Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries: A Review Emphasizing Origin and Branching
Abstract
:1. Introduction
2. Embryology
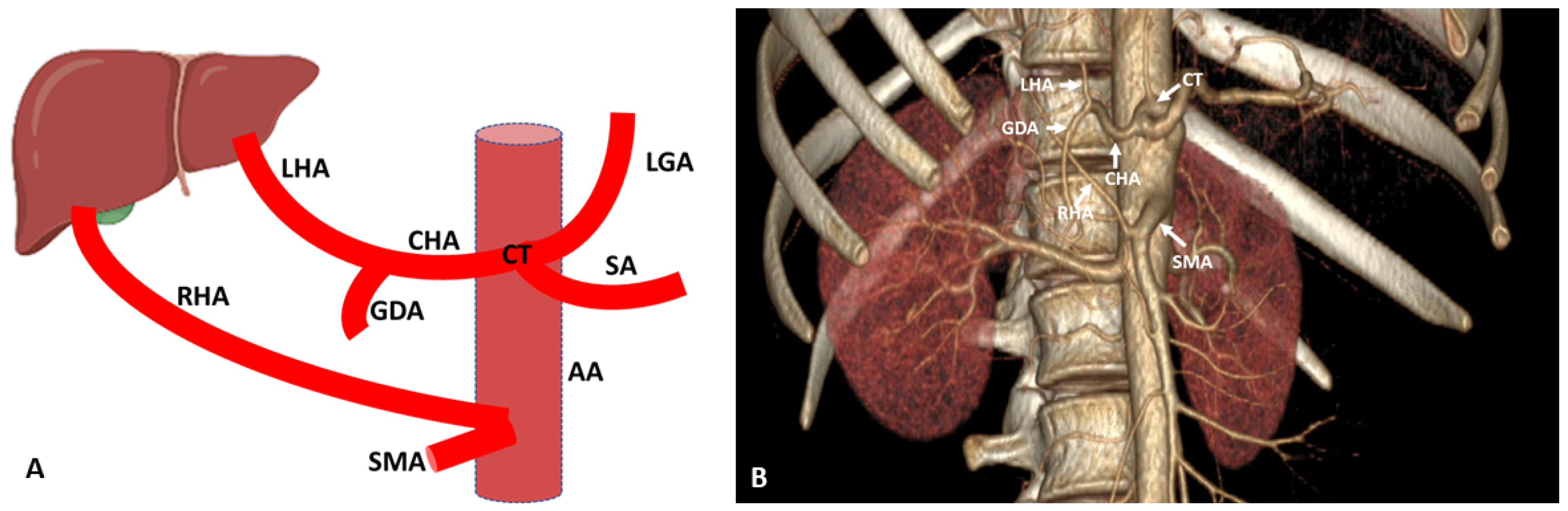
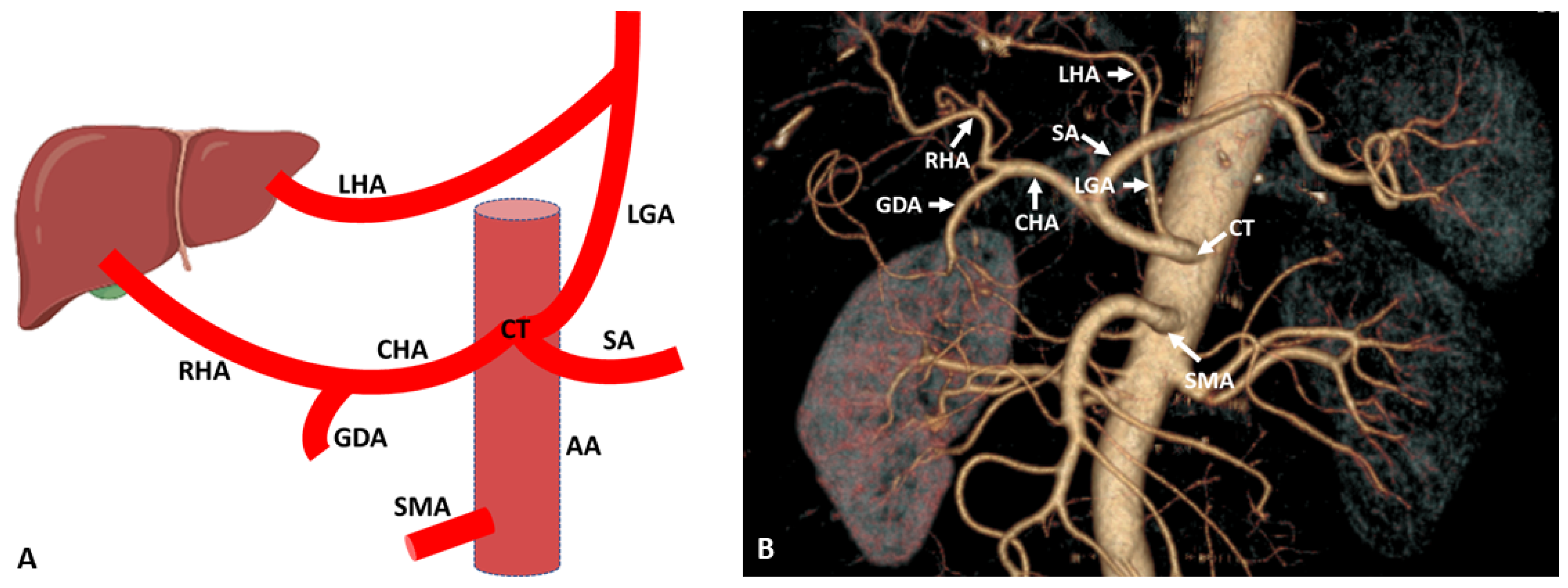
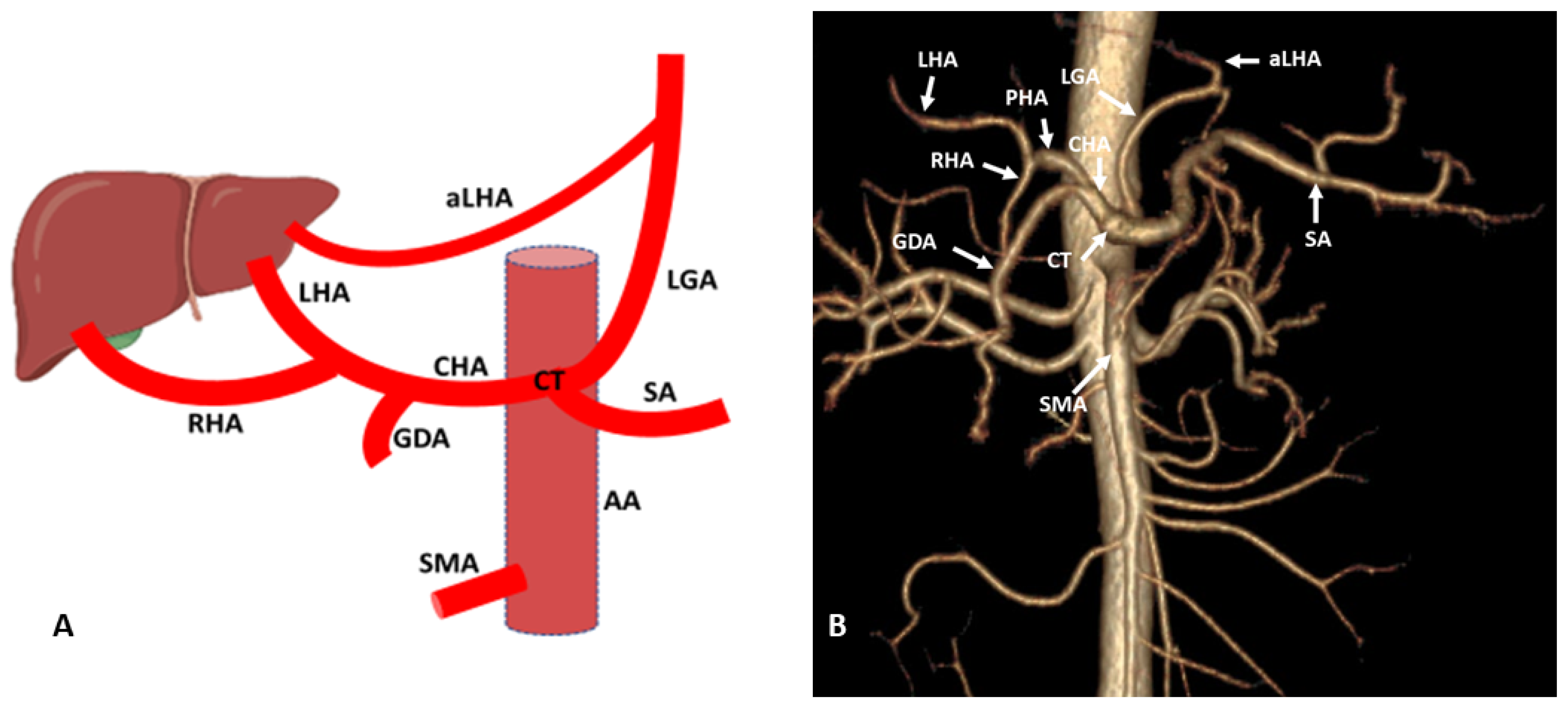
3. Hepatic Artery Variations on the Basis of Anatomical and Radiological Aspect
3.1. Variations of Common Hepatic Artery
3.2. Variations of Right Hepatic Artery
3.3. Variations of Left Hepatic Artery
4. Detection of Hepatic Artery Variations and Their Classifications
5. Clinical Applications of the Hepatic Artery Variations
5.1. Pancreatoduodenectomy
5.2. Management of Aberrant Hepatic Artery during the Surgical Procedure
5.3. Liver Transplantation
6. Hepatic Artery in Interventional Radiology
7. Comparison of Different Imaging Methods in Detecting Arterial Anatomy
8. Latest Diagnostic Imaging Modalities to Detect Liver Vascularity
8.1. Ultrasound
8.2. CT Scan
8.3. MRI
8.4. Positron Emission Tomography with Computed Tomography (PET/CT)
8.5. Digital Subtraction Angiography (DSA)
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sinnatamby, C.S. Coeliac Trunk. In Last’s Anatomy: Regional and Applied, 12th ed.; Elsevier/Churchill Livingstone Elsevier: Edinburgh, UK, 2011; p. 244e5. [Google Scholar]
- Swami, A.; Yadav, T.; Varshney, V.K.; Sreesanth, K.S.; Dixit, S.G. Hepatic arterial variations and its implication during pancreatic cancer surgeries. J. Gastrointest. Cancer 2021, 52, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Malviya, K.K.; Verma, A.; Nayak, A.K.; Mishra, A.; More, R.S. Unraveling variations in celiac trunk and hepatic artery by CT angiography to aid in surgeries of upper abdominal region. Diagnostics 2021, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Imam, A.; Karatas, C.; Mecit, N.; Karakaya, A.D.; Yildirimoglu, T.; Kalayoglu, M.; Kanmaz, T. Anatomical variations of the hepatic artery: A closer view of rare unclassified variants. Folia Morphol. 2022, 81, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Neto, O.C.L.D.; Lima, H.C.S.; Rabelo, P.; Melo, P.S.V.; Amorim, A.G.; Lacerda, C.M. Anatomic variations of hepatic artery: A study in 479 liver transplantations. Arq. Bras. Cir. Dig. 2017, 30, 35–375. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, T.T.; Joseph, S.; Ramakrishnan, S.; Mathew, A.J. Anatomical variations of the hepatic artery in it’s extra hepatic journey: A Cadaveric study with its clinical implications. Anat. Cell Biol. 2022, 55, 269–276. [Google Scholar] [CrossRef]
- Zaki, S.M.; Abdelmaksoud, A.H.K.; Khaled, B.E.A.; Abdel Kader, I.A. Anatomical variations of hepatic artery using the multidetector computed tomography angiography. Folia Morphol. 2020, 79, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Németh, K.; Deshpande, R.; Máthé, Z.; Szuák, A.; Kiss, M.; Korom, C.; Nemeskéri, Á.; Kóbori, L. Extrahepatic arteries of the human liver—Anatomical variants and surgical relevancies. Transpl. Int. 2015, 28, 1216–1226. [Google Scholar] [CrossRef]
- Polguj, M.; Podgórski, M.; Hogendorf, P.; Topol, M. Variations of the hepatobiliary vasculature including coexistence of accessory right hepatic artery with unusually arising double cystic arteries: Case report and literature review. Anat. Sci. Int. 2014, 89, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Mangieri, C.W.; Valenzuela, C.D.; Erali, R.A.; Shen, P.; Howerton, R.; Clark, C.J. Prognostic effect of aberrant right hepatic artery with pancreaticoduodenectomy: Focus on hepatic recurrence. Ann. Surg. Oncol. 2022, 29, 3219–3228. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Liu, S.; Wang, Y.; Liu, K.; Meng, L.; Chen, Q.; Jia, B.; Liu, Y. A single-center clinical study of hepatic artery variations in laparoscopic pancreaticoduodenectomy. Medicine 2020, 99, e20403. [Google Scholar] [CrossRef]
- El Amrani, M.; Pruvot, F.R.; Truant, S. Management of the right hepatic artery in pancreaticoduodenectomy: A systematic review. J. Gastrointest. Oncol. 2016, 7, 298–305. [Google Scholar] [PubMed]
- Kardile, P.B.; Ughade, J.M.; Ughade, M.N.; Dhende, A.; Ali, S.S. Anomalous origin of the hepatic artery from the hepatomesenteric trunk. J. Clin. Diagn. Res. 2013, 7, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Borruso, L.; Kotecha, K.; Singla, A.; Maitra, R.; Mittal, A.; Samra, J. Rare anastomosis between a replaced right hepatic artery and left branch of the proper hepatic artery. Surg. Radiol. Anat. 2022, 44, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bhatia, M.; Garg, A.; Jain, S.; Kumar, K. Abernethy malformation: A comprehensive review. Diagn. Interv. Radiol. 2022, 28, 21–28. [Google Scholar] [CrossRef]
