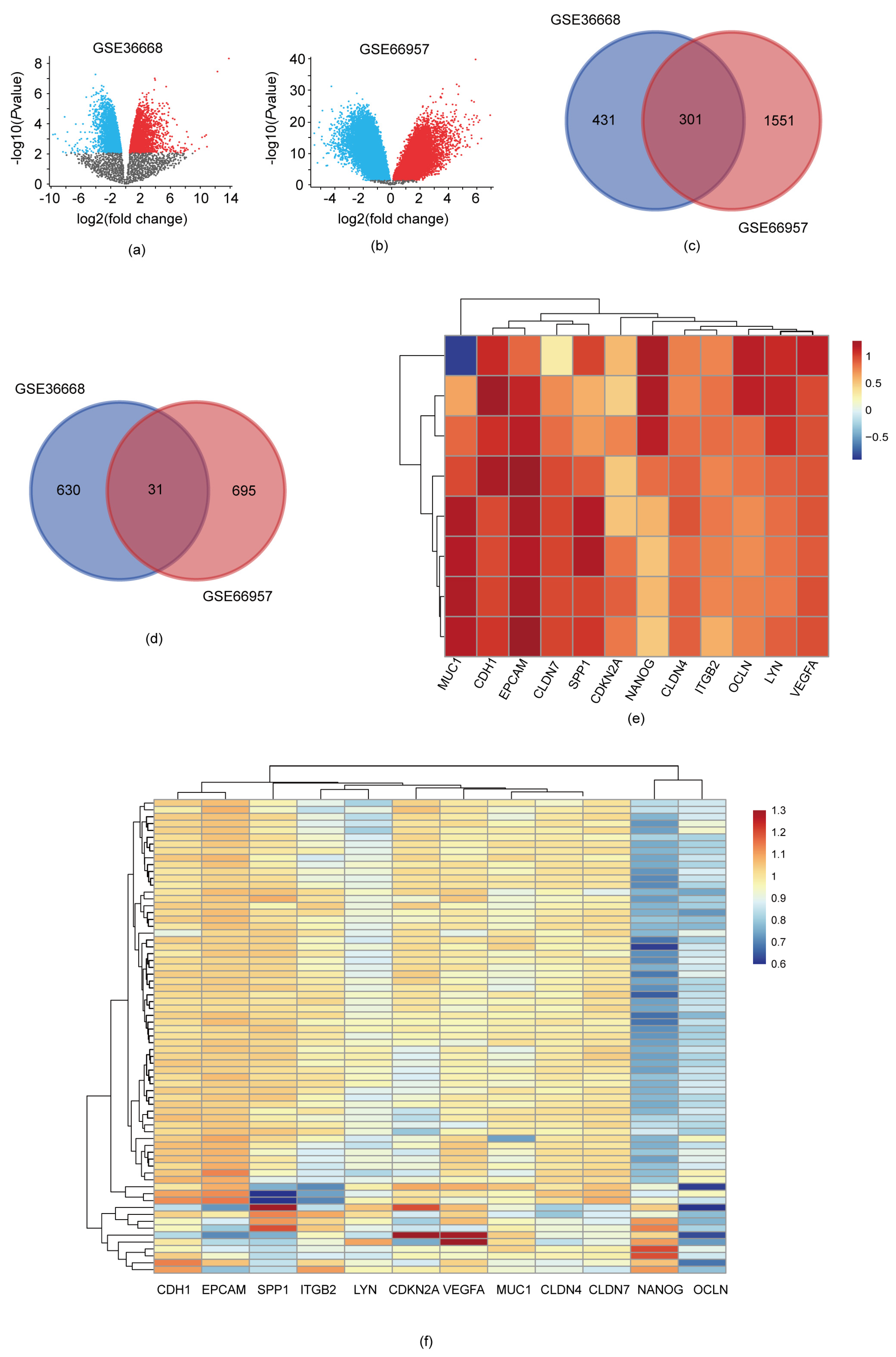
3.1. DEGs Identification
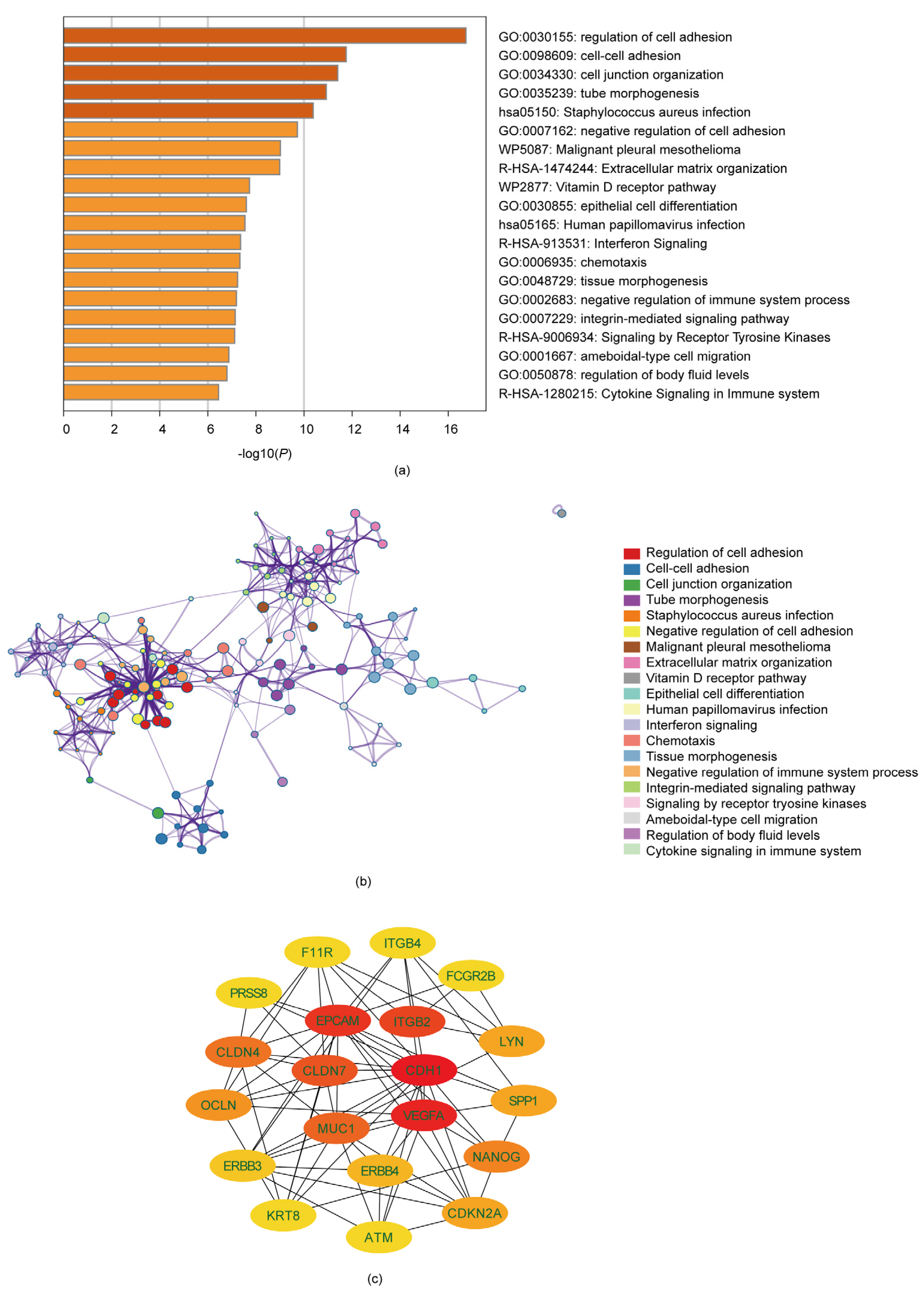
3.2. Analysis of Gene Function and Pathway Enrichment
3.3. Identification of Ten Hub Genes through PPI
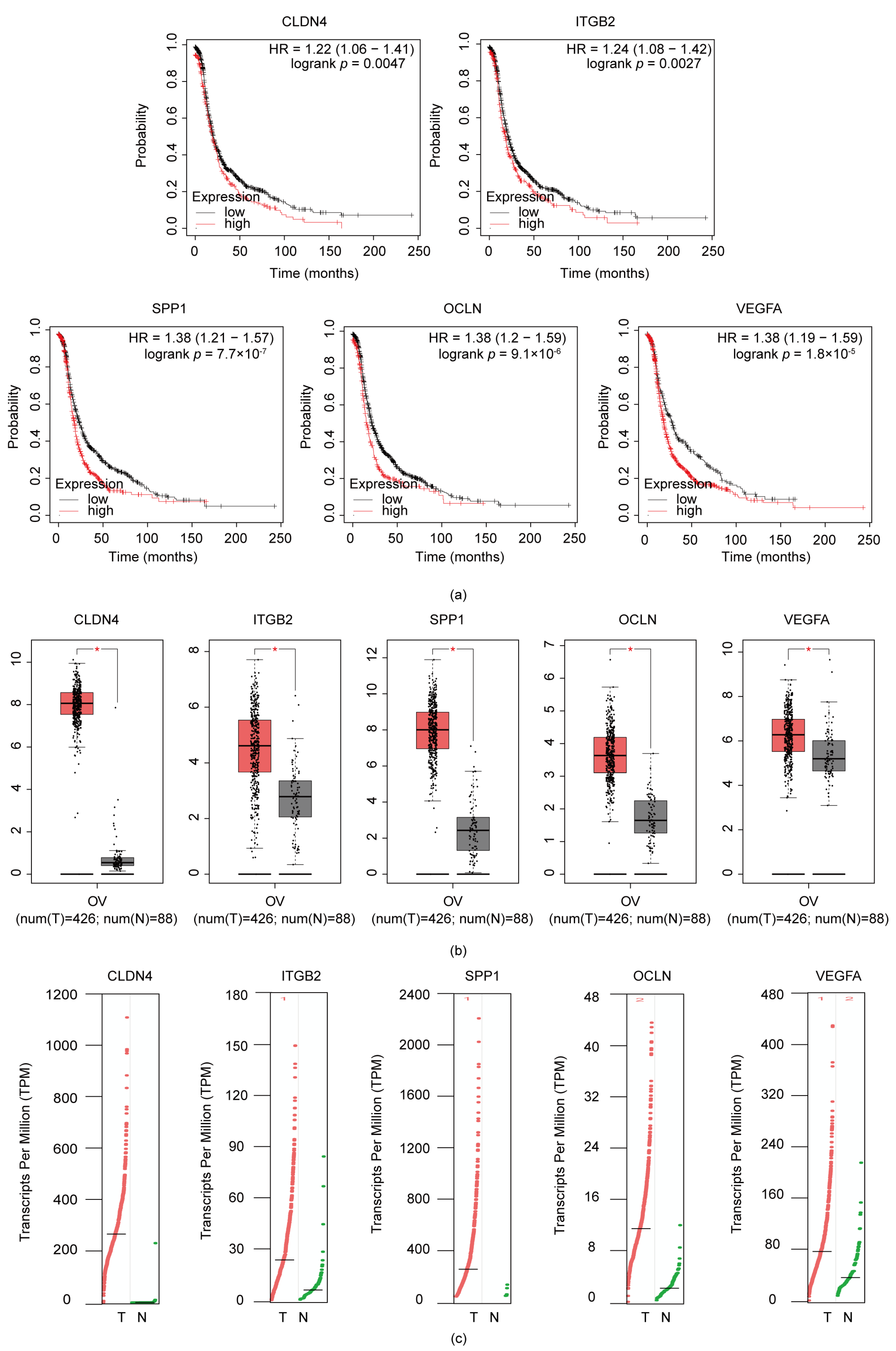
3.4. ITGB2, VEGFA, CLDN4, OCLN, and SPP1 Were Correlated with Poor Prognosis in Serous Ovarian Cancer Patients
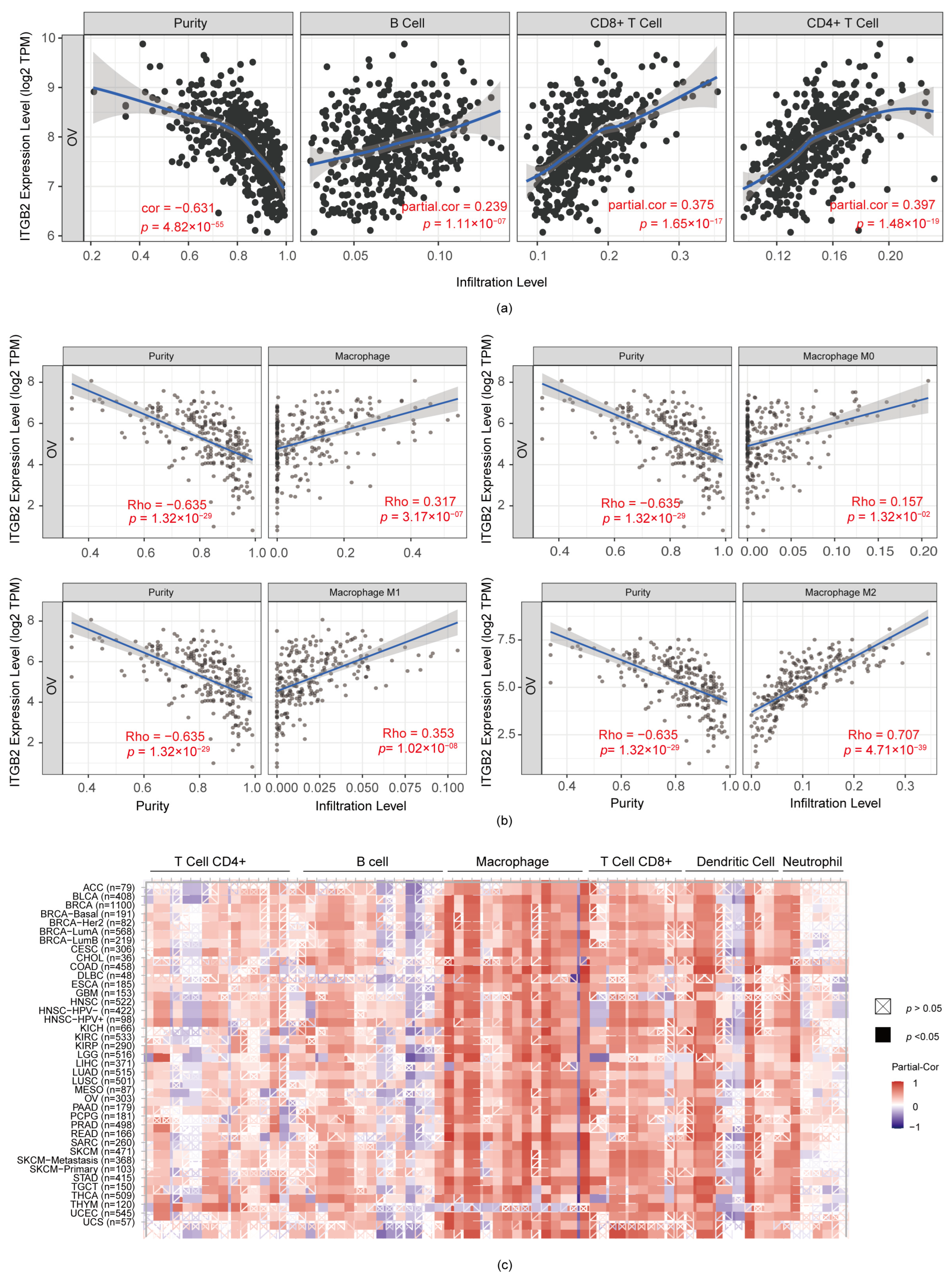
3.5. ITGB2 Was Associated with TAM (Tumor-Associated Macrophage) Infiltration in Serous Ovarian Cancer
3.6. Validation the Expression of ITGB2 in Serous Ovarian Cancer
3.7. Immune-Associated Pathways for ITGB2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Paffenholz, S.V.; Salvagno, C.; Ho, Y.J.; Limjoco, M.; Baslan, T.; Tian, S.; Kulick, A.; de Stanchina, E.; Wilkinson, J.E.; Barriga, F.M.; et al. Senescence induction dictates response to chemo- and immunotherapy in preclinical models of ovarian cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2117754119. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Del Paggio, J.C. Immunotherapy: Cancer immunotherapy and the value of cure. Nat. Rev. Clin. Oncol. 2018, 15, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Dai, X.; Ma, L.; Chen, Y.; Bian, W.; Sun, Y. Exploration of the underlying biological differences and targets in ovarian cancer patients with diverse immunotherapy response. Front. Immunol. 2022, 13, 1480. [Google Scholar] [CrossRef]
