Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion
Abstract
:1. Introduction
2. Methods and Materials
2.1. Statistical Analysis
2.2. Method of Error
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haas, A.J. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965, 35, 200–217. [Google Scholar] [CrossRef]
- Wertz, R.A. Skeletal and dental changes accompanying rapid midpalatal suture opening. Am. J. Orthod. 1970, 58, 41–66. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.A. Maxillary transverse deficiency. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 567–570. [Google Scholar] [CrossRef]
- Garrett, B.J.; Caruso, J.M.; Rungcharassaeng, K.; Farrage, J.R.; Kim, J.S.; Taylor, G.D. Skeletal effects to the maxilla after rapid maxillary expansion assessed with cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Lagravere, M.O.; Heo, G.; Major, P.W.; Flores-Mir, C. Meta-analysis of immediate changes with rapid maxillary expansion treatment. J. Am. Dent. Assoc. 2006, 137, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felippe, N.L.O.; Da Silveira, A.C.; Viana, G.; Kusnoto, B.; Smith, B.; Evans, C.A. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: Short- and long-term effects. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 370–382. [Google Scholar] [CrossRef]
- Franchi, L.; Baccetti, T.; Lione, R.; Fanucci, E.; Cozza, P. Modifications of midpalatal sutural density induced by rapid maxillary expansion: A low-dose computed tomography evaluation. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 486–488. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.W.; Hershey, G.; Turvey, T.A.; Hinton, V.A.; Hairfield, W.M. The nasal airway following maxillary expansion. Am. J. Orthod. 1987, 91, 111–116. [Google Scholar] [CrossRef]
- Da Silva, O.G.; do Prado Montes, L.A.; Torelly, L.F. Rapid maxillary expansion in the deciduous and mixed dentition evaluated through posteroanterior cephalometric analysis. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 268–275. [Google Scholar] [CrossRef]
- Cross, D.L.; McDonald, J.P. Effect of rapid maxillary expansion on skeletal, dental, and nasal structures: A postero-anterior cephalometric study. Eur. J. Orthod. 2000, 22, 519–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basciftci, F.A.; Mutlu, N.; Karaman, A.I.; Malkoc, S.; Küçükkolbasi, H. Does the timing and method of rapid maxillary expansion have an effect on the changes in nasal dimensions? Angle Orthod. 