- Kikuya, K.; Einama, T.; Miyata, Y.; Iwasaki, T.; Yamagishi, Y.; Takihata, Y.; Morimura, F.; Edo, H.; Otsuka, Y.; Mori, S.; et al. Destruction of a wandering accessory right hepatic artery in a patient with pancreatic body cancer: A case report. Clin. J. Gastroenterol. 2021, 14, 560–565. [Google Scholar] [CrossRef]
- Iacob, N.; Pusztai, A.M.; Miclăuş, G.D.; Pop, E.; Matusz, P. An anomalous origin of the gastrosplenic trunk and common hepatic artery arising independently from the abdominal aorta: A case report using MDCT angiography. Rom. J. Morphol. Embryol. 2018, 59, 353–357. [Google Scholar]
- Choi, T.W.; Chung, J.W.; Kim, H.C.; Lee, M.; Choi, J.W.; Jae, H.J.; Hur, S. Anatomic variations of the hepatic artery in 5625 patients. Radiol. Cardiothorac. Imaging 2021, 3, e210007. [Google Scholar] [CrossRef]
- Thangarajah, A.; Parthasarathy, R. Celiac axis, common hepatic and hepatic artery variants as evidenced on MDCT angiography in south indian population. J. Clin. Diagn. Res. 2016, 10, TC01. [Google Scholar] [CrossRef]
- Coco, D.; Leanza, S. Celiac Trunk and Hepatic Artery Variants in Pancreatic and Liver Resection Anatomy and Implications in Surgical Practice. Open Access Maced. J. Med. Sci. 2019, 7, 2563–2568. [Google Scholar] [CrossRef]
- Gümüs, H.; Bükte, Y.; Özdemir, E.; Sentürk, S.; Tekbas, G.; Önder, H.; Ekici, F.; Bilici, A. Variations of the celiac trunk and hepatic arteries: A study with 64-detector computed tomographic angiography. Eur. Rev. Med. Pharm. Sci. 2013, 17, 1636–1641. [Google Scholar]
- Favelier, S.; Germain, T.; Genson, P.Y.; Cercueil, J.P.; Denys, A.; Krausé, D.; Guiu, B. Anatomy of liver arteries for interventional radiology. Diagn. Interv. Imaging. 2015, 96, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Hoven, A.F.; van Leeuwen, M.S.; Lam, M.G.; van den Bosch, M.A. Hepatic arterial configuration in relation to the segmental anatomy of the liver; observations on MDCT and DSA relevant to radioembolization treatment. Cardiovasc. Interv. Radiol. 2015, 38, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilek, O.N.; Atay, A. Dealing with hepatic artery traumas: A clinical literature review. World J. Clin. Cases 2021, 9, 8425–8440. [Google Scholar] [CrossRef] [PubMed]
- Hakseven, M.; Çetindağ, Ö.; Avşar, G.; Deryol, R.; Dokçu, Ş.; Culcu, S.; Akbulut, S.; Bayar, S.; Ünal, A.E.; Demirci, S. Management of Aberrant Left Hepatic Artery During Laparoscopic Gastrectomy and Consequences. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 999–1004. [Google Scholar] [CrossRef]
- Chipaila, J.; Kato, H.; Iizawa, Y.; Motonori, N.; Noguchi, D.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Tanemura, A.; Murata, Y.; et al. Prolonged operating time is a significant perioperative risk factor for arterial pseudoaneurysm formation and patient death following hemorrhage after pancreaticoduodenectomy. Pancreatology 2020, 20, 1540–1549. [Google Scholar] [CrossRef]
- Burasakarn, P.; Higuchi, R.; Yazawa, T.; Uemura, S.; Izumo, W.; Matsunaga, Y.; Yamamoto, M. Hepatic artery resection without reconstruction in pancreatoduodenectomy. Langenbecks Arch. Surg. 2021, 406, 2081–2090. [Google Scholar] [CrossRef]
- Cho, A.; Gunji, H.; Koike, N.; Narumoto, S.; Asano, T.; Yamamoto, H.; Kainuma, O.; Ryu, M.; Mori, C.; Murakami, G.; et al. Intersegmental arterial communication between the medial and left lateral segments of the liver. Dig. Surg. 2007, 24, 328–330. [Google Scholar] [CrossRef]
- Miyata, T.; Yamamoto, Y.; Sugiura, T.; Okamura, Y.; Ito, T.; Ashida, R.; Uemura, S.; Kato, Y.; Ohgi, K.; Kohga, A.; et al. Combined resection of the transpancreatic common hepatic artery preserving the gastric arterial arcade without arterial reconstruction in hepatopancreatoduodenectomy: A case report. Surg. Case Rep. 2018, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Vas, D.; Moreno Rojas, J.; Solà Garcia, M. Replaced right hepatic artery arising from the distal renal artery, a new variation. Surg. Radiol. Anat. 2022, 44, 1339–1342. [Google Scholar] [CrossRef]