- Lee, Y.J.; Woo, H.Y.; Kim, Y.N.; Park, J.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T.; Park, E.; Joung, J.G.; et al. Dynamics of the Tumor Immune Microenvironment during Neoadjuvant Chemotherapy of High-Grade Serous Ovarian Cancer. Cancers 2022, 14, 2308. [Google Scholar] [CrossRef]
- Yigit, R.; Massuger, L.F.; Figdor, C.G.; Torensma, R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol. Oncol. 2010, 117, 366–372. [Google Scholar] [CrossRef]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef]
- Odunsi, K. Immunotherapy in ovarian cancer. Ann. Oncol. 2017, 28, viii1–viii7. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Wang, S.; Fu, Y.; Kuerban, K.; Liu, J.; Huang, X.; Pan, D.; Chen, H.; Zhu, Y.; Ye, L. Discoidin domain receptor 1 is a potential target correlated with tumor invasion and immune infiltration in gastric cancer. Front. Immunol. 2022, 13, 933165. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Li, M.; Zhou, R.; Zhang, J.; Sun, H.; Shi, M.; Bin, J.; Liao, Y.; Rao, J.; Liao, W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol. Res. 2019, 7, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.W.D.; Keane, C.; Mollee, P.; Birch, S.; Gould, C.; Gunawardana, J.; Hoang, T.; Ma, T.; Abro, E.U.; Shanavas, M.; et al. The Tumor Microenvironment Is Independently Prognostic of Conventional and Clinicogenetic Risk Models in Follicular Lymphoma. Blood 2017, 130, 728. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, Q.; Wang, W. Intimate communications within the tumor microenvironment: Stromal factors function as an orchestra. J. Biomed. Sci. 2023, 30, 1. [Google Scholar] [CrossRef]
