A Comparative Evaluation of Mediastinal Nodal SUVmax and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases in Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
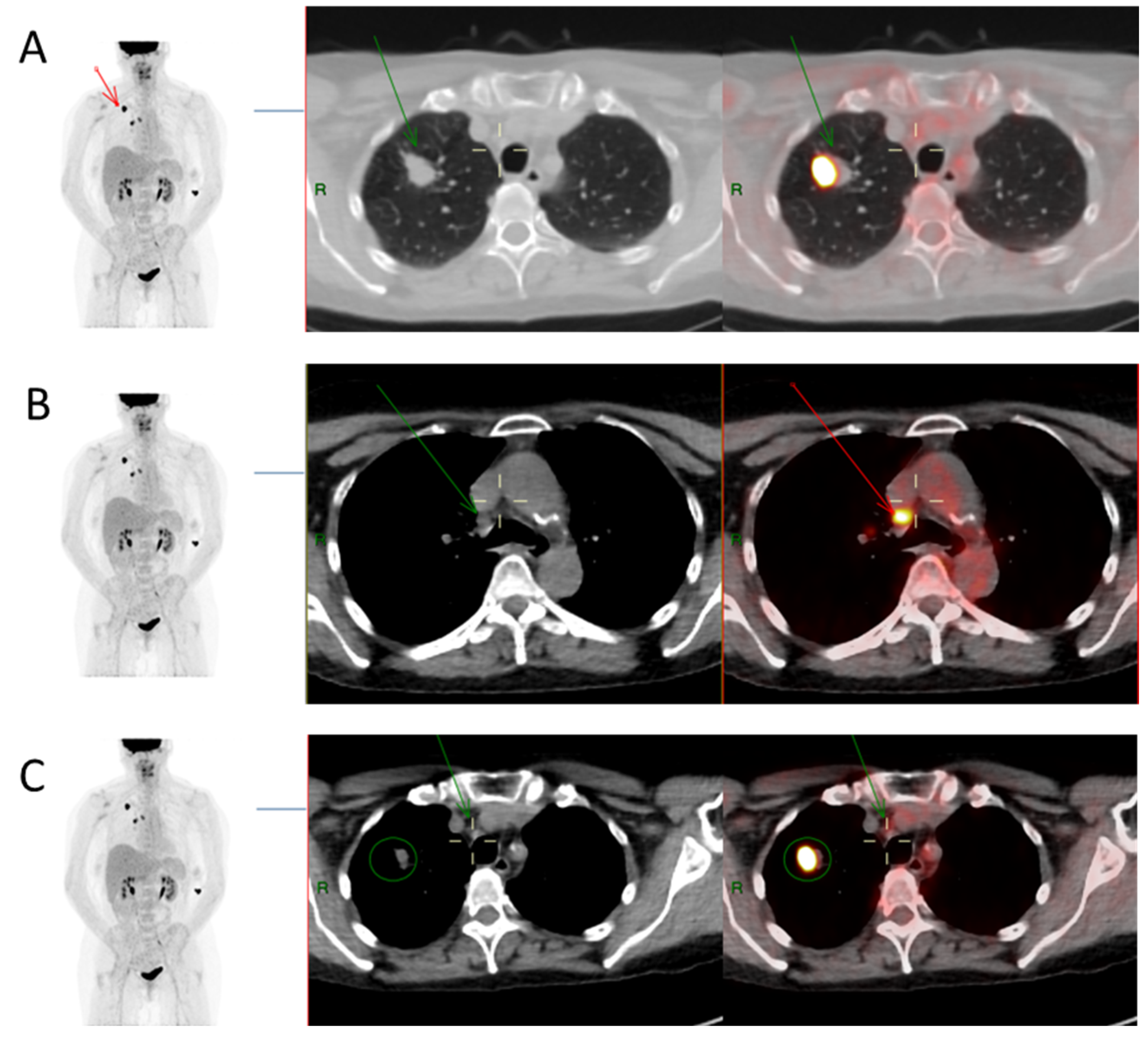
2.2. 18F-FDG PET/CT Imaging and Image Analysis
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Cohort and Tumour Characteristics
3.2. Correlative Analysis of Lymph Node Involvement
3.3. ROC Curve Comparison and Selected Threshold Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kanitkar, A.A.; Schwartz, A.G.; George, J.; Soubani, A.O. Causes of death in long-term survivors of non-small cell lung cancer: A regional surveillance, epidemiology, and end results study. Ann. Thorac. Med. 2018, 13, 76. [Google Scholar] [PubMed]
- Grant, M.J.; Herbst, R.S.; Goldberg, S.B. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat. Rev. Clin. Oncol. 2021, 18, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J. Emerging immunotherapies in the treatment of non–small cell lung cancer (NSCLC): The role of immune checkpoint inhibitors. Am. J. Clin. Oncol. 2015, 38, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wang, K.; Zhang, T.; Shen, H.; Dong, W.; Liu, Q.; Du, J. Long-term outcomes of stage I NSCLC (≤3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: A PSM based analysis. J. Thorac. Dis. 2017, 9, 4561. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.J.; Bleetman, D.; Messenger, D.E.; Joshi, N.A.; Wood, L.; Rasburn, N.J.; Batchelor, T.J. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 155, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242. [Google Scholar]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Prim. 2015, 1, 15009. [Google Scholar] [CrossRef]
- Kuipers, H.; Hoogwater, F.J.; Holtman, G.A.; Slangen, J.J.; de Haas, R.J.; de Boer, M.T. Diagnostic performance of preoperative CT in differentiating between benign and malignant origin of suspicious gallbladder lesions. Eur. J. Radiol. 2021, 138, 109619. [Google Scholar] [CrossRef]
- Ma, J.; He, N.; Yoon, J.H.; Ha, R.; Li, J.; Ma, W.; Meng, T.; Lu, L.; Schwartz, L.H.; Wu, Y. Distinguishing benign and malignant lesions on contrast-enhanced breast cone-beam CT with deep learning neural architecture search. Eur. J. Radiol. 2021, 142, 109878. [Google Scholar] [CrossRef]
- Shao, D.; Gao, Q.; Tian, X.-W.; Wang, S.-Y.; Liang, C.-H.; Wang, S.-X. Differentiation and diagnosis of benign and malignant testicular lesions using 18F-FDG PET/CT. Eur. J. Radiol. 2017, 93, 114–120. [Google Scholar] [CrossRef] [PubMed]