- Cawich, S.O.; Sinanan, A.; Gosein, M.; Pearce, N.; Deshpande, R.; Mohammed, F.; Naraynsingh, V.; Fortune, M.; Rampersad, F. An investigative study of hepatic arterial anomalies in a West Indian population. Radiol. Res. Pract. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, B.; Yuan, W.; Zhang, J.; Xiao, J.; Sha, Y. A rare case with multiple arterial variations of the liver complicated laparoscopic pancreaticoduodenectomy. BMC Gastroenterol. 2022, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sahni, D.; Kumar, H.; Yadav, T.D.; Aggarwal, A.; Gupta, T. The segmental branching of the hepatic arteries in the liver: A cadaveric study. Anat. Sci. Int. 2019, 94, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Helton, W.S. An analytical review ofvasculobiliary injury in laparoscopic and open cholecystectomy. HPB 2011, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, H.; Asano, Y.; Ito, M.; Kawabe, N.; Arakawa, S.; Shimura, M.; Koike, D.; Hayashi, C.; Ochi, T.; Kamio, K.; et al. Efficacy of Hepatic Artery Resection without Reconstruction in Pancreaticoduodenectomy, Paying Attention to the Anomaly of the Artery. Gan Kagaku Ryoho 2022, 49, 478–481. [Google Scholar]
- Jin, W.; Dong, M.; Pan, J.; Zhang, Q.; Li, M.; Guo, D.; Gao, Y.; Lv, Z.; Tan, T.; Ma, J. Rare combined variations of accessory left hepatic artery and accessory right hepatic artery: A case report and literature review. Surg. Radiol. Anat. 2020, 42, 443–447. [Google Scholar] [CrossRef]
- Raj, G.; Kaushik, N.; Singh, R.; Singh, N.; Chauhan, A.; Narayan, S.; Kumar, T.; Dixit, N.A. Assessment of Celiac Axis and Hepatic Artery Variations in Hepatobiliary and Pancreatic Malignancy with Multidetector Computed Tomography Angiography. Asian J. Oncol. 2020, 6, 134–143. [Google Scholar] [CrossRef]
- Ang, R.R.G.; Lee, H.J.; Bae, J.S.; Zhu, C.C.; Berlth, F.; Kim, T.H.; Park, S.H.; Suh, Y.S.; Kong, S.H.; Kim, S.H.; et al. Safety of Ligation of Aberrant Left Hepatic Artery Originating from Left Gastric Artery in Laparoscopic Gastrectomy for Gastric Cancer. Sci. Rep. 2020, 10, 5856. [Google Scholar] [CrossRef] [Green Version]
- Cirocchi, R.; D’Andrea, V.; Amato, B.; Renzi, C.; Henry, B.M.; Tomaszewski, K.A.; Gioia, S.; Lancia, M.; Artico, M.; Randolph, J. Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surgeon 2020, 18, 100–112. [Google Scholar] [CrossRef]
- Onashvili, N.; Mizandari, M.; Azrumelashvili, T.; Ingorokva, A.; Ubiria, G. Multidetector computed tomography angiography in the management of transarterial embolization of primary and secondary liver malignancy. Minerva Gastroenterol. Dietol. 2016, 62, 11–18. [Google Scholar]
- Yang, F.; Di, Y.; Li, J.; Wang, X.-Y.; Yao, L.; Hao, S.-J.; Jiang, Y.-J.; Jin, C.; Fu, D.-L. Accuracy of routine multidetector computed tomography to identify arterial variants in patients scheduled for pancreaticoduodenectomy. World J. Gastroenterol. 2015, 21, 969. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Sun, D.; Zhang, Z. Aberrant hepatic arteries running through pancreatic parenchyma encountered during pancreatoduodenectomy: Two rare case reports and strategies for surgical treatment. Medicine 2016, 95, e3867. [Google Scholar] [CrossRef]
- Pallisera, A.; Morales, R.; Ramia, J.M. Tricks and tips in pancreatoduodenectomy. World J. Gastrointest. Oncol. 2014, 6, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, Q.; Lai, Q.Q.; Huang, W.H.; Wu, H.; Li, W.C. Preoperative evaluation value of aortic arch lesions by multidetector computed tomography angiography in type A aortic dissection. Medicine 2016, 95, e4984. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Otsubo, T.; Koizumi, S.; Ariizumi, S.; Katagiri, S.; Watanabe, T.; Nakano, H.; Yamamoto, M. Anatomic variations of hepatic artery and new clinical classification based on abdominal angiographic images of 1200 cases. Hepatogastroenterology 2014, 61, 2345–2348. [Google Scholar] [PubMed]
- Osman, A.M.; Abdrabou, A. Celiac trunk and hepatic artery variants: A retrospective preliminary MSCT report among Egyptian patients. Egypt J. Radiol. Nucl. Med. 2016, 47, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Yelamanchi, R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World. J. Gastroenterol. 2021, 27, 3158–3181. [Google Scholar] [CrossRef] [PubMed]
- Eshuis, W.J.; Olde Loohuis, K.M.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB 2011, 13, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Rammohan, A.; Palaniappan, R.; Pitchaimuthu, A.; Rajendran, K.; Perumal, S.K.; Balaraman, K.; Ramasamy, R.; Sathyanesan, J.; Govindan, M. Implications of the presence of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J. Gastrointest. Surg. 2014, 6, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Zaima, M.; Yamamoto, H.; Harada, H.; Kawamura, J.; Yamada, M.; Yazawa, T.; Kawasoe, J. Liver necrosis shortly after pancreaticoduodenectomy with resection of the replaced left hepatic artery. World J. Surg. Oncol. 2017, 15. [Google Scholar] [CrossRef]
