MDCT Diagnosis and Staging of Xanthogranulomatous Pyelonephritis
Abstract
1. Introduction
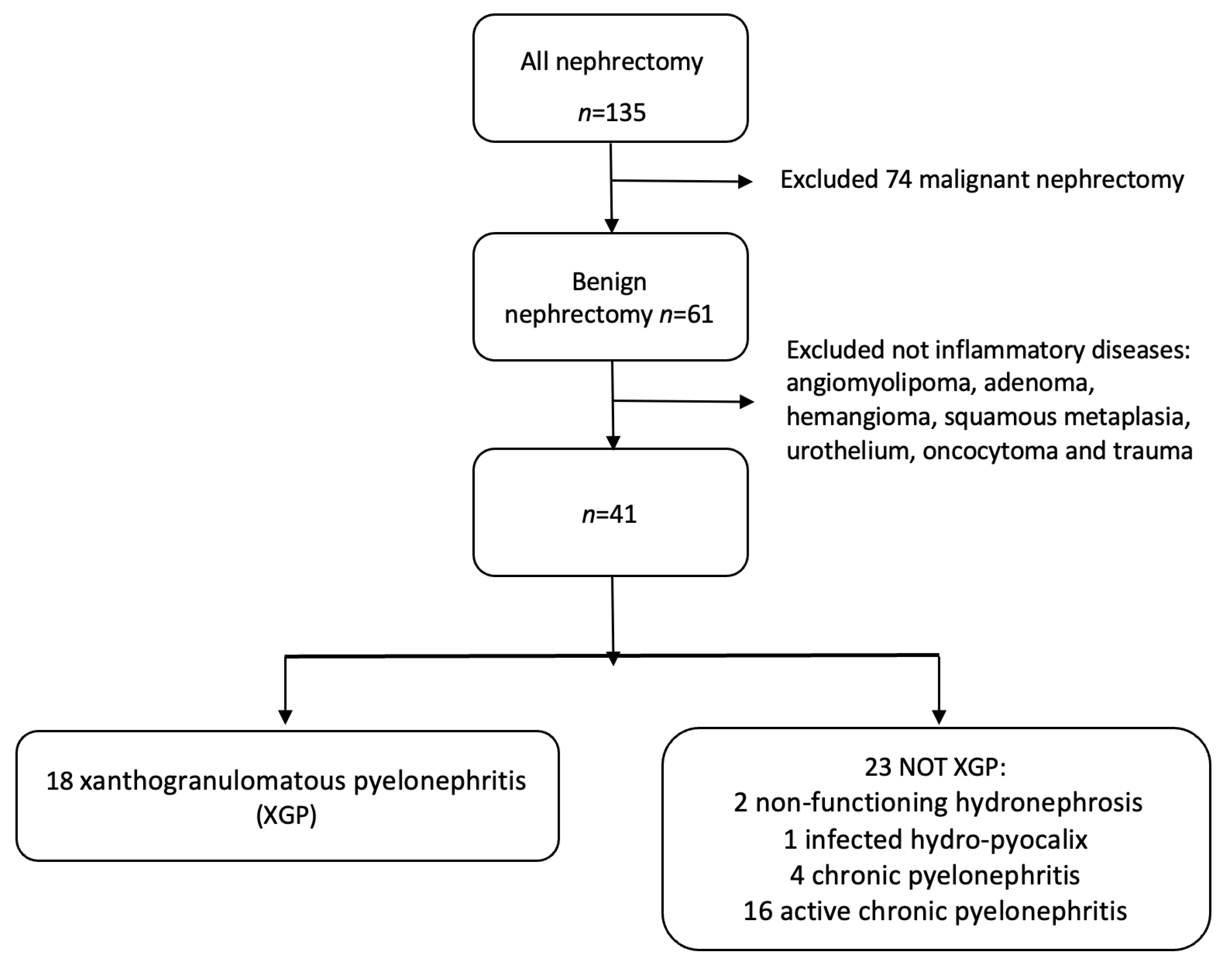
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. CT Protocol
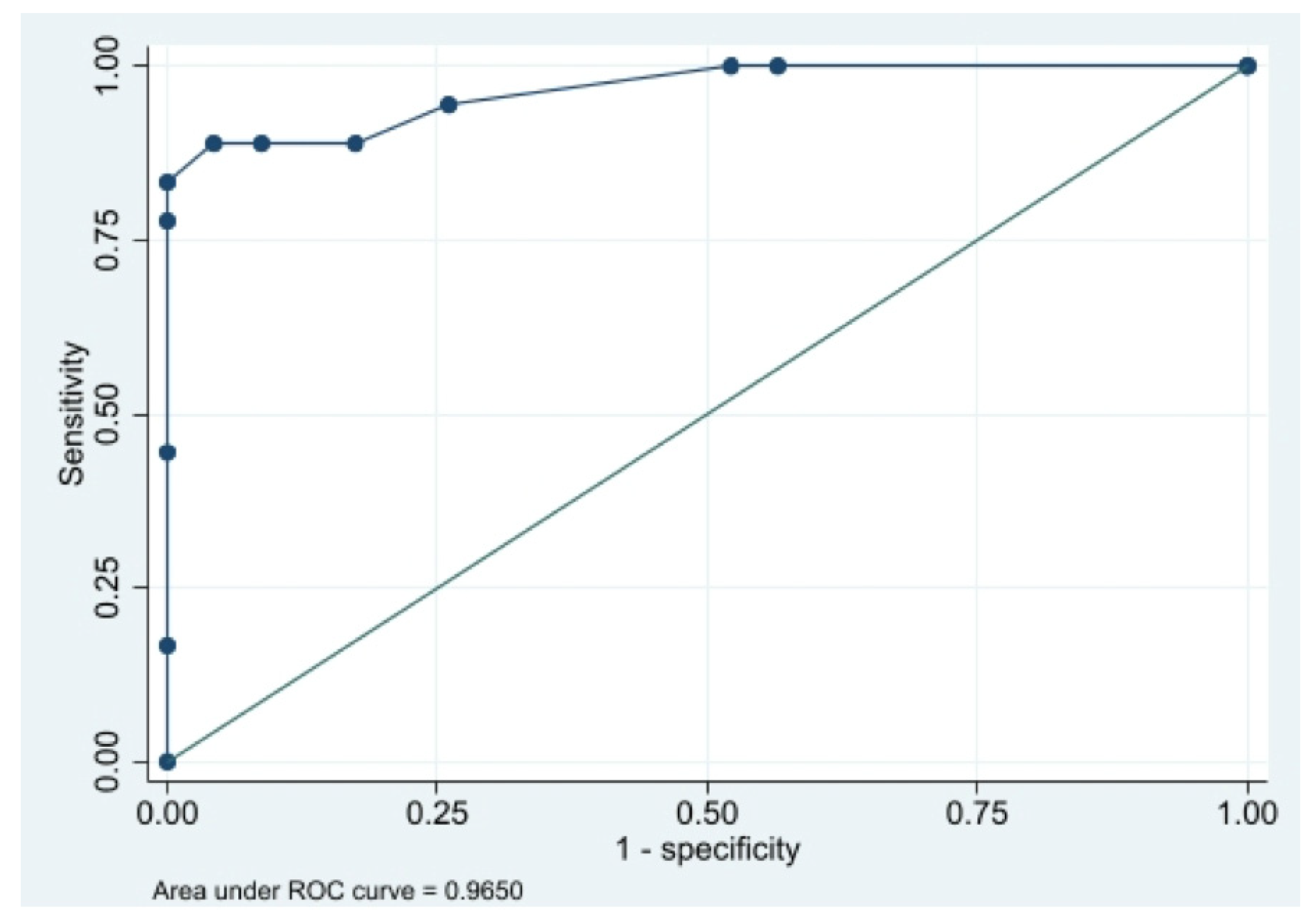
2.3. Statistical Analysis
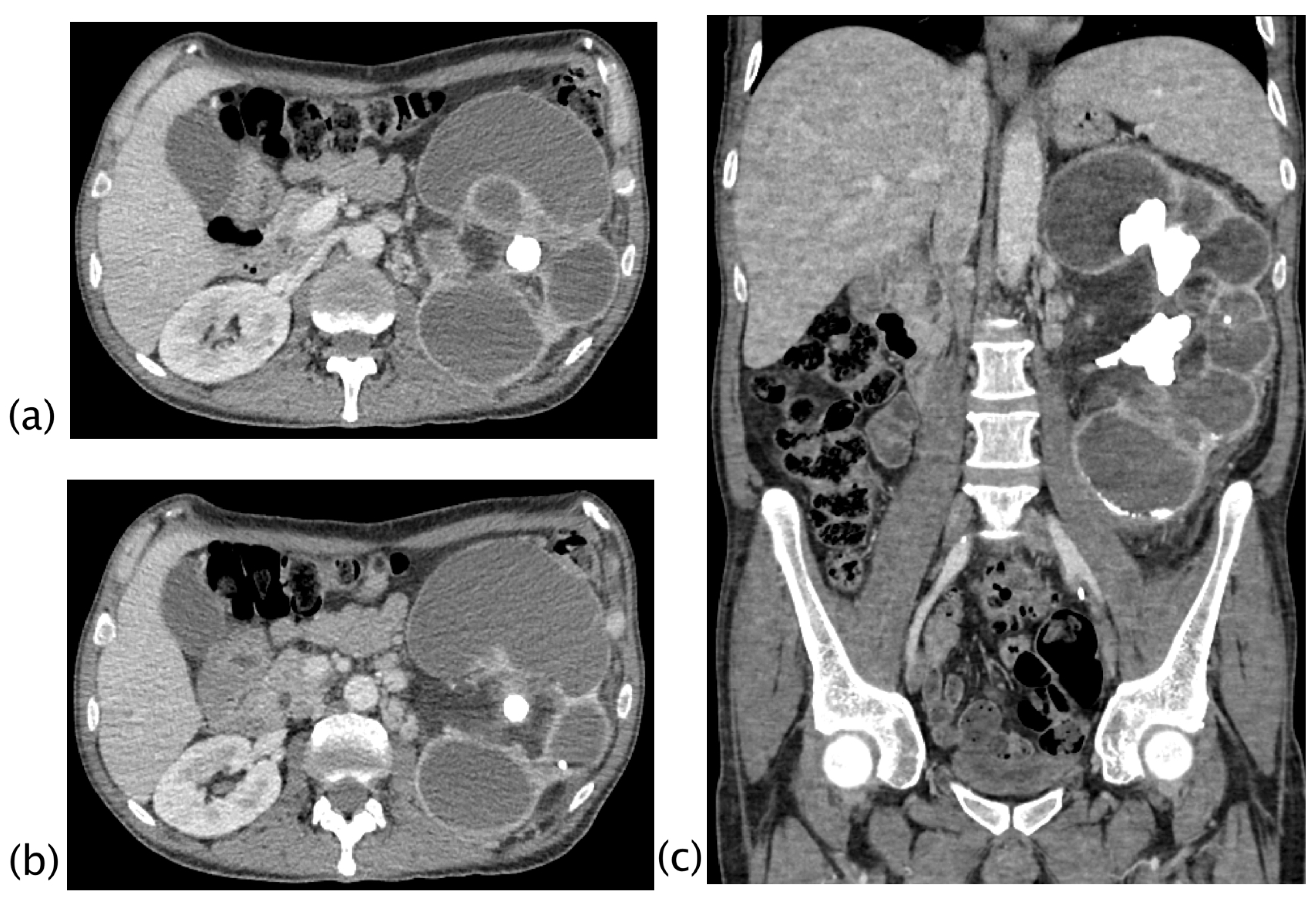
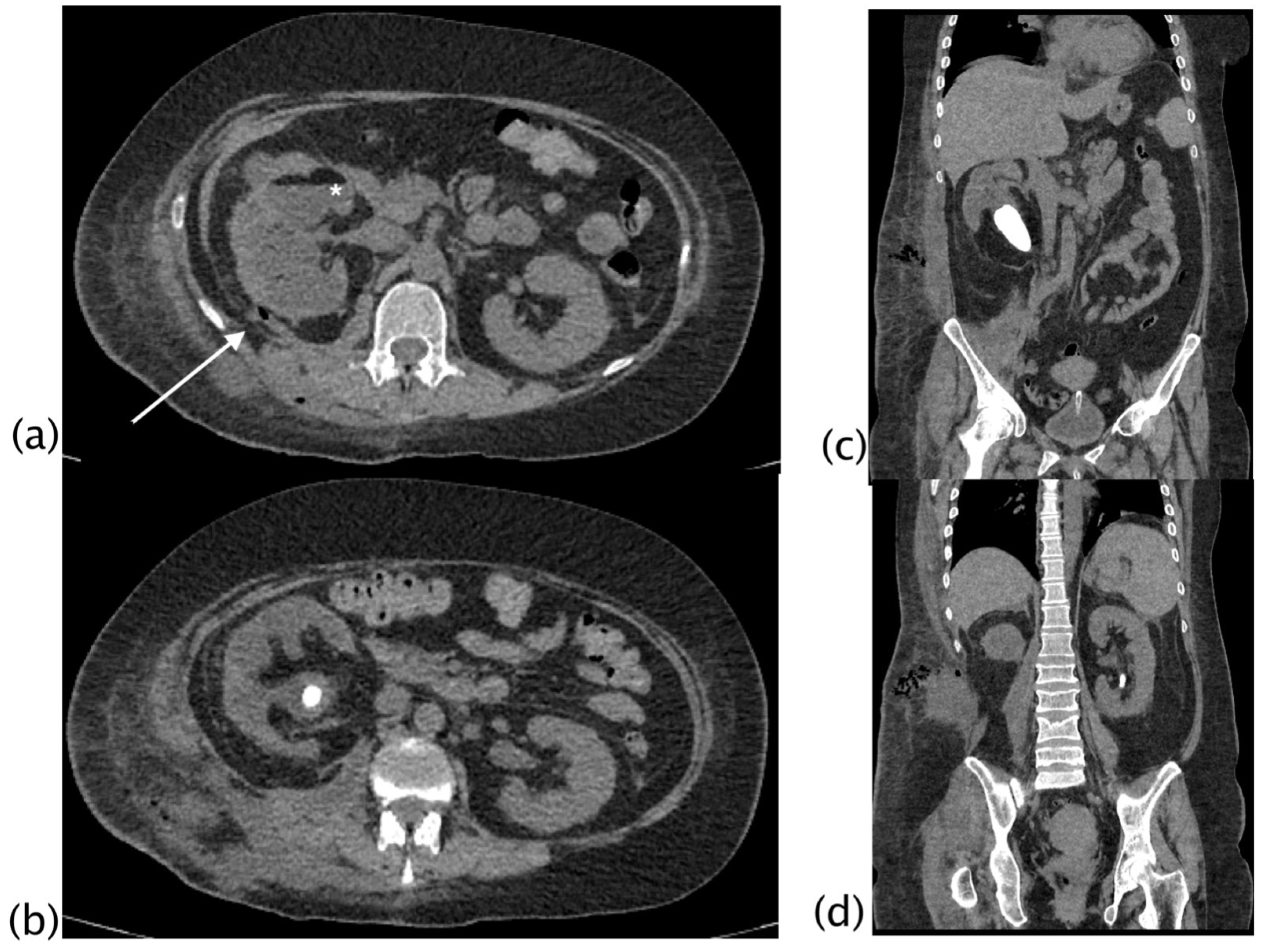
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shoag, J.; Tasian, G.E.; Goldfarb, D.S.; Eisner, B.H. The new epidemiology of nephrolithiasis. Adv. Chronic Kidney Dis. 2015, 22, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Danilovic, A.; Ferreira, T.A.C.; Vicentini, F.C.; Torricelli, F.C.M.; Marchini, G.S.; Mazzucchi, E.; Nahas, W.C.; Srougi, M. Laparoscopic nephrectomy for urolithiasis: When is better to avoid it. Rev. Col. Bras. Cir. 2019, 46, e20192092. [Google Scholar] [CrossRef] [PubMed]
- Zelhof, B.; McIntyre, I.G.; Fowler, S.M.; Napier-Hemy, R.D.; Burke, D.M.; Grey, B.R.; British Association of Urological Surgeons. Nephrectomy for benign disease in the UK: Results from the British Association of Urological Surgeons nephrectomy database. BJU Int. 2016, 117, 138–144. [Google Scholar] [CrossRef]