- De Langen, A.J.; Vincent, A.; Velasquez, L.M.; Van Tinteren, H.; Boellaard, R.; Shankar, L.K.; Boers, M.; Smit, E.F.; Stroobants, S.; Weber, W.A. Repeatability of 18F-FDG uptake measurements in tumors: A metaanalysis. J. Nucl. Med. 2012, 53, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Mattes, M.D.; Moshchinsky, A.B.; Ahsanuddin, S.; Rizk, N.P.; Foster, A.; Wu, A.J.; Ashamalla, H.; Weber, W.A.; Rimner, A. Ratio of lymph node to primary tumor SUV on PET/CT accurately predicts nodal malignancy in non–small-cell lung cancer. Clin. Lung Cancer 2015, 16, e253–e258. [Google Scholar] [CrossRef] [PubMed]
- Paquet, N.; Albert, A.; Foidart, J.; Hustinx, R. Within-patient variability of 18F-FDG: Standardized uptake values in normal tissues. J. Nucl. Med. 2004, 45, 784–788. [Google Scholar] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Lacoeuille, F.; Fosse, P.; Vervueren, L.; Cahouet-Vannier, A.; Dabli, D.; Bouchet, F.; Couturier, O. For avid glucose tumors, the SUV peak is the most reliable parameter for [18 F] FDG-PET/CT quantification, regardless of acquisition time. EJNMMI Res. 2016, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 1–8. [Google Scholar] [CrossRef]
- Sherman, M.; Cessie, S.l. A comparison between bootstrap methods and generalized estimating equations for correlated outcomes in generalized linear models. Commun. Stat.-Simul. Comput. 1997, 26, 901–925. [Google Scholar] [CrossRef]
- Heineman, D.J.; Berge, M.G.T.; Daniels, J.M.; Versteegh, M.I.; Marang-van de Mheen, P.J.; Wouters, M.W.; Schreurs, W.H. The quality of staging non-small cell lung cancer in the Netherlands: Data from the Dutch Lung Surgery Audit. Ann. Thorac. Surg. 2016, 102, 1622–1629. [Google Scholar] [CrossRef] [Green Version]
- De Wever, W.; Ceyssens, S.; Mortelmans, L.; Stroobants, S.; Marchal, G.; Bogaert, J.; Verschakelen, J. Additional value of PET-CT in the staging of lung cancer: Comparison with CT alone, PET alone and visual correlation of PET and CT. Eur. Radiol. 2007, 17, 23–32. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Forde, P.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.; Felip, E.; Broderick, S.; Brahmer, J.; Swanson, S. CheckMate 816 Investigators. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Koksal, D.; Demirag, F.; Bayiz, H.; Ozmen, O.; Tatci, E.; Berktas, B.; Aydoğdu, K.; Yekeler, E. The correlation of SUVmax with pathological characteristics of primary tumor and the value of Tumor/Lymph node SUVmax ratio for predicting metastasis to lymph nodes in resected NSCLC patients. J. Cardiothorac. Surg. 2013, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Divisi, D.; Barone, M.; Crisci, R. Current role of standardized uptake valuemax-derived ratios in N2 fluorine-18 fluorodeoxyglucose positron-emission tomography non-small cell lung cancer. J. Thorac. Dis. 2018, 10, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousema, J.E.; Heineman, D.J.; Dijkgraaf, M.G.; Annema, J.T.; van den Broek, F.J. Adherence to the mediastinal staging guideline and unforeseen N2 disease in patients with resectable non-small cell lung cancer: Nationwide results from the Dutch Lung Cancer Audit-Surgery. Lung Cancer 2020, 142, 51–58. [Google Scholar] [CrossRef]
- Cho, J.; Choe, J.G.; Pahk, K.; Choi, S.; Kwon, H.R.; Eo, J.S.; Seo, H.J.; Kim, C.; Kim, S. Ratio of mediastinal lymph node SUV to primary tumor SUV in 18 F-FDG PET/CT for nodal staging in non-small-cell lung cancer. Nucl. Med. Mol. Imaging 2017, 51, 140–146. [Google Scholar] [CrossRef]
- Lee, A.Y.; Choi, S.J.; Jung, K.P.; Park, J.S.; Lee, S.M.; Bae, S.K. Characteristics of metastatic mediastinal lymph nodes of non-small cell lung cancer on preoperative F-18 FDG PET/CT. Nucl. Med. Mol. Imaging 2014, 48, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Namura, K.; Minamimoto, R.; Yao, M.; Makiyama, K.; Murakami, T.; Sano, F.; Hayashi, N.; Tateishi, U.; Ishigaki, H.; Kishida, T. Impact of maximum standardized uptake value (SUVmax) evaluated by 18-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18 F-FDG-PET/CT) on survival for patients with advanced renal cell carcinoma: A preliminary report. BMC Cancer 2010, 10, 667. [Google Scholar] [CrossRef] [Green Version]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Yao, Y.; Ma, C.; Ma, X.; Wang, Z.; Lv, T.; Xiao, X.; Yin, J.; Song, Y. Ratio of maximum standardized uptake value to primary tumor size is a prognostic factor in patients with advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2015, 4, 18. [Google Scholar]
- Hofheinz, F.; Bütof, R.; Apostolova, I.; Zöphel, K.; Steffen, I.G.; Amthauer, H.; Kotzerke, J.; Baumann, M.; van den Hoff, J. An investigation of the relation between tumor-to-liver ratio (TLR) and tumor-to-blood standard uptake ratio (SUR) in oncological FDG PET. EJNMMI Res. 2016, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirai, K.; Abe, T.; Saitoh, J.I.; Mizukami, T.; Irie, D.; Takakusagi, Y.; Shiba, S.; Okano, N.; Ebara, T.; Ohno, T. Maximum standardized uptake value on FDG-PET predicts survival in stage I non-small cell lung cancer following carbon ion radiotherapy. Oncol. Lett. 2017, 13, 4420–4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variables | % (Number of Cases in Bracket) |