- Crocetti, D.; Sapienza, P.; Ossola, P.; Tarallo, M.; Cavallaro, G.; Serra, R.; Grande, R.; Mingoli, A.; Fiori, E.; De Toma, G. Does Aberrant Right Hepatic Artery Influence the Surgical Short- and Long-term Outcome of Pancreatoduodenectomy? In Vivo 2019, 33, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Hackert, T.; Stampfl, U.; Schulz, H.; Strobel, O.; Büchler, M.W.; Werner, J. Clinical significance of liver ischaemia after pancreatic resection. J. Br. Surg. 2011, 98, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Yang, F.; Fu, D.L. Clinical significance of variant hepatic artery in pancreatic resection: A comprehensive review. World J. Gastroenterol. 2022, 28, 2057–2075. [Google Scholar] [CrossRef] [PubMed]
- Ramesh Babu, C.S.; Sharma, M. Biliary tract anatomy and its relationship with venous drainage. J. Clin. Exp. Hepatol. 2014, 4, S18–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, T. Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer. World J. Gastroenterol. 2022, 28, 985–1008. [Google Scholar] [CrossRef]
- Castaldi, A.; Chierici, A.; El Zibawi, M.; Rosso, E.; Iannelli, A. Management of an aberrant right hepatic artery arising from the superior mesenteric artery during minimally-invasive pancreatoduodenectomy—A narrative review. Dig. Med. Res. 2022, 5, 34. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ambo, Y.; Kodama, Y.; Takada, M.; Kato, K.; Nakamura, F.; Hirano, S. Preoperative embolization strategy for the combined resection of replaced right hepatic artery in pancreaticoduodenectomy: A small case series. Surg. Case Rep. 2022, 8, 49. [Google Scholar] [CrossRef]
- Marichez, A.; Turrini, O.; Fernandez, B.; Garnier, J.; Lapuyade, B.; Ewald, J.; Adam, J.P.; Marchese, U.; Chiche, L.; Delpero, J.R.; et al. Does pre-operative embolization of a replaced right hepatic artery before pancreaticoduodenectomy for pancreatic adenocarcinoma affect postoperative morbidity and R0 resection? A bi-centric French cohort study. HPB 2021, 23, 1683–1691. [Google Scholar] [CrossRef]
- Okada, K.I.; Kawai, M.; Hirono, S.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Tani, M.; Yamaue, H. A replaced right hepatic artery adjacent to pancreatic carcinoma should be divided to obtain R0 resection in pancreaticoduodenectomy. Langenbeck Arch. Surg. 2015, 400, 57–65. [Google Scholar] [CrossRef]
- Yoshidome, H.; Shimizu, H.; Ohtsuka, M.; Yoshitomi, H.; Kato, A.; Furukawa, K.; Miyazaki, M. Pancreaticoduodenetomy combined with hepatic artery resection following preoperative hepatic arterial embolization. J. Hepatobil. Pancreat. Sci. 2014, 21, 850–855. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Chandra, V.; Louie, J.D.; Rao, S.; Visser, B.C. Preoperative embolization of replaced right hepatic artery prior to pancreaticoduodenectomy. J. Surg. Oncol. 2012, 106, 509–512. [Google Scholar] [CrossRef]
- Ichida, A.; Sakamoto, Y.; Akahane, M.; Ishizawa, T.; Kaneko, J.; Aoki, T.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Successful case of pancreaticoduodenectomy with resection of the hepatic arteries preserving a single aberrant hepatic artery for a pancreatic neuroendocrine tumor: Report of a case. Surg. Today 2015, 45, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.Z.; Ch’ng, J.K. Use of the gastroduodenal artery in hepatic artery reconstruction for iatrogenic hepatic artery injury during laparoscopic total gastrectomy: Case report and review of the literature. Ann. Vasc. Surg. 2020, 66, 666. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Inoue, Y.; Takahashi, Y.; Mise, Y.; Ishizawa, T.; Tanakura, K.; Ito, H.; Saiura, A. Distal Pancreatectomy with Celiac Axis Resection Combined with Reconstruction of the Left Gastric Artery. J. Gastrointest. Surg. 2017, 21, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Weitz, J.; Büchler, M.W. Splenic artery use for arterial reconstruction in pancreatic surgery. Langenbeck Arch. Surg. 2014, 399, 667–671. [Google Scholar] [CrossRef]
- Chamberlain, R.S.; El-Sedfy, A.; Rajkumar, D. Aberrant hepatic arterial anatomy and the whipple procedure: Lessons learned. Am. Surg. 2011, 77, 517–526. [Google Scholar] [CrossRef]