2002, 72, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hershey, H.G.; Stewart, B.L.; Warren, D.W. Changes in nasal airway resistance associated with rapid maxillary expansion. Am. J. Orthod. 1976, 69, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.A.; Itikawa, C.E.; Valera, F.C.; Faria, G.; Anselmo-Lima, W.T. Long-term effects of rapid maxillary expansion on nasal area and nasal airway resistance. Am. J. Rhinol. Allergy 2010, 24, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.M.; Dalci, O.; Ali Darendeliler, M.; Papageorgiou, S.N.; Papadopoulou, A.K. Volumetric upper airway changes after rapid maxillary expansion: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Cappellette, M.; Nagai, L.H.Y.; Gonçalves, R.M.; Yuki, A.K.; Pignatari, S.S.N.; Fujita, R.R. Skeletal effects of RME in the transverse and vertical dimensions of the nasal cavity in mouth-breathing growing children. Dent. Press. J. Orthod. 2017, 22, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Hartgerink, D.V.; Vig, P.S.; Orth, D.; Abbott, D.W. The effect of rapid maxillary expansion on nasal airway resistance. Am. J. Orthod. Dentofac. Orthop. 1987, 92, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Timms, D.J.; Preston, C.B.; Daly, P.F. A computed tomographic assessment of maxillary movement induced by rapid expansion. A pilot study. Eur. J. Orthod. 1982, 4, 123–127. [Google Scholar] [CrossRef]
- Doruk, C.; Sökücü, O.; Sezer, H.; Canbay, E.I. Evaluation of nasal airway resistance during rapid maxillary expansion using acoustic rhinometry. Eur. J. Orthod. 2004, 26, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Clement, P.A.R.; Gordts, F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology 2005, 43, 169–179. [Google Scholar]
- Ceroni Compadretti, G.; Tasca, I.; Alessandri-Bonetti, G.; Peri, S.; D’Addario, A. Acoustic rhinometric measurements in children undergoing rapid maxillary expansion. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 27–34. [Google Scholar] [CrossRef]
- Enoki, C.; Valera, F.C.P.; Lessa, F.C.R.; Elias, A.M.; Matsumoto, M.A.N.; Anselmo-Lima, W.T. Effect of rapid maxillary expansion on the dimension of the nasal cavity and on nasal air resistance. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1225–1230. [Google Scholar] [CrossRef]
- Montgomery, W.M.; Vig, P.S.; Staab, E.V.; Matteson, S.R. Computed tomography: A three-dimensional study of the nasal airway. Am. J. Orthod. 1979, 76, 363–375. [Google Scholar] [CrossRef]