|---|---|
| Gender (%) | |
| Male | 45.3% (n = 24) |
| Female | 54.7% (n = 29) |
| Age (years); Mean, SD (range) | |
| 67.52, 8.31 (53–87) | |
| Tumour location (%) | |
| RUL (right upper lobe) | 35.8% (n = 19) |
| Right hilar | 7.5% (n = 4) |
| RML (right middle lobe) | 3.8% (n = 2) |
| RLL (right lower lobe) | 24.5% (n = 13) |
| LUL (left upper lobe) | 24.5% (n = 13) |
| LLL (left lower lobe) | 3.8% (n = 2) |
| Types of Surgical Resection (%) | |
| Lobectomy | 77.4% (n = 41) |
| Lobectomy + wedge resection | 13.2% (n = 7) |
| Pneumonectomy | 7.5% (n = 4) |
| Wedge resection | 1.9% (n = 1) |
| Tumour histology (%) | |
| Adenocarcinoma | 54.7% (n = 29) |
| Squamous cell carcinoma | 30.2% (n = 16) |
| Others | 15.1% (n = 8) |
| Tumour grade (%) | |
| Moderately differentiated | 60.4% (n = 32) |
| Poorly differentiated | 26.4% (n = 14) |
| Unknown | 13.2% (n = 7) |
| (A) Nodal stations and pathologic status | |
|---|---|
| Mediastinal lymph node stations; % (number, n) | |
| Upper paratracheal (station 2) | 4.4% (n = 5) |
| Pre-vascular and retro-tracheal (station 3) | 0.9% (n = 1) |
| Lower para-tracheal (station 4) | 28.9% (n = 33) |
| Sub-aortic (station 5) | 9.6% (n= 11) |
| Para-aortic (station 6) | 4.4% (n= 5) |
| Sub-carinal (station 7) | 35.1% (n= 40) |
| Para-esophageal (station 8) | 9.6% (n= 11) |
| Pulmonary ligament (station 9) | 7.0% (n= 8) |
| Mediastinal lymph node histology; % (number, n) | |
| Negative mediastinal | 67.5% (n = 77) |
| Positive mediastinal | 32.5% (n = 37) |
| (B) Summary of parameters associated with pathologically involved lymph nodes | |
| Parameters | Mean, SD |
| Mediastinal lymph nodes | |
| LN size (short axis dimension, mm) | 8.2, 5.3 |
| LN SUVmax | 5.1, 5.1 |
| Other parameters | |
| Tumour SUVmax | 16.7, 8.21 |
| Liver SUVmax | 4.0, 0.83 |
| Mediastinum SUVmax | 2.6, 0.56 |
| Tumour size (longest dimension, mm) | 37.10, 22.71 |
| Tumour pathological size (largest dimension, mm) | 42.61, 26.21 |
| Characteristics (Mean ± SD) | Positive | Negative | p-Value |
|---|---|---|---|
| LN size (short axis dimension, cm) | 0.98 ± 0.18 | 0.76 ± 0.25 | <0.001 |
| SUVmax of LN | 8.22 ± 6.45 | 3.62 ± 3.36 | <0.001 |
| SR (LN SUV/LN size) | 7.91 ± 5.36 | 4.87 ± 4.25 | <0.001 |
| LR (LN SUVmax/liver SUVmax) | 2.15 ± 1.72 | 0.98 ± 1.10 | <0.001 |
| MR (LN SUVmax/mediastinum SUVmax) | 3.36 ± 2.71 | 1.49 ± 1.70 | <0.001 |