- Shibuya, K.; Kamachi, H.; Orimo, T.; Nagatsu, A.; Shimada, S.; Wakayama, K.; Yokoo, H.; Kamiyama, T.; Taketomi, A. Pancreaticoduodenectomy with Preservation of Collateral Circulation or Revascularization for Biliary Pancreatic Cancer with Celiac Axis Occlusion: A Report of 2 Cases. Am. J. Case Rep. 2018, 19, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Son, T.; Song, J.H.; Choi, S.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Adverse Effects of Ligation of an Aberrant Left Hepatic Artery Arising from the Left Gastric Artery during Radical Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis. J. Gastric Cancer. 2021, 21, 74–83. [Google Scholar] [CrossRef]
- Kundaragi, N.G.; Asthana, S.; Reddy, J.; Lochan, R. Hepatic arterial communicating arcades-Case series and review of literature. Indian J. Radiol. Imaging 2019, 29, 462–467. [Google Scholar] [CrossRef]
- Mine, T.; Murata, S.; Ueda, T.; Takeda, M.; Onozawa, S.; Yamaguchi, H.; Kawano, Y.; Kumita, S. Contribution of extrahepatic collaterals to liver parenchymal circulation after proper hepatic artery embolization. J. Gastroenterol. Hepatol. 2014, 29, 1515–1521. [Google Scholar] [CrossRef]
- Egorov, V.; Kim, P.; Kharazov, A.; Dzigasov, S.; Popov, P.; Rykova, S.; Zelter, P.; Demidova, A.; Kondratiev, E.; Grigorievsky, M.; et al. Hemodynamic, Surgical and Oncological Outcomes of 40 Distal Pancreatectomies with Celiac and Left Gastric Arteries Resection (DP CAR) without Arterial Reconstructions and Preoperative Embolization. Cancers 2022, 14, 1254. [Google Scholar] [CrossRef]
- Kim, P.T.W.; Temple, S.; Atenafu, E.G.; Cleary, S.P.; Moulton, C.-A.; McGilvray, I.D.; Gallinger, S.; Greig, P.D.; Wei, A.C. Aberrant right hepatic artery in pancreaticoduodenectomy for adenocarcinoma: Impact on resectability and postoperative outcomes. HPB 2014, 16, 204–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winer, J.; Can, M.F.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. The current state of robotic-assisted pancreatic surgery. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Appanraj, P.; Mathew, A.P.; Kandasamy, D.; Venugopal, M. CT reporting of relevant vascular variations and its implication in pancreatoduodenectomy. Abdom. Radiol. 2021, 46, 3935–3945. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Zenati, M.S.; Boone, B.A.; Steve, J.; Hogg, M.E.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. Robotic pancreaticoduodenectomy in the presence of aberrant or anomalous hepatic arterial anatomy: Safety and oncologic outcomes. HPB 2015, 17, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Gonzalez-Heredia, R.; Daskalaki, D.; Rashdan, M.; Masrur, M.; Giulianotti, P.C. Totally replaced right hepatic artery in pancreaticoduodenectomy: Is this anatomical condition a contraindication to minimally invasive surgery? HPB 2016, 18, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, B.T.; Birk, J.W. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J. Clin. Transl. Hepatol. 2019, 7, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Mourad, M.M.; Liossis, C.; Gunson, B.K.; Mergental, H.; Isaac, J.; Muiesan, P.; Mirza, D.F.; Perera, M.T.; Bramhall, S.R. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transplant. 2014, 20, 713–723. [Google Scholar]
- Roos, F.J.M.; Poley, J.W.; Polak, W.G.; Metselaar, H.J. Biliary complications after liver transplantation; recent developments in etiology, diagnosis and endoscopic treatment. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 227–235. [Google Scholar] [CrossRef]
- Dala Riva, D.F.; Nacif, L.S.; Fernandes, M.R.; Silva, N.A.; Pinheiro, R.S.; Rocha-Santos, V.; De Martino, R.B.; Waisberg, D.R.; Macedo, R.A.; Ducatti, L.; et al. Relationship of anatomic variations and arterial reconstruction of the hepatic artery: Prevalence and effect on orthotopic liver transplantation. Transplant. Proc. 2022, 54, 1313–1315. [Google Scholar] [PubMed]
- Atay, A.; Gungor, F.; Sur, Y.; Gunes, O.; Dilek, F.H.; Karasu, Ş.; Dilek, O.N. Management of hepatic artery trauma during hepato-pancreato-biliary procedures: Evolving approaches, clinical outcomes, and literature review. Ulus Travma Acil. Cerrahi. Derg. 2022, 28, 1549–1557. [Google Scholar]
- Gauthier, S.V.; Voskanov, M.A.; Monakhov, A.R.; Semash, K.O. The role of endovascular and endobiliary methods in the treatment of complications after liver transplantation. Bull. Transpl. Artif. Organs 2021, 22, 140–148. [Google Scholar]
