Evaluation of Trabecular Microstructure of Cancellous Bone Using Quarter-Detector Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. CT Imaging
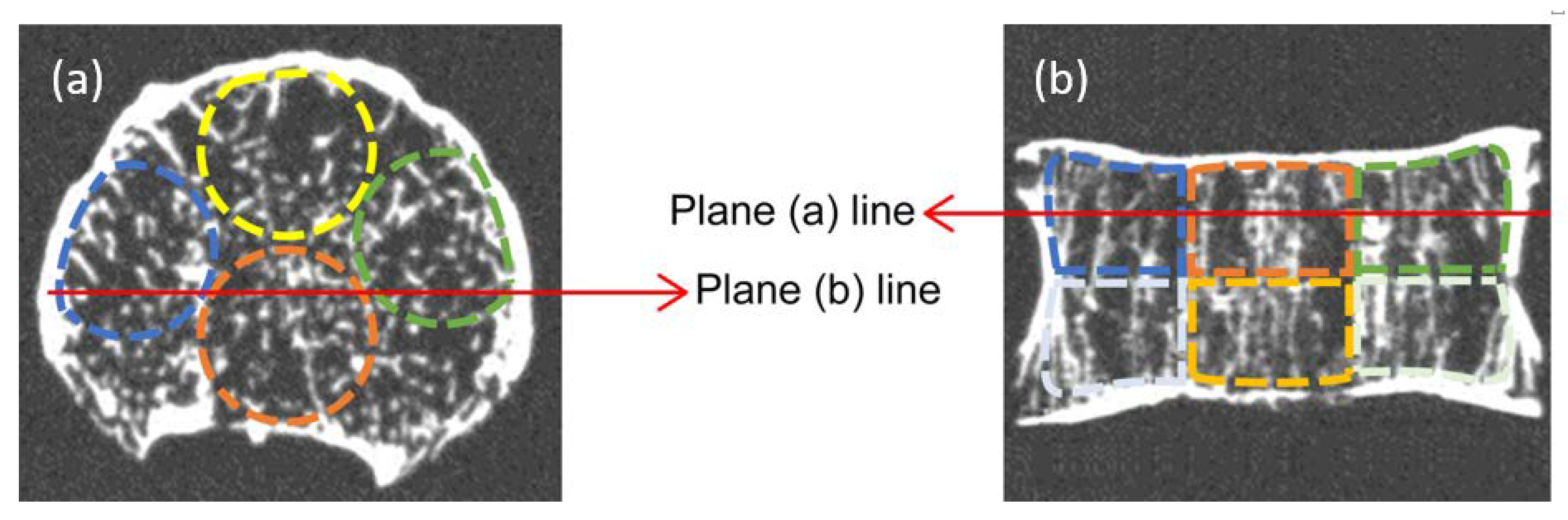
2.2. Bone Morphometry
2.3. Statistical Analysis
3. Results
3.1. Morphometric Parameters of Cancellous Bone Microstructure
3.2. Relationship between Cancellous Bone Morphometric Parameters as Measured by Different Modalities
3.3. Characteristics of Cancellous Bone Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oostveen, L.J.; Boedeker, K.L.; Brink, M.; Prokop, M.; de Lange, F.; Sechopoulos, I. Physical evaluation of an ultra-high-resolution CT scanner. Eur. Radiol. 2020, 30, 2552–2560. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, M.; Hata, A.; Honda, O.; Kikuchi, N.; Miyata, T.; Uranishi, A.; Tsukagoshi, S.; Tomiyama, N. Subjective and objective comparisons of image quality between ultra-high-resolution CT and conventional area detector CT in phantoms and cadaveric human lungs. Eur. Radiol. 2018, 28, 5060–5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, Y.; Kurokawa, R.; Maeda, E.; Mori, H.; Amemiya, S.; Sato, J.; Ino, K.; Torigoe, R.; Abe, O. Evaluation of peripheral bronchiole visualization using model-based iterative reconstruction in quarter-detector computed tomography. PLoS ONE 2020, 15, e0239459. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Murayama, K.; Suzuki, S.; Watanabe, A.; Hayakawa, M.; Saito, Y.; Katada, K.; Toyama, H. Initial clinical experience of a prototype ultra-high-resolution CT for assessment of small intracranial arteries. Jpn. J. Radiol. 2019, 37, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ito, H.; Sarai, M.; Nagahara, Y.; Miyajima, K.; Matsumoto, R.; Doi, Y.; Kataoka, Y.; Takahashi, H.; Ozaki, Y.; et al. Ultra-High-resolution computed tomography angiography for assessment of coronary artery stenosis. Circ. J. 2018, 82, 1844–1851. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Matsumoto, N.; Momosaka, D.; Nakatake, H.; Sakai, Y.; Hond, A.H. Ultrahigh-resolution CT scan of the temporal bone. Eur. Arch. Otorhinolaryngol. 2018, 275, 2797–2803. [Google Scholar] [CrossRef]
- Kleerekoper, M.; Villanueva, A.R.; Stanciu, J.; Rao, D.S.; Parfitt, A.M. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif. Tissue. Int. 1985, 37, 594–597. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Mathews, C.H.; Villanueva, A.R.; Kleerekoper, M.; Frame, B.; Rao, D.S. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J. Clin. Investig. 1983, 72, 1396–1409. [Google Scholar] [CrossRef]
- Chiba, K.; Ito, M.; Osaki, M.; Uetani, M.; Shindo, H. In vivo structural analysis of subchondral trabecular bone in osteoarthritis of the hip using multi-detector row CT. Osteoarthr. Cartil. 2011, 19, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Goff, E.; Buccino, F.; Bregoli, C.; McKinley, J.P.; Aeppli, B.; Recker, R.R.; Shane, E.; Cohen, A.; Kuhn, G.; Müller, R. Large-scale quantification of human osteocyte lacunar morphological biomarkers as assessed by ultra-high-resolution desktop micro-computed tomography. Bone 2021, 152, 116094. [Google Scholar] [CrossRef]
- Goff, E.; Cohen, A.; Shane, E.; Recker, R.R.; Kuhn, G.; Müller, R. Large-scale osteocyte lacunar morphological analysis of transiliac bone in normal and osteoporotic premenopausal women. Bone 2022, 160, 116424. [Google Scholar] [CrossRef]
- Klintström, E.; Klintström, B.; Spångeus, A.; Sandborg, M.; Woisetschläger, M. Trabecular bone microstructure analysis on data from a novel twin robotic X-ray device. Acta Radiol. 2022, 13. [Google Scholar] [CrossRef]