- Podesser, B.; Williams, S.; Bantleon, H.P.; Imhof, H. Quantitation of transverse maxillary dimensions using computed tomography: A methodological and reproducibility study. Eur. J. Orthod. 2004, 26, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, G.; Carey, J.P.; Flores-Mir, C.; Secanell, M.; Hart, A.; Dietrich, N.; Lagravère-Vich, M.O. Three-dimensional cephalometric superimposition of the nasomaxillary complex. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Haralambidis, A.; Ari-Demirkaya, A.; Acar, A.; Küçükkeleş, N.; Ateş, M.; Özkaya, S. Morphologic changes of the nasal cavity induced by rapid maxillary expansion: A study on 3-dimensional computed tomography models. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nguyen, M.; Gohl, E.; Mah, J.K.; Sameshima, G.; Enciso, R. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 71–78. [Google Scholar] [CrossRef]
- Cordasco, G.; Nucera, R.; Fastuca, R. Effects of orthopedic maxillary expansion on nasal cavity size in growing subjects: A low dose computer tomography clinical trial. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.N.C.; De Paiva, J.B.; Rino-Neto, J.; Illipronti-Filho, E.; Trivino, T.; Fantini, S.M. Upper airway expansion after rapid maxillary expansion evaluated with cone beam computed tomography. Angle Orthod. 2012, 82, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Ghoneima, A.; Stewart, K. Three-dimensional computed tomography analysis of airway volume changes after rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 618–626. [Google Scholar] [CrossRef]
- Chang, Y.; Koenig, L.J.; Pruszynski, J.E.; Bradley, T.G.; Bosio, J.A.; Liu, D. Dimensional changes of upper airway after rapid maxillary expansion: A prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 462–470. [Google Scholar] [CrossRef] [Green Version]
- El, H.; Palomo, J.M. Three-dimensional evaluation of upper airway following rapid maxillary expansion A CBCT study. Angle Orthod. 2014, 84, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.M.; Rosenblatt, M.; Witmans, M.; Carey, J.P.; Heo, G.; Major, P.W. Rapid palatal expansion effects on nasal airway dimensions as measured by acoustic rhinometry. Angle Orthod. 2009, 79, 1000–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, T.; Saitoh, I.; Takemoto, Y.; Inada, E.; Kakuno, E.; Kanomi, R.; Hayasaki, H.; Yamasaki, Y. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: A cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, A.; Meneghel, M.; Fastuca, R.; Zecca, P.A.; Nucera, R.; Nosetti, L. Rapid maxillary expansion in growing patients: Correspondence between 3-dimensional airway changes and polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ballanti, F.; Lione, R.; Baccetti, T.; Franchi, L.; Cozza, P. Treatment and posttreatment skeletal effects of rapid maxillary expansion investigated with low-dose computed tomography in growing subjects. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 311–317. [Google Scholar] [CrossRef]
- Podesser, B.; Williams, S.; Crismani, A.G.; Bantleon, H.P. Evaluation of the effects of rapid maxillary expansion in growing children using computer tomography scanning: A pilot study. Eur. J. Orthod. 2007, 29, 37–44. [Google Scholar] [CrossRef]
- Weissheimer, A.; de Menezes, L.M.; Mezomo, M.; Dias, D.M.; de Lima, E.M.; Rizzatto, S.M. Immediate effects of rapid maxillary expansion with Haas-type and hyrax-type expanders: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 366–376. [Google Scholar] [CrossRef]
- Da Silva, O.G.; Lara, T.S.; Silva, H.C.; Bertoz, F.A. Comportamento da sutura palatina mediana em crianças submetidas à expansão rápida da maxila: Avaliação mediante imagem de tomografia computadorizada. Rev. Dent. Press. Ortodon. Ortop. Fac. 2007, 12, 94–103. [Google Scholar] [CrossRef]
- Kartalian, A.; Gohl, E.; Adamian, M.; Enciso, R. Cone-bean computadorized tomography evaluation of the maxillary dentoskeletal complex after rapid palatal expansion. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 786–792. [Google Scholar] [CrossRef]
- Caldas, L.D.; Takeshita, W.M.; Machado, A.W.; Bittencourt, M.A.V. Effect of rapid maxillary expansion on nasal cavity assessed with cone-beam computed tomography. Dent. Press. J. Orthod. 2020, 25, 39–45. [Google Scholar] [CrossRef]
- Inoue, N.; Oyama, K.; Ishiguro, K.; Azuma, M.; Ozaki, T. Radiographic observation of rapid expansion of human maxilla. Bull. Tokyo Med. Dent. Univ. 1970, 17, 249–261. [Google Scholar] [PubMed]
- Iseri, H.; Tekkaya, A.E.; Oztan, O.; Bilgiη, S. Biomechanics effects of rapid maxillary expansion on the craniofacial skeleton, studied by the finite element. Eur. J. Orthod. 1998, 20, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, K.F.; Boucher, N.; Chung, C.H. Effects of bonded rapid palatal expansion on the transverse dimensions of the maxilla: A cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 79–85. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, X. A prospective CBCT study of upper airway changes after rapid maxillary expansion. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1805–1810. [Google Scholar] [CrossRef]