- Delgado-Moraleda, J.J.; Ballester-Vallés, C.; Marti-Bonmati, L. Role of imaging in the evaluation of vascular complications after liver transplantation. Insights Imaging 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andraus, W.; Haddad, L.B.P.; Ducatti, L.; Martino, R.B.; Santos, V.R.; D’Albuquerque, L.A. Reconstrução arterial no transplante hepático: A Melhor Reconstrução Para Variação da Artéria Hepática direita. ABCD Arq. Bras. Cir. Dig. 2013, 26, 62–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Gómez, L.M.; Bernal-Bellido, C.; Alamo-Martínez, J.M.; Porras-López, F.M.; Suárez-Artacho, G.; Serrano-Diaz-Canedo, J.; Padillo-Ruiz, J.; Gómez-Bravo, M.A. Intraoperative hepatic artery blood flow predicts early hepatic artery thrombosis after liver transplantation. Transpl. Proc. 2012, 44, 2078–2081. [Google Scholar] [CrossRef]
- Castiñeiras, M.B.; Linero, I.B.; Zarcero, V.S.; Castellano, G.F.; Suárez-Artacho, G.; Romero, J.L. Hepatic artery thrombosis after orthotopic liver transplant: Experience in the last 10 years. Transplant. Proc. 2022, 54, 51–53. [Google Scholar] [CrossRef]
- Pérez-Saborido, B.; Pacheco-Sánchez, D.; Barrera Rebollo, A.; Pinto Fuentes, P.; Asensio Díaz, E.; Labarga Rodriguez, F.; Sarmentero Prieto, J.C.; Martínez Díez, R.; Rodríguez Vielba, P.; Gonzálo Martín, M.; et al. Incidence of hepatic artery variations in liver transplantation: Does it really influence short- and long-term results? Transpl. Proc. 2012, 44, 2606–2608. [Google Scholar] [CrossRef]
- Ozsoy, M.; Zeytunlu, M.; Kilic, M.; Alper, M.; Sozbilen, M. The results of vascular and biliary variations in turks liver donors: Comparison with others. ISRN Surg. 2011, 2011, 367083. [Google Scholar] [CrossRef] [Green Version]
- Fouzas, I.; Papanikolaou, C.; Katsanos, G.; Antoniadis, N.; Salveridis, N.; Karakasi, K.; Vasileiadou, S.; Fouza, A.; Mouloudi, E.; Imvrios, G.; et al. Hepatic Artery Anatomic Variations and Reconstruction in Liver Grafts Procured in Greece: The Effect on Hepatic Artery Thrombosis. Transpl. Proc. 2019, 51, 416–420. [Google Scholar] [CrossRef]
- Karakoyun, R.; Romano, A.; Yao, M.; Dlugosz, R.; Ericzon, B.G.; Nowak, G. Impact of Hepatic Artery Variations and Reconstructions on the Outcome of Orthotopic Liver Transplantation. World J. Surg. 2020, 44, 1954–1965. [Google Scholar] [CrossRef] [Green Version]
- Schroering, J.R.; Kubal, C.A.; Fridell, J.A.; Hathaway, T.J.; Robinson, R.C.; Mangus, R.S. Impact of Variant Donor Hepatic Arterial Anatomy on Clinical Graft Outcomes in Liver Transplantation. Liver Transpl. 2018, 24, 1481–1484. [Google Scholar] [CrossRef] [Green Version]
- Werner, M.J.M.; de Meijer, V.E.; Adelmeijer, J.; de Kleine, R.H.J.; Scheenstra, R.; Bontemps, S.T.H.; Reyntjens, K.M.E.M.; Hulscher, J.B.F.; Lisman, T.; Porte, R.J. Evidence for a rebalanced hemostatic system in pediatric liver transplantation: A prospective cohort study. Am. J. Transplant. 2020, 20, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberkofler, C.E.; Raptis, D.A.; Müller, P.C.; Sousa da Silva, R.X.; Lehmann, K.; Ito, T.; Owen, T.; Pollok, J.M.; Parente, A.; Schlegel, A.; et al. Low-dose aspirin confers protection against acute cellular allograft rejection after primary liver transplantation. Liver Transpl. 2022, 28, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Shay, R.; Taber, D.; Pilch, N.; Meadows, H.; Tischer, S.; McGillicuddy, J.; Bratton, C.; Baliga, P.; Chavin, K. Early aspirin therapy may reduce hepatic artery thrombosis in liver transplantation. Transplant. Proc. 2013, 45, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, H.; Almaas, R.; Thorgersen, E.B.; Foss, A.; Line, P.D.; Sanengen, T.; Bergmann, G.B.; Ohlin, P.; Waelgaard, L.; Grindheim, G.; et al. Clinical experience with microdialysis catheters in pediatric liver transplants. Liver Transpl. 2013, 19, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, O.; Osman, A.M.A.; Hosny, K.A.; Emad-Eldin, S.; Serour, D.K.; Mostafa, M. Management of early hepatic artery thrombosis following living-donor liver transplantation: Feasibility, efficacy and potential risks of endovascular therapy in the first 48 hours post-transplant—A retrospective cohort study. Transpl. Int. 2021, 34, 1134–1149. [Google Scholar] [CrossRef]
- Lau, N.S.; Jacques, A.; McCaughan, G.; Crawford, M.; Liu, K.; Pulitano, C. Addressing the challenges of split liver transplantation through technical advances. A systematic review. Transpl. Rev. 2021, 35, 100627. [Google Scholar] [CrossRef]
- Abdelrahman, H.; Ajaj, A.; Atique, S.; El-Menyar, A.; Al-Thani, H. Conservative management of major liver necrosis after angioembolization in a patient with blunt trauma. Case Rep. Surg. 2013, 2013, 954050. [Google Scholar] [CrossRef]