- Kim, S.W.; Yoon, B.I.; Ha, U.S.; Sohn, D.W.; Cho, Y.H. Xanthogranulomatous pyelonephritis: Clinical experience with 21 cases. J. Infect. Chemother. 2013, 19, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.L.; Cheng, C. Xanthogranulomatous inflammation of urachus mimicking urachal carcinoma. Urology 2009, 73, 443.e13–443.e14. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Baumal, R.; Eddy, A.A. Xanthogranulomatous pyelonephritis in children. Etiology, pathogenesis, clinical and radiologic features, and management. Clin. Pediatr. 1994, 33, 360–366. [Google Scholar] [CrossRef]
- Hou, J.; Herlitz, L.C. Renal Infections. Surg. Pathol. Clin. 2014, 7, 389–408. [Google Scholar] [CrossRef]
- Li, L.; Parwani, A.V. Xanthogranulomatous pyelonephritis. Arch. Pathol. Lab. Med. 2011, 135, 671–674. [Google Scholar] [CrossRef]
- Herrera-Bedoya, M.; Avendano-Capriles, C.A.; Zakzuk-Martinez, E.; Barrera, J. Xanthogranulomatous Pyelonephritis and Its Differential Diagnoses: An In-Depth Case Review. Cureus 2021, 13, e19133. [Google Scholar] [CrossRef]
- Jha, S.K.; Aeddula, N.R. Pyelonephritis Xanthogranulomatous; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ballard, D.H.; De Alba, L.; Migliaro, M.; Previgliano, C.H.; Sangster, G.P. CT imaging spectrum of infiltrative renal diseases. Abdom. Radiol. 2017, 42, 2700–2709. [Google Scholar] [CrossRef]