- Kandalaft, L.E.; Dangaj Laniti, D.; Coukos, G. Immunobiology of high-grade serous ovarian cancer: Lessons for clinical translation. 2022, 22, 640–656. Nat. Rev. Cancer 2022, 22, 640–656. [Google Scholar] [CrossRef]
- Rajtak, A.; Ostrowska-Lesko, M.; Zak, K.; Tarkowski, R.; Kotarski, J.; Okla, K. Integration of local and systemic immunity in ovarian cancer: Implications for immunotherapy. Front. Immunol. 2022, 13, 1018256. [Google Scholar] [CrossRef]
- Zhao, Y.; Mei, S.; Huang, Y.; Chen, J.; Zhang, X.; Zhang, P. Integrative analysis deciphers the heterogeneity of cancer-associated fibroblast and implications on clinical outcomes in ovarian cancers. Comput. Struct. Biotechnol. J. 2022, 20, 6403–6411. [Google Scholar] [CrossRef]
- Siminzar, P.; Tohidkia, M.R.; Eppard, E.; Vahidfar, N.; Tarighatnia, A.; Aghanejad, A. Recent Trends in Diagnostic Biomarkers of Tumor Microenvironment. Mol. Imaging Biol. 2022, 1–19. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor Microenvironment in Ovarian Cancer: Function and Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 758. [Google Scholar] [CrossRef]
- Colvin, E.K. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front. Oncol. 2014, 4, 137. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Spitler, R.; Hutter, G.; Ho, J.Q.; Pauliah, M.; Mahmoudi, M. Tumor-associated macrophages, nanomedicine and imaging: The axis of success in the future of cancer immunotherapy. Immunotherapy 2017, 9, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Truxova, I.; Cibula, D.; Spisek, R.; Fucikova, J. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J. Immunother. Cancer 2023, 11, e005968. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, X.; Ni, Y.; Zhao, X.; Liang, X. Metabolic reprogramming of the tumor immune microenvironment in ovarian cancer: A novel orientation for immunotherapy. Front. Immunol. 2022, 13, 1030831. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, A.; Han, X.; Li, Y.; Zhang, Z.; Song, L.; Wang, W.; Lou, M. ITGB2 as a prognostic indicator and a predictive marker for immunotherapy in gliomas. Cancer Immunol. Immunother. 2022, 71, 645–660. [Google Scholar] [CrossRef]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β2 Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef]
- Brücher, B.L.D.M.; Jamall, I.S. Cell-Cell Communication in the Tumor Microenvironment, Carcinogenesis, and Anticancer Treatment. Cell. Physiol. Biochem. 2014, 34, 213–243. [Google Scholar] [CrossRef]
- Wei, J.; Huang, X.J.; Huang, Y.; Xiong, M.Y.; Yao, X.Y.; Huang, Z.N.; Li, S.N.; Zhou, W.J.; Fang, D.L.; Deng, D.H.; et al. Key immune-related gene ITGB2 as a prognostic signature for acute myeloid leukemia. Ann. Transl. Med. 2021, 9, 1386. [Google Scholar] [CrossRef]