- Di Saverio, S.; Moore, E.E.; Tugnoli, G.; Naidoo, N.; Ansaloni, L.; Bonilauri, S.; Cucchi, M.; Catena, F. Non operative management of liver and spleen traumatic injuries: A giant with clay feet. World J. Emerg. Surg. 2012, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Zago, T.M.; Tavares Pereira, B.M.; Araujo Calderan, T.R.; Godinho, M.; Nascimento, B.; Fraga, G.P. Nonoperative management for patients with grade IV blunt hepatic trauma. World J. Emerg. Surg. 2012, 7, S8. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.; Sheth, R.A. Hepatic trauma. Ann. Transl. Med. 2021, 9, 1195. [Google Scholar] [CrossRef]
- Kanani, A.; Sandve, K.O.; Søreide, K. Management of severe liver injuries: Push, pack, pringle—And plug! Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment Strategies for Hepatocellular Carcinoma—A Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsin, I.-F.; Hsu, C.-Y.; Huang, H.-C.; Huang, Y.-H.; Lin, H.-C.; Lee, R.-C.; Chiang, J.-H.; Lee, F.-Y.; Huo, T.-I.; Lee, S.-D. Liver Failure After Transarterial Chemoembolization for Patients With Hepatocellular Carcinoma and Ascites: Incidence, Risk Factors, and Prognostic Prediction. J. Clin. Gastroenterol. 2011, 45, 556–562. [Google Scholar] [CrossRef]
- Fu, Y.; Lewis, M.R.; Mitchao, D.P.; Benjamin, E.R.; Wong, M.; Demetriades, D. Gunshot wound versus blunt liver injuries: Different liver-related complications and outcomes. Eur. J. Trauma Emerg. Surg. 2023, 49, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Gruber-Rouh, T. HCC: Transarterial Therapies-What the Interventional Radiologist Can Offer. Dig. Dis. Sci. 2019, 64, 959–967. [Google Scholar] [CrossRef]
- Leonhardt, H.; Thilander-Klang, A.; Båth, J.; Johannesson, M.; Kvarnström, N.; Dahm-Kähler, P.; Brännström, M. Imaging evaluation of uterine arteries in potential living donors for uterus transplantation: A comparative study of MRA, CTA, and DSA. Eur. Radiol. 2022, 32, 2360–2371. [Google Scholar] [CrossRef]
- Feng, Y.; Shu, S.J. Diagnostic value of low-dose 256-slice spiral CT angiography, MR angiography, and 3D-DSA in cerebral aneurysms. Dis. Mark. 2020, 2020, 8536471. [Google Scholar] [CrossRef]
- Lescher, S.; Gehrisch, S.; Klein, S.; Berkefeld, J. Time-resolved 3D rotational angiography: Display of detailed neurovascular anatomy in patients with intracranial vascular malformations. J. NeuroInterv. Surg. 2017, 9, 887–894. [Google Scholar] [CrossRef]
- Sailer, A.M.; Grutters, J.P.; Wildberger, J.E.; Hofman, P.A.; Wilmink, J.T.; van Zwam, W.H. Cost-effectiveness of CTA, MRA and DSA in patients with non-traumatic subarachnoid haemorrhage. Insights Imaging 2013, 4, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, G.A.; Sotiropoulos, A.; Lianos, G.D.; Zigouris, A.; Metaxas, D.; Nasios, A.; Michos, E.; Mitsis, M.; Pachatouridis, D.; Voulgaris, S. Blood glucose levels may aid the decision for CT scan in minor head trauma. Dis. Mark. 2019, 2019, 1065254. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, Y.; Tong, H.; Dong, Y.; Ma, D.; Xu, L.; Yang, C. Meta-analysis of computed tomography angiography versus magnetic resonance angiography for intracranial aneurysm. Medicine 2018, 97, e10771. [Google Scholar] [CrossRef] [PubMed]
- El-Nakeep, S.; Ziska, S.K. Doppler Liver Assessment, Protocols, and Interpretation of Results. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Torres Rojas, A.M.; Lorente, S.; Hautefeuille, M.; Sanchez-Cedillo, A. Hierarchical Modeling of the Liver Vascular System. Front. Physiol. 2021, 12, 733165. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. 2001 2020, 47, 239–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver—Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.M.; Fung, A.; Kambadakone, A.R.; Yeh, B.M. Computed Tomography Techniques, Protocols, Advancements, and Future Directions in Liver Diseases. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 305–320. [Google Scholar] [CrossRef]
- Chartampilas, E.; Rafailidis, V.; Georgopoulou, V.; Kalarakis, G.; Hatzidakis, A.; Prassopoulos, P. Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers 2022, 14, 3997. [Google Scholar] [CrossRef]
- Ueda, K.; Matsui, O.; Kitao, A.; Kobayashi, S.; Nakayama, J.; Miyagawa, S.; Kadoya, M. Tumor Hemodynamics and Hepa tocarcinogenesis: Radio-Pathological Correlations and Outcomes of Carcinogenic Hepatocyte Nodules. ISRN Hepatol. 2014, 2014, 607628. [Google Scholar] [CrossRef] [Green Version]