| TR (LN SUVmax/tumour SUVmax) | 0.56 ± 0.36 | 0.23 ± 0.20 | <0.001 |
| Parameters | Threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| LN_SUVmax | 4.4 | 67.6 | 83.1 |
| LN_Size (mm) | 5.5 | 91.9 | 49.4 |
| Tumor_SUVmax | 37.9 | 2.7 | 98.7 |
| Mediastinum_SUVmax | 3.1 | 16.2 | 87.0 |
| Liver_SUVmax | 4.0 | 46.0 | 59.7 |
| SR (LN_SUVmax/LN_Size) | 0.4 | 81.1 | 46.8 |
| LR (LN_SUVmax/Liver_SUVmax) | 0.8 | 89.2 | 67.5 |
| MR (LN_SUVmax/Mediastinum_SUVmax) | 1.3 | 81.1 | 68.8 |
| TR (LN_SUVmax/Tumor_SUVmax) | 0.2 | 83.8 | 70.1 |
| p-Values (with Adjusted p-Values) for Sensitivity and Specificity | ||
|---|---|---|
| Comparison | Sensitivity | Specificity |
| TR-LR | 0.083 (0.332) | 0.157 (1.000) |
| TR-SR | <0.001 (0.005) | 0.317 (1.000) |
| TR-MR | 0.157 (0.471) | 0.157 (1.000) |
| LR-SR | <0.001 (0.005) | 0.317 (1.000) |
| LR-MR | 0.317 (0.634) | 0.157 (1.000) |
| SR-MR | <0.001 (0.005) | 0.317 (1.000) |
| LR-LN SUV | <0.001 (0.005) | 0.157 (1.000) |
| TR-LN SUV | <0.001 (0.005) | 0.157 (1.000) |
| SR-LN SUV | 0.317 (0.634) | 0.157 (1.000) |
| MR-LNSUV | <0.001 (0.005) | 0.157 (1.000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlRasheedi, M.; Han, S.; Thygesen, H.; Neilson, M.; Hendry, F.; Alkarn, A.; Maclay, J.D.; Leung, H.Y. A Comparative Evaluation of Mediastinal Nodal SUVmax and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases in Non-Small Cell Lung Cancer. Diagnostics 2023, 13, 1209. https://doi.org/10.3390/diagnostics13071209
AlRasheedi M, Han S, Thygesen H, Neilson M, Hendry F, Alkarn A, Maclay JD, Leung HY. A Comparative Evaluation of Mediastinal Nodal SUVmax and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases in Non-Small Cell Lung Cancer. Diagnostics. 2023; 13(7):1209. https://doi.org/10.3390/diagnostics13071209
Chicago/Turabian StyleAlRasheedi, Maha, Sai Han, Helene Thygesen, Matt Neilson, Fraser Hendry, Ahmed Alkarn, John D. Maclay, and Hing Y. Leung. 2023. "A Comparative Evaluation of Mediastinal Nodal SUVmax and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases in Non-Small Cell Lung Cancer" Diagnostics 13, no. 7: 1209. https://doi.org/10.3390/diagnostics13071209
APA StyleAlRasheedi, M., Han, S., Thygesen, H., Neilson, M., Hendry, F., Alkarn, A., Maclay, J. D., & Leung, H. Y. (2023). A Comparative Evaluation of Mediastinal Nodal SUVmax and Derived Ratios from 18F-FDG PET/CT Imaging to Predict Nodal Metastases in Non-Small Cell Lung Cancer. Diagnostics, 13(7), 1209. https://doi.org/10.3390/diagnostics13071209