- Varga, G.; Balkow, S.; Wild, M.K.; Stadtbaeumer, A.; Krummen, M.; Rothoeft, T.; Higuchi, T.; Beissert, S.; Wethmar, K.; Scharffetter-Kochanek, K.; et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood 2006, 109, 661–669. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Luo, T.; Zhen, Z.; Liu, L.; Zheng, Y.; Zhang, C.; Hu, X. Correlation between ITGB2 expression and clinical characterization of glioma and the prognostic significance of its methylation in low-grade glioma(LGG). Front. Endocrinol. 2022, 13, 1106120. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Feng, X.; Shen, Z.; Sun, J.; Zhang, X.; Bu, F.; Xu, M.; Tan, C.; Wang, Z. Stanniocalcin-2 promotes cell EMT and glycolysis via activating ITGB2/FAK/SOX6 signaling pathway in nasopharyngeal carcinoma. Cell Biol. Toxicol. 2022, 38, 259–272. [Google Scholar] [CrossRef]
- Benedicto, A.; Marquez, J.; Herrero, A.; Olaso, E.; Kolaczkowska, E.; Arteta, B. Decreased expression of the beta2 integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. BMC Cancer 2017, 17, 827. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 1472. [Google Scholar] [CrossRef]
- Zu, L.; He, J.; Zhou, N.; Zeng, J.; Zhu, Y.; Tang, Q.; Jin, X.; Zhang, L.; Xu, S. The Profile and Clinical Significance of ITGB2 Expression in Non-Small-Cell Lung Cancer. J. Clin. Med. 2022, 11, 6421. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Menden, H.; Xia, S.; Yu, W.; Holmes, A.; Johnston, J.; Reid, K.J.; Josephson, C.D.; Patel, R.M.; Ahmed, A.; et al. ITGB2 (Integrin beta2) Immunomodulatory Gene Variants in Premature Infants With Necrotizing Enterocolitis. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e37–e41. [Google Scholar] [CrossRef]