- Legrand, E.; Chappard, D.; Pascaretti, C.; Duquenne, M.; Krebs, S.; Rohmer, V.; Basle, M.F.; Audran, M. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J. Bone Min. Res. 2000, 15, 13–19. [Google Scholar] [CrossRef]
- Legrand, E.; Audran, M.; Guggenbuhl, P.; Levasseur, R.; Chalès, G.; Baslé, M.F.; Chappard, D. Trabecular bone microarchitecture is related to the number of risk factors and etiology in osteoporotic men. Microsc. Res. Tech. 2007, 70, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Ikeda, K.; Nishiguchi, M.; Shindo, H.; Uetani, M.; Hosoi, T.; Orimo, H. Multi-detector row CT imaging of vertebral microstructure for evaluation of fracture risk. J. Bone Min. Res. 2005, 20, 1828–1836. [Google Scholar] [CrossRef]
- Cortet, B.; Dubois, P.; Boutry, N.; Bourel, P.; Cotton, A.; Marchandise, X. Image analysis of the distal radius trabecular network using computed tomography. Osteoporos. Int. 1999, 9, 410–419. [Google Scholar] [CrossRef]
- Gordon, C.L.; Lang, T.F.; Augat, P.; Genant, H.K. Image-based assessment of spinal trabecular bone structure from high-resolution CT images. Osteoporos. Int. 1998, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Boutroy, S.; Bouxsein, M.L.; Munoz, F.; Delmas, P.D. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J. Clin. Endocrinol. Metab. 2005, 90, 6508–6515. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.E. Quantitative computed tomography. Eur. J. Radiol. 2009, 71, 415–424. [Google Scholar] [CrossRef]
- Metcalf, L.M.; Dall’Ara, E.; Paggiosi, M.A.; Rochester, J.R.; Vilayphiou, N.; Kemp, G.J.; McCloskey, E.V. Validation of calcaneus trabecular microstructure measurements by HR-pQCT. Bone 2018, 10, 69–77. [Google Scholar] [CrossRef]
- Powles, T.J.; Hickish, T.; Kanis, J.A.; Tidy, A.; Ashley, S. Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J. Clin. Oncol. 1996, 14, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdóttir, A.; Fornander, T.; Jacobsson, H.; von Schoultz, E.; Rutqvist, L.E. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J. Clin. Oncol. 2004, 22, 3694–3699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, C.L.; Manola, J.; Leboff, M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J. Clin. Oncol. 2001, 19, 3306–3311. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Goode, M.; Zietman, A.L.; McGovern, F.J.; Lee, H.; Finkelstein, J.S. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: Effects on bone mineral density and body composition. J. Clin. Oncol. 2004, 22, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Sieber, P.R.; Keiller, D.L.; Kahnoski, R.J.; Gallo, J.; McFadden, S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. J. Urol. 2004, 171, 2272–2276. [Google Scholar] [CrossRef]
- Hillner, B.E.; Ingle, J.N.; Chlebowski, R.T.; Gralow, J.; Yee, G.C.; Janjan, N.A.; Cauley, J.A.; Blumenstein, B.A.; Albain, K.S.; Lipton, A.; et al. American Society of Clinical Oncology. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003, 21, 4042–4057. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Nango, N.; Kubota, S.; Horiguchi, Y. Three-dimensional microstructural measurement for predicting the risk of osteoporotic fracture. In Osteoporotic Fracture and Systemic Skeletal Disorders; Takahashi, H.E., Burr, D.B., Yamamoto, N., Eds.; Springer: Singapore, 2022; pp. 129–160. [Google Scholar]
- Odgaard, A.; Gundersen, H.J. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 1993, 14, 173–182. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Lynch, J.A.; Hawkes, D.J.; Buckland-Wright, J.C. Analysis of texture in macroradiographs of osteoarthritic knees using the fractal signature. Phys. Med. Biol. 1991, 36, 709–722. [Google Scholar] [CrossRef]