- Rimola, J.; Forner, A.; Tremosini, S.; Reig, M.; Vilana, R.; Bianchi, L.; Rodríguez-Lope, C.; Solé, M.; Ayuso, C.; Bruix, J. Non-invasive diagnosis of hepatocellular carcinoma ≤2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J. Hepatol. 2012, 56, 1317–1323. [Google Scholar] [CrossRef]
- Ronot, M.; Clift, A.K.; Vilgrain, V.; Frilling, A. Functional imaging in liver tumours. J. Hepatol. 2016, 65, 1017–1030. [Google Scholar] [CrossRef] [Green Version]
- Cannella, R.; Sartoris, R.; Grégory, J.; Garzelli, L.; Vilgrain, V.; Ronot, M.; Dioguardi Burgio, M. Quantitative magnetic resonance imaging for focal liver lesions: Bridging the gap between research and clinical practice. Br. J. Radiol. 2021, 94, 20210220. [Google Scholar] [CrossRef]
- Pauwels, E.K.; Coumou, A.W.; Kostkiewicz, M.; Kairemo, K. [18F] fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging in oncology: Initial staging and evaluation of cancer therapy. Med. Princ. Pract. 2013, 22, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.; Royalty, K.; Kowarschik, M.; Rohkohl, C.; Oberstar, E.; Aagaard-Kienitz, B.; Niemann, D.; Ozkan, O.; Strother, C.; Mistretta, C. 4D Digital Subtraction Angiography: Implementation and Demonstration of Feasibility. Am. J. Neuroradiol. 2013, 34, 1914–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meram, E.; Shaughnessy, G.; Longhurst, C.; Hoffman, C.; Wagner, M.; Mistretta, C.A.; Speidel, M.A.; Laeseke, P.F. Optimization of quantitative time-resolved 3D (4D) digital subtraction angiography in a porcine liver model. Eur. Radiol. Exp. 2020, 4, 37. [Google Scholar] [CrossRef] [PubMed]




| Anatomical Variation of Hepatic Artery | Michel Classification | Hiatt Classification |
|---|---|---|
| Normal anatomy | Type I | Type I |
| LHA branch LGA | Type II | Type II |
| RHA branch SMA | Type III | Type III |
| Type I and II association | Type IV | Type IV |
| LHA accessory LGA | Type V | Type II |
| RHA accessory SMA | Type VI | Type III |
| LHA accessory LGA + RHA accessory SMA | Type VII | Type IV |
| LHA accessory LGA+ RHA branch SMA | Type VIII | Type IV |
| CHA branch SMA | Type IX | Type V |
| RHA and LHA branch LGA | Type X | ------ |
| CHA aorta branch | ------ | Type VI |
| S. No. | Category | Subcategory |
|---|---|---|
| 1. | “Y” | (i) “Y”; CHA (normal anatomy) |
| (ii) “Y”; CHA-CMA (celiomesentric trunk type) | ||
| (iii) “Y”; CHA-SMA (CHA from SMA) | ||
| (iv) “Y”; CHA-Ao (CHA from aorta) | ||
| 2. | “Y plus I” | (i) “I, Y”; SMA, CHA (accessory RHA from SMA) |
| (ii) “I, Y”; Ao, CHA (accessory RHA from aorta) | ||
| (iii) “I, Y”; GDA, CHA (accessory RHA from GDA) | ||
| (iv) “I, Y”; CHA, LGA (accessory LHA from LGA) | ||
| (v) “I, Y”, I”; SMA, CHA, LGA (accessory RHA from SMA and accessory LHA from LGA) | ||
| 3. | “I-I” | (i) “I-I”; SMA, CHA (RHA from SMA) |
| (ii) “I-I”; GDA, CHA (RHA from GDA) | ||
| (iii) “I-I”; CHA, LGA (LHA from LGA) | ||
| (iv) “I-I”; CHA, GDA (LHA from GDA) | ||
| (v) “I-I”; CHA, LGA-Ao (LHA from LGA and LGA from aorta) | ||
| (vi) “I-I”; CHA, Ao (RHA & LHA separately from aorta and GDA from RHA) | ||
| (vii) “I-I”; Ao, CHA (RHA & LHA separately from aorta and GDA from LHA) | ||
| (viii) “I-I”; SMA, GDA (LHA from GDA and RHA from SMA) | ||
| (ix) “I-I”; SMA, Ao (LHA from aorta and RHA from SMA) | ||
| (x) “I-I”; SMA, LGA (LHA from LGA and RHA from SMA) | ||
| 4. | “I-I plus I” | (i) “I-I, I”; SMA, CHA, LGA (accessory LHA from LGA and RHA from SMA) |
| (ii) “I, I-I”; SMA, CHA, LGA (LHA from LGA and accessory RHA from SMA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malviya, K.K.; Verma, A. Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries: A Review Emphasizing Origin and Branching. Diagnostics 2023, 13, 1233. https://doi.org/10.3390/diagnostics13071233
Malviya KK, Verma A. Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries: A Review Emphasizing Origin and Branching. Diagnostics. 2023; 13(7):1233. https://doi.org/10.3390/diagnostics13071233
Chicago/Turabian StyleMalviya, Kapil Kumar, and Ashish Verma. 2023. "Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries: A Review Emphasizing Origin and Branching" Diagnostics 13, no. 7: 1233. https://doi.org/10.3390/diagnostics13071233
APA StyleMalviya, K. K., & Verma, A. (2023). Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries: A Review Emphasizing Origin and Branching. Diagnostics, 13(7), 1233. https://doi.org/10.3390/diagnostics13071233