- Goldman, S.M.; Hartman, D.S.; Fishman, E.K.; Finizio, J.P.; Gatewood, O.M.; Siegelman, S.S. CT of xanthogranulomatous pyelonephritis: Radiologic-pathologic correlation. AJR Am. J. Roentgenol. 1984, 142, 963–969. [Google Scholar] [CrossRef]
- Jones, P.; Lazic, D.; Somani, B.K.; Hawary, A. Xanthogranulomatous pyelonephritis: An overview and management guide for clinicians. Br. J. Hosp. Med. 2021, 82, 1–8. [Google Scholar] [CrossRef]
- Alzanbagi, A.; Alhazmi, G.A.; Alghamdi, S.; Alosaimi, G.; Shariff, M.K. Infliximab-Associated Xanthogranulomatous Pyelonephritis: A Rare Complication. Cureus 2022, 14, e21051. [Google Scholar] [CrossRef]
- Ozkayin, N.; Inan, M.; Aladag, N.; Kaya, M.; Iscan, B.; Yalcin, O. Complicated xanthogranulomatous pyelonephritis in a child. Pediatr. Int. 2010, 52, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Gupta, M.; Goyal, R. Xanthogranulomatous pyelonephritis: A rare entity. N. Am. J. Med. Sci. 2011, 3, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Grainger, R.G.; Longstaff, A.J.; Parsons, M.A. Xanthogranulomatous pyelonephritis: A reappraisal. Lancet 1982, 1, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Bolger, M.P.; Hennebry, J.; Byrne, C.; Greene, L.; Stroiescu, A.; Heneghan, J.; Ryan, A.G. Xanthogranulomatous Pyelonephritis: A Narrative Review with Current Perspectives on Diagnostic Imaging and Management, Including Interventional Radiology Techniques. Int. J. Nephrol. Renov. Dis. 2021, 14, 359–369. [Google Scholar] [CrossRef]
- Tamburrini, S.; Fiorini, V.; Lugara, M.; Napodano, G.; Del Biondo, D.; Squame, F.; Sarti, G.; Quassone, P.; Coppola, M.G.; Iannuzzi, M.; et al. Nephrobronchial fistula a case report and review of the literature. Radiol. Case Rep. 2021, 16, 3470–3477. [Google Scholar] [CrossRef]
- O’Neill, S.; Motyer, R.; O’Neill, H.; Brennan, I.; Ryan, J.M.; Guiney, M. “Uroptysis!”—A case report of xanthogranulomatous pyelonephritis with nephrobronchial fistulation. Int. J. Surg. Case Rep. 2022, 98, 107551. [Google Scholar] [CrossRef]
- Ciccarese, F.; Brandi, N.; Corcioni, B.; Golfieri, R.; Gaudiano, C. Complicated pyelonephritis associated with chronic renal stone disease. Radiol. Med. 2021, 126, 505–516. [Google Scholar] [CrossRef]
- Shadman, A.; Bastani, B. Kidney Calculi: Pathophysiology and as a Systemic Disorder. Iran. J. Kidney Dis. 2017, 11, 180–191. [Google Scholar]