- Bazargani, F.; Feldmann, I.; Bondemark, L. Three-dimensional analysis of effects of rapid maxillary expansion on facial sutures and bones: A systematic review. Angle Orthod. 2013, 7, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, T.; Zou, W. Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur. J. Orthod. 2015, 37, 651–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caprioglio, A.; Fastuca, R.; Zecca, P.A.; Beretta, M.; Mangano, C.; Piattelli, A.; Macchi, A.; Lezzi, G. Cellular Midpalatal suture changes after rapid maxillary expansion in growing subjects: A case report. Int. J. Mol. Sci. 2017, 18, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fastuca, R.; Lorusso, P.; Lagravère, M.O.; Michelotti, A.; Portelli, M.; Zecca, P.A.; D’ Antò, V.; Militi, A.; Nucera, R.; Caprioglio, A. Digital evaluation of nasal changes induced by rapid maxillary expansion with different anchorage and appliance design. BMC Oral. Health 2017, 17, 113. [Google Scholar] [CrossRef] [Green Version]
- Palaisa, J.; Ngan, P.; Martin, C.; Razmus, T. Use of conventional tomography to evaluate changes in the nasal cavity with rapid palatal expansion. Am. J. Orthod. 2007, 132, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Truong, C.T.; Jeon, H.H.; Sripinun, P.; Tierney, A.; Boucher, N.S. Short-term and long-term effects of rapid maxillary expansion on the nasal soft and hard tissue. Angle Orthod. 2021, 91, 46–53. [Google Scholar] [CrossRef]
- Guilleminault, C.; Lee, J.H.; Chan, A. Pediatric obstructive sleep apnea syndrome. Arch. Pediatr. Adolesc. Med. 2005, 159, 775–785. [Google Scholar] [CrossRef]
- Oniani, N.; Sakhelashvili, I.; Supatashvili, M.; Basishvili, T.; Eliozishvili, M.; Maisuradze, L.; Cervena, K. Relationship between sleep disorders and health related quality of life-results from the Georgia SOMNUS study. Int. J. Environ. Res. Public Health 2018, 15, 1588. [Google Scholar] [CrossRef] [Green Version]
- Vogler, K.; Daboul, A.; Obst, A.; Fietze, I.; Ewert, R.; Biffar, R.; Krüger, M. Quality of life in patients with obstructive sleep apnea: Results from the study of health in Pomerania. J. Sleep. Res. 2023, 32, e13702. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Lindsey, C.; Elliott, B.A.; Avis, K.T.; Schwebel, D.C.; Goodin, B.R. Quality of life in youth with obstructive sleep apnea syndrome (OSAS) treated with continuous positive airway pressure (CPAP) therapy. Behav. Sleep. Med. 2019, 17, 238–245. [Google Scholar] [CrossRef]