- Hahn, M.; Vogel, M.; Pompesius-Kempa, M.; Delling, G. Trabecular bone pattern factor--a new parameter for simple quantification of bone microarchitecture. Bone 1992, 13, 327–330. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput. Methods Biomech. Biomed. Eng. 1997, 1, 15–23. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, D.; Li, C.; Janz, K.F.; Burns, T.L.; Torner, J.C.; Levy, S.M.; Saha, P.K. A robust algorithm for thickness computation at low resolution and its application to in vivo trabecular bone CT imaging. IEEE Trans. Biomed. Eng. 2014, 61, 2057–2069. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Subramanian, S.; Cao, Q.; Demehri, S.; Siewerdsen, J.H.; Zbijewski, W. Application of a novel ultra-high resolution multi-detector CT in quantitative imaging of trabecular microstructure. Proc. SPIE Int. Soc. Opt. Eng. 2020, 11317, 113171E. [Google Scholar] [CrossRef]
- Klintström, E.; Smedby, O.; Moreno, R.; Brismar, T.B. Trabecular bone structure parameters from 3D image processing of clinical multi-slice and cone-beam computed tomography data. Skelet. Radiol. 2014, 43, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Guha, I.; Klintström, B.; Klintström, E.; Zhang, X.; Smedby, Ö.; Moreno, R.; Saha, P.K. A comparative study of trabecular bone micro-structural measurements using different CT modalities. Phys. Med. Biol. 2020, 65, 235029. [Google Scholar] [CrossRef]
- Pumberger, M.; Issever, A.S.; Diekhoff, T.; Schwemmer, C.; Berg, S.; Palmowski, Y.; Putzier, M. Bone structure determined by HR-MDCT does not correlate with micro-CT of lumbar vertebral biopsies: A prospective cross-sectional human in vivo study. J. Orthop. Surg. Res. 2020, 15, 398. [Google Scholar] [CrossRef]
- Klintström, B.; Henriksson, L.; Moreno, R.; Malusek, A.; Smedby, Ö.; Woisetschläger, M.; Klintström, E. Photon-counting detector CT and energy-integrating detector CT for trabecular bone microstructure analysis of cubic specimens from human radius. Eur. Radiol. Exp. 2022, 6, 31. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone quality--the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, G.; Henebry, G.M. Lacunarity analysis of spatial pattern in CT images of vertebral trabecular bone for assessing osteoporosis. Med. Eng. Phys. 2002, 24, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhang, X.; Guo, J.; Jin, D.; Letuchy, E.M.; Burns, T.L.; Levy, S.M.; Hoffman, E.A.; Saha, P.K. Quantitative imaging of peripheral trabecular bone microarchitecture using MDCT. Med. Phys. 2018, 45, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Jinnai, H.; Watashiba, H.; Kajihara, T.; Nishikawa, Y.; Takahashi, M.; Ito, M. Surface curvatures of trabecular bone microarchitecture. Bone 2002, 30, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Scherf, H.; Tilgner, R. A new high-resolution computed tomography (CT) segmentation method for trabecular bone architectural analysis. Am. J. Phys. Anthr. 2009, 140, 39–51. [Google Scholar] [CrossRef]


| Bone Morphometric Parameters (Abbreviations) | Description |
|---|---|
| Tissue volume (TV) | The volume, including the bone marrow cavity near the cancellous bone; bone tissue volume |
| Bone volume (BV) | Volume of the trabecular cancellous bone |
| Bone surface (BS) | Surface area of the trabecular cancellous bone |
| Bone volume fraction (BV/TV) | Volume of the cancellous bone relative to the bone marrow cavity; trabecular bone volume fraction |
| Connectivity density (Conn.D) [29,30] | Connectivity density of trabecular bone structure; when b is the number of branches of the trabecular bone structure, it is calculated as b/TV |