- Dashti, N.; Mahmoudi, M.; Gharibdoost, F.; Kavosi, H.; Rezaei, R.; Imeni, V.; Jamshidi, A.; Aslani, S.; Mostafaei, S.; Vodjgani, M. Evaluation of ITGB2 (CD18) and SELL (CD62L) genes expression and methylation of ITGB2 promoter region in patients with systemic sclerosis. Rheumatol. Int. 2018, 38, 489–498. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Deng, T.; Cao, J.; Zhou, Y.; Luo, X.; Feng, Y.; Huang, H.; Liu, J. Identifying ITGB2 as a Potential Prognostic Biomarker in Ovarian Cancer. Diagnostics 2023, 13, 1169. https://doi.org/10.3390/diagnostics13061169
Li C, Deng T, Cao J, Zhou Y, Luo X, Feng Y, Huang H, Liu J. Identifying ITGB2 as a Potential Prognostic Biomarker in Ovarian Cancer. Diagnostics. 2023; 13(6):1169. https://doi.org/10.3390/diagnostics13061169
Chicago/Turabian StyleLi, Chanyuan, Ting Deng, Junya Cao, Yun Zhou, Xiaolin Luo, Yanling Feng, He Huang, and Jihong Liu. 2023. "Identifying ITGB2 as a Potential Prognostic Biomarker in Ovarian Cancer" Diagnostics 13, no. 6: 1169. https://doi.org/10.3390/diagnostics13061169
APA StyleLi, C., Deng, T., Cao, J., Zhou, Y., Luo, X., Feng, Y., Huang, H., & Liu, J. (2023). Identifying ITGB2 as a Potential Prognostic Biomarker in Ovarian Cancer. Diagnostics, 13(6), 1169. https://doi.org/10.3390/diagnostics13061169