- Marcus, C.L.; Carroll, J.L.; Koerner, C.B.; Hamer, A.; Lutz, J.; Loughlin, G.M. Determinants of growth in children with the obstructive sleep apnea syndrome. J. Pediatr. 1994, 125, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Melendres, C.S.; Lutz, J.M.; Rubin, E.D.; Marcus, C.L. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics 2004, 114, 768–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]


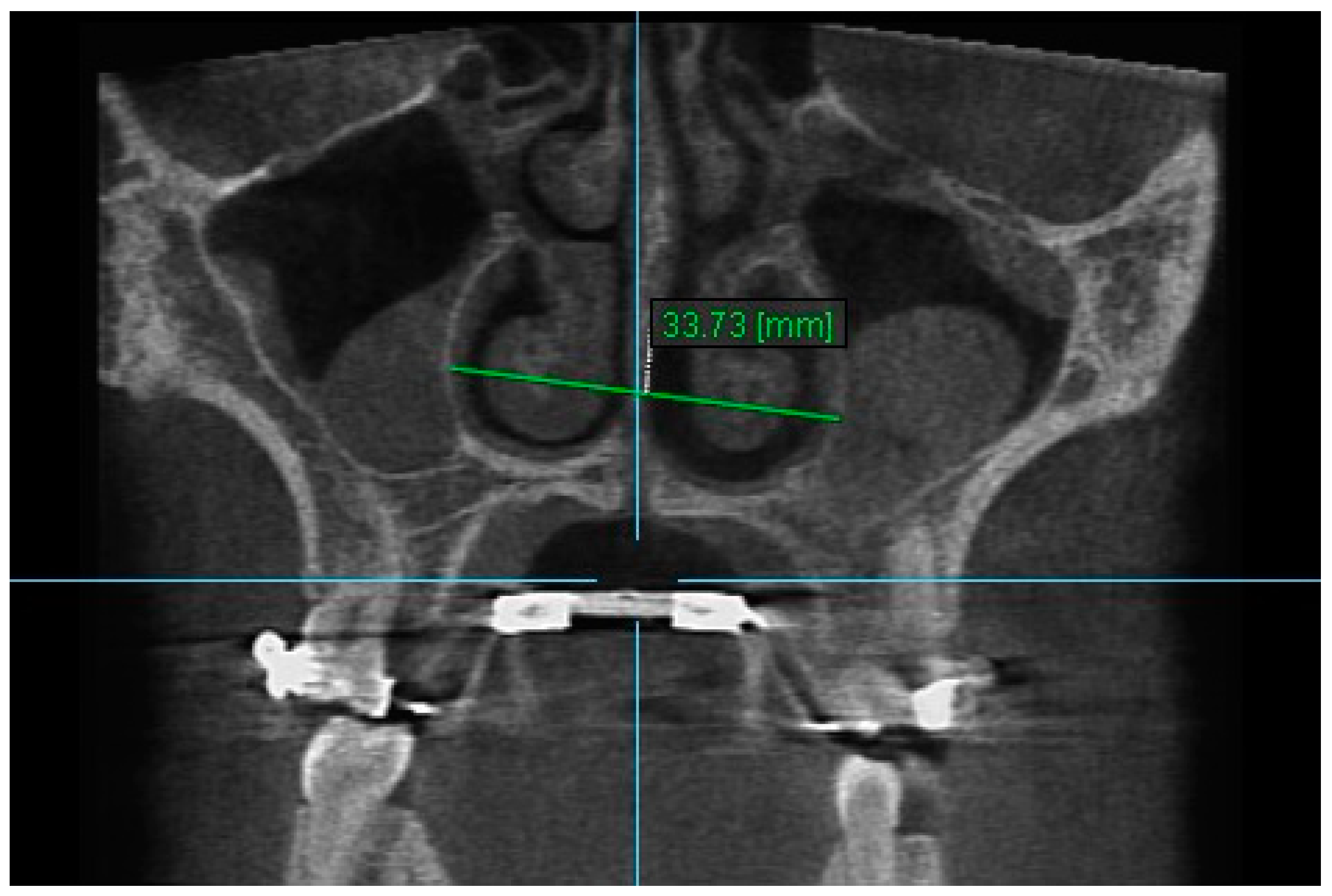

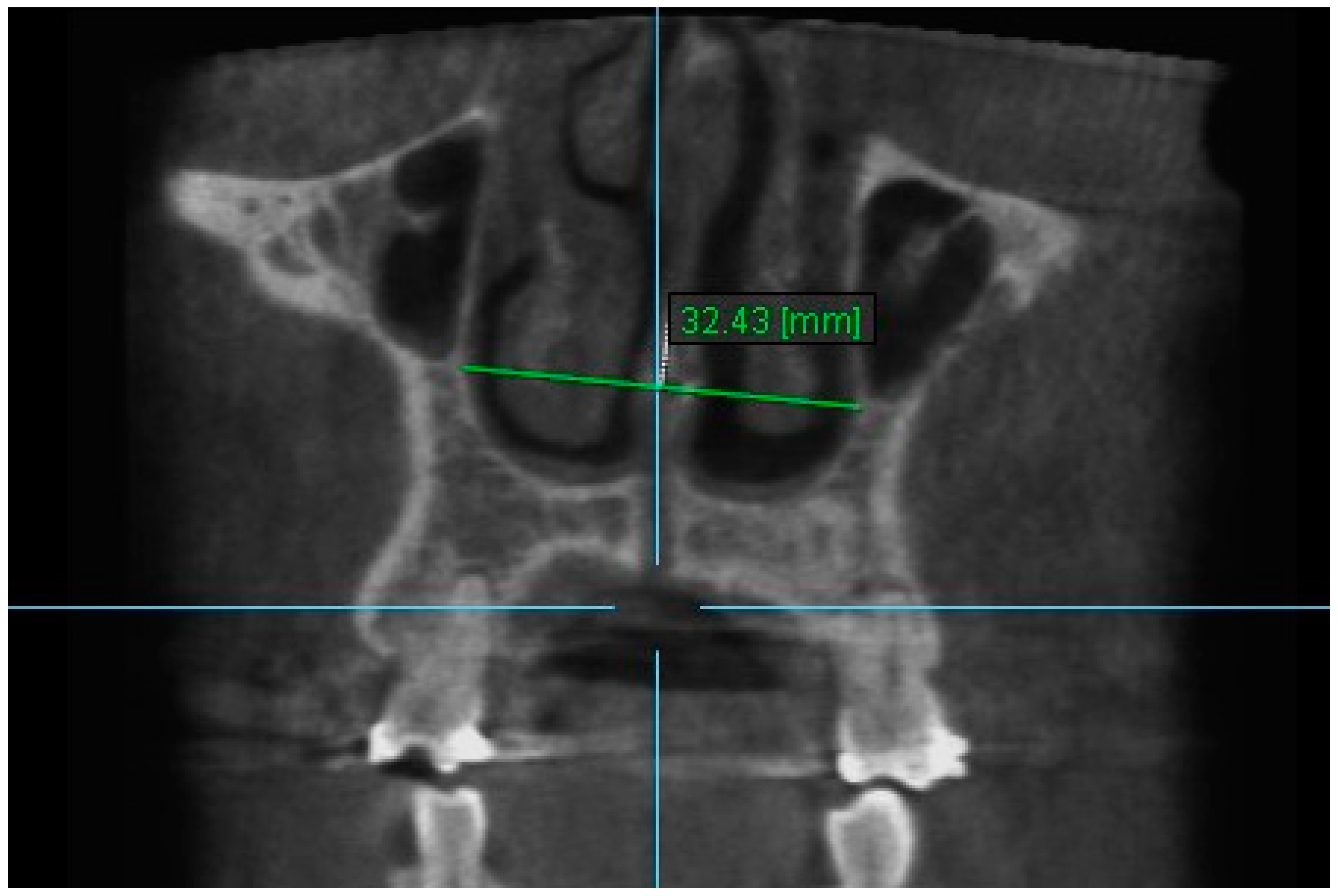

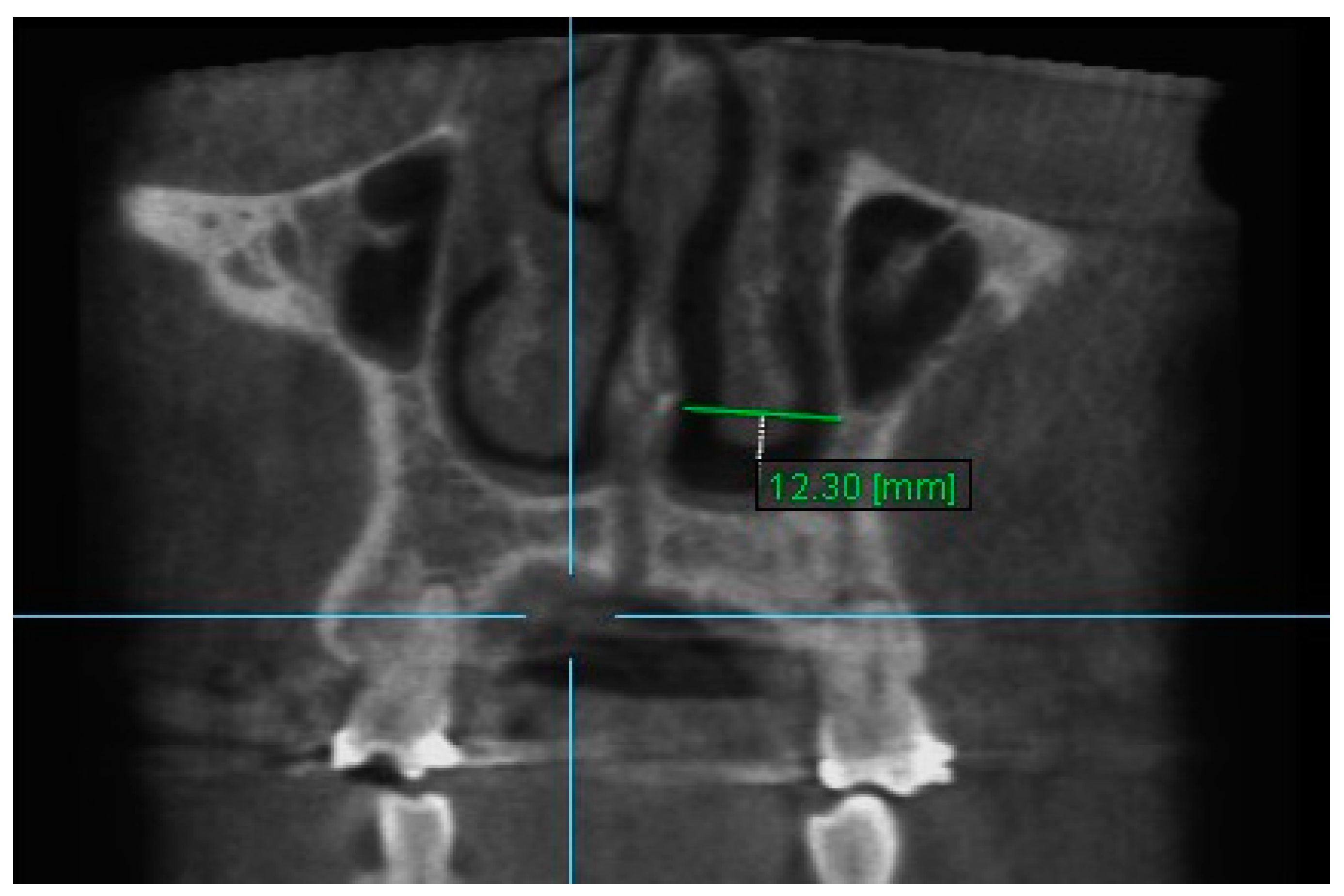
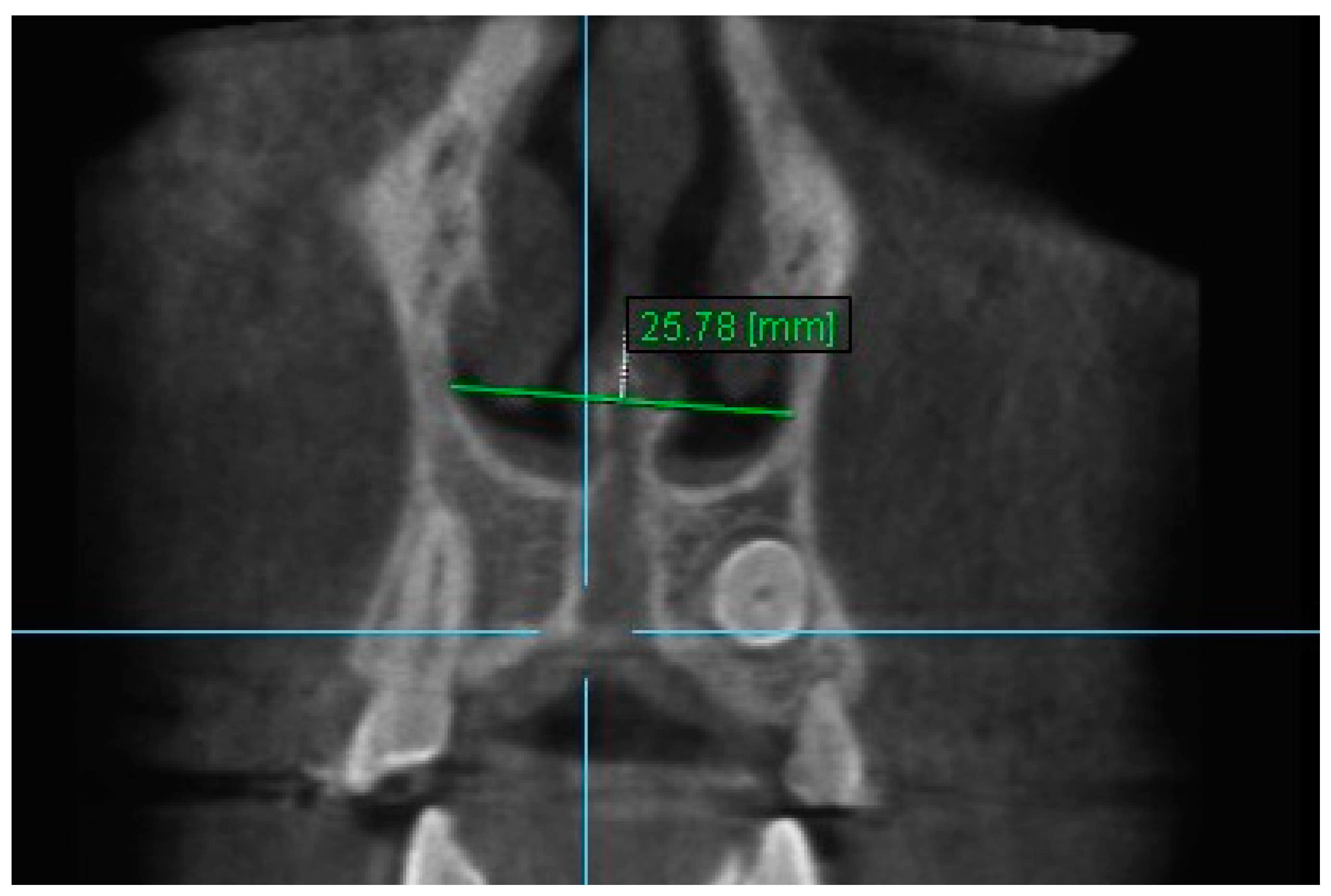
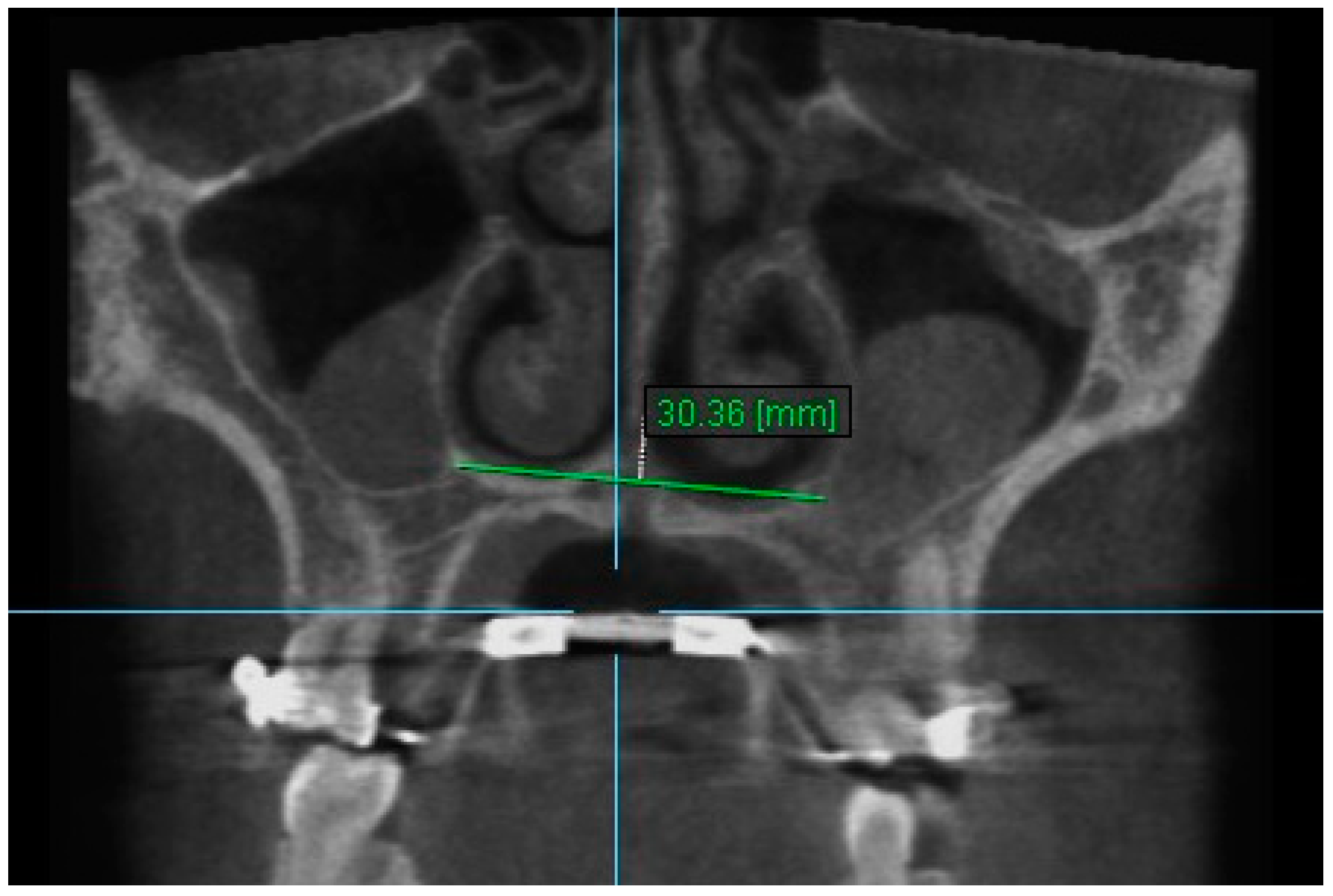
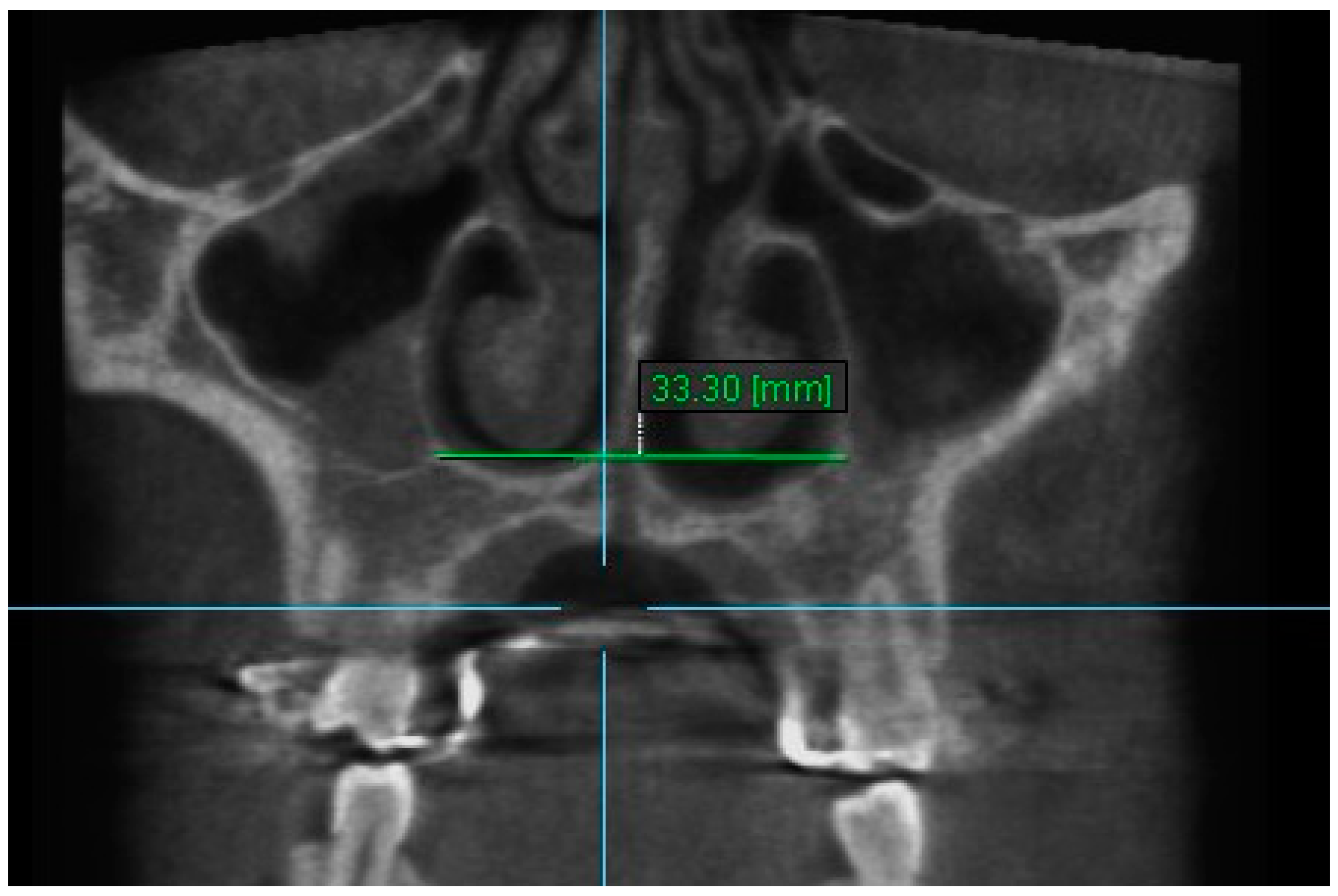

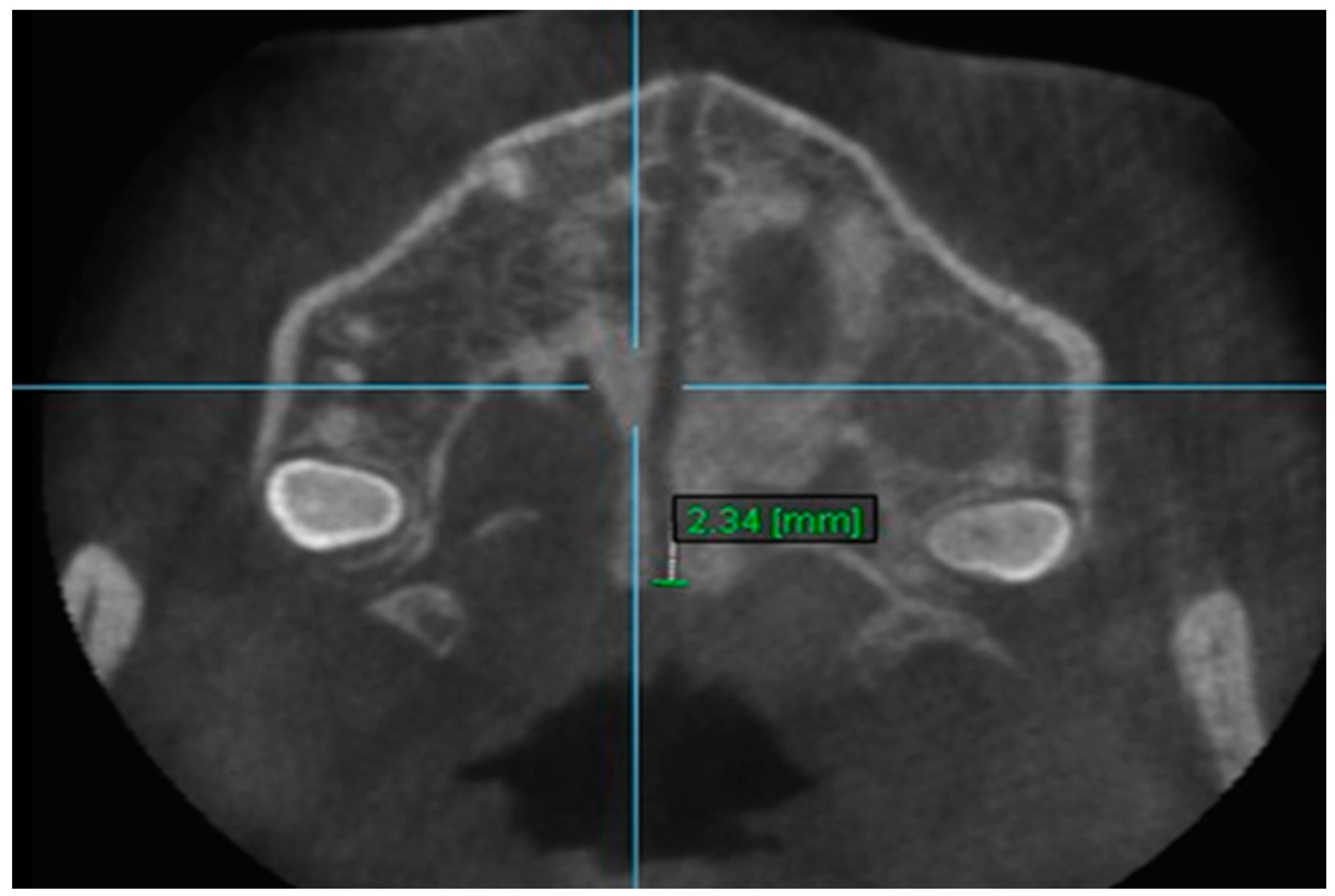
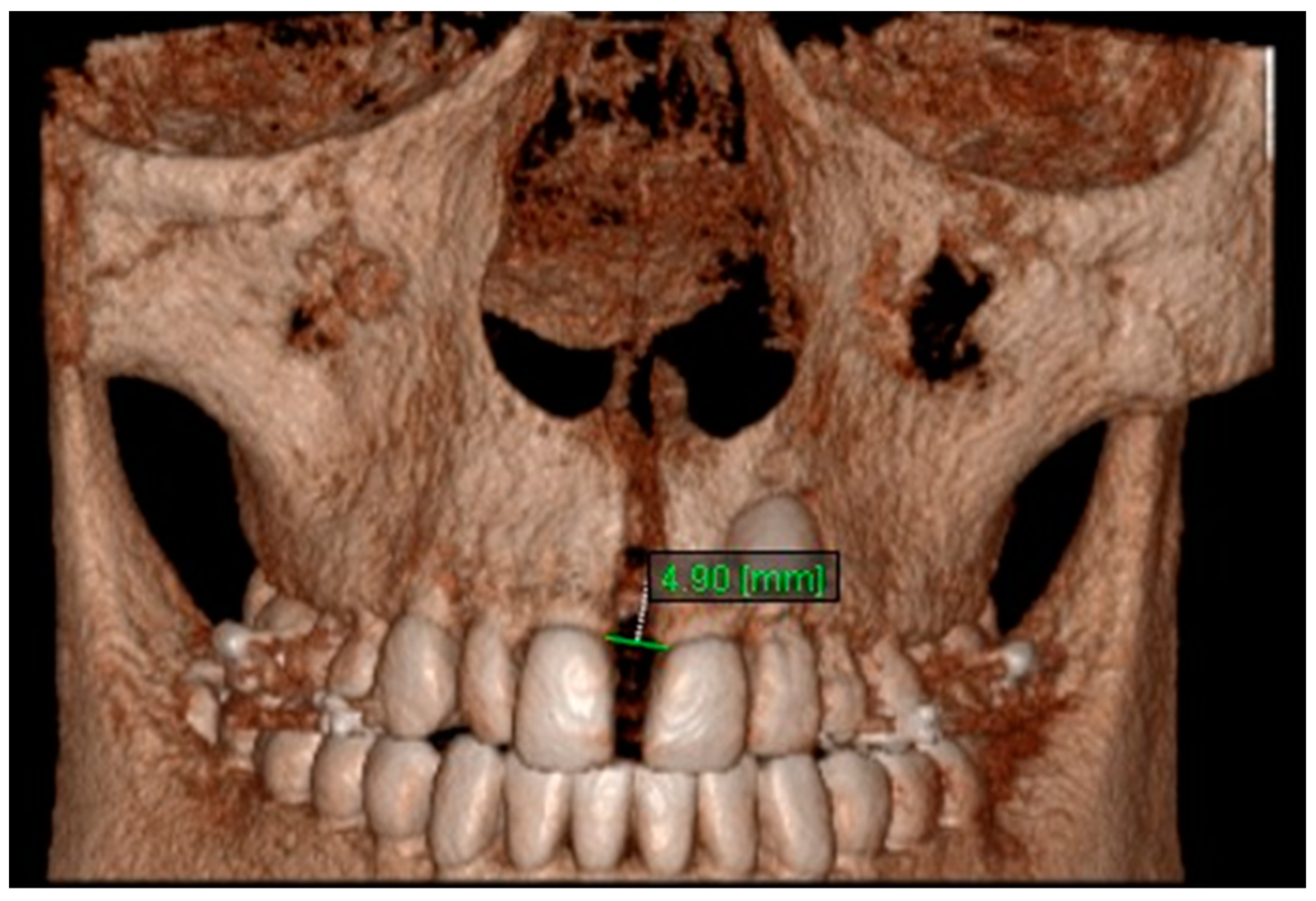
| Variables | Time Interval | 1st Measurement (mm) | 2nd Measurement (mm) | ICC (95% CI) * |
|---|---|---|---|---|
| Nasal Width 1 | T1 | 30.36 ± 2.76 | 30.44 ± 2.69 | 0.998 (0.992–0.999) |
| T2 | 32.46± 2.69 | 32.47 ± 2.57 | 0.997 (0.989–0.999) | |
| T3 | 31.71 ± 2.76 | 31.72 ± 2.77 | 0.998 (0.993–0.999) | |
| Nasal Width 2 | T1 | 30.54 ± 3.09 | 30.60 ± 3.03 | 0.996 (0.990–0.999) |
| T2 | 32.59 ± 3.10 | 32.69 ± 3.13 | 0.997 (0.992–0.999) | |
| T3 | 31.73 ± 2.88 | 31.71 ± 2.90 | 0.994 (0.981–0.998) | |
| Nasal Width 3 | T1 | 28.32 ± 3.13 | 28.34 ± 2.98 | 0.996 (0.988–0.998) |
| T2 | 30.83 ± 2.91 | 35.93 ± 2.90 | 0.993 (0.980–0.998) | |
| T3 | 29.69 ± 2.65 | 29.65 ± 2.65 | 0.990 (0.968–0.996) | |
| Nasal width 4 | T1 | 12.84 ± 1.58 | 12.83 ± 1.51 | 0.982 (0.943–0.994) |
| T2 | 13.75 ± 1.33 | 13.59 ± 1.25 | 0.988 (0.947–0.996) | |
| T3 | 13.17 ± 1.66 | 13.21 ± 1.70 | 0.988 (0.962–0.996) | |
| Nasal Width 5 | T1 | 12.84 ± 1.84 | 12.83 ± 1.71 | 0.989 (0.967–0.996) |
| T2 | 14.21 ± 1.87 | 13.99 ± 1.85 | 0.982 (0.915–0.995) | |
| T3 | 13.28 ± 1.34 | 12.90 ± 1.12 | 0.993 (0.976–0.998) | |
| Nasal Width 6 | T1 | 23.58 ± 1.52 | 23.70 ± 1.51 | 0.979 (0.934–0.993) |
| T2 | 26.61 ± 1.79 | 26.65 ± 1.69 | 0.991 (0.970–0.997) | |
| T3 | 25.16 ± 1.70 | 25.25 ± 1.76 | 0.988 (0.965–0.996) | |
| Nasal Floor 7 | T1 | 28.62 ± 2.81 | 28.68 ± 2.83 | 0.998 (0.994–0.999) |
| T2 | 30.84 ± 2.57 | 30.89 ± 2.59 | 0.997 (0.993–0.999) | |
| T3 | 30.28 ± 2.86 | 30.19 ± 2.76 | 0.995 (0.985–0.998) | |
| Nasal Floor 8 | T1 | 28.44 ± 3.95 | 28.39 ± 3.90 | 0.997 (0.992–0.999) |