- Harley, F.; Wei, G.; O’Callaghan, M.; Wong, L.M.; Hennessey, D.; Kinnear, N. Xanthogranulomatous pyelonephritis: A systematic review of treatment and mortality in >1000 cases. BJU Int. 2023, 131, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, T.J.; Bivalacqua, T.J.; Pierorazio, P.M.; Varkarakis, J.; Schaeffer, E.M.; Allaf, M.E. Xanthogranulomatous pyelonephritis: Presentation and management in the era of laparoscopy. BJU Int. 2009, 104, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Addison, B.; Zargar, H.; Lilic, N.; Merrilees, D.; Rice, M. Analysis of 35 cases of Xanthogranulomatous pyelonephritis. ANZ J. Surg. 2015, 85, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.S.; Elder, J.S. Xanthogranulomatous pyelonephritis: A critical analysis of 26 cases and of the literature. J. Urol. 1978, 119, 589–593. [Google Scholar] [CrossRef]
- Loffroy, R.; Varbedian, O.; Guiu, B.; Delgal, A.; Michel, F.; Cercueil, J.P.; Krause, D. Xanthogranulomatous pyelonephritis: Main imaging features. Prog. Urol. 2008, 18, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Korkes, F.; Favoretto, R.L.; Broglio, M.; Silva, C.A.; Castro, M.G.; Perez, M.D. Xanthogranulomatous pyelonephritis: Clinical experience with 41 cases. Urology 2008, 71, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J. Classic signs in uroradiology. Radiographics 2004, 24 (Suppl. 