| Total strut length (TSL) [29] | The total distance of the skeletal network; represented by classifying trabeculae into connection points between the trabeculae, end points that are not connected with other trabeculae, and connection points with the cortical bone |
| Trabecular thickness (Tb.Th) [29,31] | Trabecular bone width; the distance directly measured to the trabecular surface within the trabecular bone; the diameter of the sphere contained in the trabecular bone is twice the resulting value; trabecular thickness is the average maximum diameter of these spheres |
| Trabecular number (Tb.N) [29,31] | Number of trabecular bones; it represents the number of trabeculae per unit length that crosses a straight line perpendicular to the longitudinal direction of the trabecular bone |
| Trabecular separation (Tb.Sp) [29,31] | Trabecular spacing; the distance to the trabecular surface is directly measured within the bone marrow region; the diameter of the spheres contained in the bone marrow is twice the resulting value; trabecular spacing is the mean maximum diameter of these spheres |
| Fractal dimension (FD) [29,32] | Fractal dimensionality; it represents the complexity of the unevenness of the trabecular surface |
| Trabecular bone pattern factor (TBPf) [29,33] | A quantitative index of the three-dimensional concave structure; it represents the change in surface area relative to the change in near-surface volume of trabecular bone; this value is 0 in plate-like structures |
| Structure model index (SMI) [29,34] | An index of the three-dimensional morphology of trabecular bone; 0, 3, and 4 points indicate ideal plate-like, rod-like, and sphere-like structures, respectively |
| Marrow space star volume (V*m.space) [29,35] | An index of continuity in the bone marrow cavity based on the star-line algorithm; it is the average volume of the marrow cavity that can be traced from a central point in the marrow cavity in all directions |
| Trabecular star volume (V*tr) [29,35] | An index of continuity in trabecular bone based on the star-line algorithm; it is the average volume from a central point in the trabecular bone to the trabecular edge in all directions. |
| Bone Morphometric Parameters | Micro-CT | QDCT | p-Value |
|---|---|---|---|
| TV (mm3) | 2102 ± 135 | 2102 ± 134 | 1.00 |
| BV (mm3) | 198.9 ± 26.9 | 655.2 ± 97.5 | * |
| BS (mm2) | 2364 ± 254 | 2988 ± 314 | * |
| BV/TV (%) | 9.470 ± 1.237 | 31.22 ± 4.61 | * |
| Conn.D (1/mm3) | 0.3752 ± 0.0758 | 0.3839 ± 0.0929 | 0.875 |
| TSL (mm) | 2590 ± 324 | 2147 ± 392 | 0.0611 |
| Tb.Th (μm) | 267.6 ± 8.2 | 663.8 ± 58.3 | * |
| Tb.N (1/mm) | 0.2860 ± 0.0317 | 0.2667 ± 0.0290 | 0.270 |
| Tb.Sp (μm) | 996.1 ± 95.1 | 1126.5 ± 220.7 | 0.270 |
| Fractal dimension | 2.104 ± 0.026 | 2.275 ± 0. 014 | * |
| TBPf (1/mm) | 5.483 ± 0.573 | 3.800 ± 0.128 | * |
| SMI | 2.890 ± 0.197 | 5.395 ± 0.413 | * |
| V*m.space (mm3) | 64.91 ± 11.82 | 48.51 ± 16.18 | 0.0661 |
| V*tr (mm3) | 1.425 ± 0.311 | 16.28 ± 4.67 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, H.; Nango, N.; Machida, M. Evaluation of Trabecular Microstructure of Cancellous Bone Using Quarter-Detector Computed Tomography. Diagnostics 2023, 13, 1240. https://doi.org/10.3390/diagnostics13071240
Hasegawa H, Nango N, Machida M. Evaluation of Trabecular Microstructure of Cancellous Bone Using Quarter-Detector Computed Tomography. Diagnostics. 2023; 13(7):1240. https://doi.org/10.3390/diagnostics13071240
Chicago/Turabian StyleHasegawa, Hiroaki, Nobuhito Nango, and Masafumi Machida. 2023. "Evaluation of Trabecular Microstructure of Cancellous Bone Using Quarter-Detector Computed Tomography" Diagnostics 13, no. 7: 1240. https://doi.org/10.3390/diagnostics13071240