| T2 | 30.92 ± 4.23 | 30.82 ± 4.18 | 0.997 (0.993–0.999) | |
| T3 | 29.99 ± 4.21 | 29.99 ± 4.11 | 0.995 (0.987–0.998) | |
| Nasal Floor 9 | T1 | 15.75 ± 3.69 | 15.67 ± 3.51 | 0.995 (0.984–0.998) |
| T2 | 18.52 ± 3.21 | 18.42 ± 2.88 | 0.991 (0.974–0.997) | |
| T3 | 17.18 ± 3.51 | 17.37 ± 3.39 | 0.990 (0.958–0.997) | |
| Aperture of Midpalatal Suture 10 | T1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0 |
| T2 | 2.52 ± 0.92 | 2.54 ± 0.98 | 0.993 (0.978–0.997) | |
| T3 | 1.65 ± 0.77 | 1.67 ± 0.76 | 0.982 (0.945–0.994) | |
| Aperture of Midpalatal Suture 11 | T1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0 |
| T2 | 5.46 ± 1.51 | 5.24 ± 1.41 | 0.973 (0.920–0.994) | |
| T3 | 2.48 ± 0.78 | 2.40 ± 0.79 | 0.994 (0.982–0.998) |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.13 | 0.82 | 2.07–2.19 | <0.001 | *** |
| T3–T1 | 1.30 | 1.02 | 1.26–1.34 | <0.001 | *** |
| T3–T2 | −0.83 | 0.59 | −0.85–−0.81 | 0.003 | ** |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.92 | 1.46 | 2.74–3.09 | <0.001 | *** |
| T3–T1 | 1.52 | 0.99 | 1.39–1.64 | 0.003 | ** |
| T3–T2 | −1.39 | 1.38 | −1.45–−1.35 | 0.003 | ** |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.08 | 0.69 | 2.05–2.10 | <0.001 | *** |
| T3–T1 | 1.28 | 1.15 | 1.61–1.41 | <0.001 | *** |
| T3–T2 | −0.79 | 0.093 | −0.94–−0.64 | 0.074 | NS |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.77 | 1.65 | 2.68–2.86 | <0.001 | *** |
| T3–T1 | 1.61 | 1.16 | 1.35–1.88 | <0.001 | *** |
| T3–T2 | −1.15 | 1.67 | −1.34–−0.94 | 0.061 | NS |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 1.05 | 1.01 | 0.91–1.19 | 0.012 | * |
| T3–T1 | 0.28 | 0.77 | 0.24–0.33 | 0.651 | NS |
| T3–T2 | −0.76 | 0.88 | −0.95–−0.58 | 0.036 | * |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 1.39 | 0.70 | 1.37–1.40 | 0.004 | ** |
| T3–T1 | 0.68 | 0.88 | 0.41–0.95 | 0.077 | NS |
| T3–T2 | −0.71 | 1.18 | −1.01–−0.42 | 0.327 | NS |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.32 | 1.03 | 2.18–2.47 | <0.001 | *** |
| T3–T1 | 1.59 | 1.26 | 1.57–1.61 | <0.002 | ** |
| T3–T2 | −0.73 | 0.62 | −0.89–−0.57 | 0.004 | ** |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.37 | 0.91 | 2.19–2.55 | <0.001 | *** |
| T3–T1 | 1.45 | 0.89 | 1.28–1.61 | 0.003 | *** |
| T3–T2 | −0.92 | 0.62 | −0.94–−0.91 | 0.001 | ** |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 2.99 | 1.48 | 2.72–3.27 | 0.005 | ** |
| T3–T1 | 1.51 | 0.97 | 1.40–1.61 | <0.002 | ** |
| T3–T2 | −1.49 | 1.54 | −1.66–−1.32 | 0.001 | ** |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 1.90 | 0.88 | 1.37–2.43 | 0.003 | ** |
| T3–T1 | 1.16 | 0.72 | 0.72–1.59 | 0.003 | ** |
| T3–T2 | −0.75 | 0.49 | −0.84–−0.65 | 0.003 | ** |
| Time Interval | Mean Difference | Standard Deviation | 95% Confidence Interval | p Value | p Significance |
|---|---|---|---|---|---|
| T2–T1 | 4.57 | 1.41 | 3.73–5.41 | 0.003 | ** |
| T3–T1 | 2.08 | 0.76 | 1.62–2.53 | 0.003 | ** |
| T3–T2 | −2.49 | 1.07 | −2.88–−2.11 | 0.003 | ** |
| Variables | Mean T1 | Mean T2 | Mean T3 | Mean Difference (T2-T1) | p | Mean Difference (T3-T1) | p | Mean Difference (T3-T2) | p |
|---|---|---|---|---|---|---|---|---|---|
| Nasal Width 1 | 28.83 | 30.97 | 30.14 | 2.13 | *** | 1.30 | *** | −0.83 | ** |
| Nasal Width 2 | 28.84 | 30.92 | 30.13 | 2.08 | *** | 1.28 | *** | −0.79 | (NS) |
| Nasal Width 3 | 26.45 | 29.22 | 28.07 | 2.77 | *** | 1.61 | *** | −1.15 | (NS) |
| Nasal Width 4 | 11.92 | 12.97 | 12.21 | 1.05 | * | 0.28 | (NS) | −0.76 | * |
| Nasal Width 5 | 11.75 | 13.14 | 12.43 | 1.39 | * | 0.68 | (NS) | −0.71 | (NS) |
| Nasal Width 6 | 22.73 | 25.64 | 24.24 | 2.92 | *** | 1.52 | ** | −1.39 | ** |
| Nasal Floor 7 | 27.05 | 29.37 | 28.61 | 2.32 | *** | 1.59 | *** | −0.73 | ** |
| Nasal Floor 8 | 26.27 | 28.64 | 27.72 | 2.37 | *** | 1.45 | ** | −0.92 | ** |
| Nasal Floor 9 | 13.61 | 16.60 | 15.11 | 2.99 | ** | 1.51 | *** | −1.49 | ** |
| Aperture of Midpalatal Suture 10 | 0 | 1.90 | 1.16 | 1.90 | ** | 1.16 | ** | −0.75 | ** |
| Aperture of Midpalatal Suture 11 | 0 | 4.57 | 2.08 | 4.57 | *** | 2.08 | ** | −2.49 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiadis, T.; Angelopoulos, C.; Papadopoulos, M.A.; Kolokitha, O.-E. Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion. Diagnostics 2023, 13, 1322. https://doi.org/10.3390/diagnostics13071322
Georgiadis T, Angelopoulos C, Papadopoulos MA, Kolokitha O-E. Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion. Diagnostics. 2023; 13(7):1322. https://doi.org/10.3390/diagnostics13071322
Chicago/Turabian StyleGeorgiadis, Thomas, Christos Angelopoulos, Moschos A. Papadopoulos, and Olga-Elpis Kolokitha. 2023. "Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion" Diagnostics 13, no. 7: 1322. https://doi.org/10.3390/diagnostics13071322
APA StyleGeorgiadis, T., Angelopoulos, C., Papadopoulos, M. A., & Kolokitha, O.-E. (2023). Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion. Diagnostics, 13(7), 1322. https://doi.org/10.3390/diagnostics13071322