1), S247–S280. [Google Scholar] [CrossRef]
- Parsons, M.A. Xanthogranulomatous gastritis: An entity or a secondary phenomenon? J. Clin. Pathol. 1993, 46, 580–581. [Google Scholar] [CrossRef]
- Matthews, G.J.; McLorie, G.A.; Churchill, B.A.; Steckler, R.E.; Khoury, A.E. Xanthogranulomatous pyelonephritis in pediatric patients. J. Urol. 1995, 153, 1958–1959. [Google Scholar] [CrossRef]
- Ichaoui, H.; Saadi, A.; Chakroun, M.; Ayed, H.; Bouzouita, A.; Cherif, M.; Derouiche, A.; Ben Slama, M.R.; Chebil, M. Xanthogranulomatous pyelonephritis in adults: Clinical, biological, radiological and therapeutic main findings in diffuse and focal forms. About 42 cases. Tunis. Med. 2018, 96, 495–500. [Google Scholar] [PubMed]
- Titus, B.; Gupta, S.; Edpao, P.; Psutka, S.P.; Limaye, A.P.; Bakthavatsalam, R.; Rakita, R.M. Xanthogranulomatous Pyelonephritis with Direct Extension Into the Liver. Am. J. Med. 2020, 133, 1054–1055. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Opazo, V.; Bassa, C.; Lopez, L.; Araos, F.; Madrid, P.; Morales, I. Xanthogranulomatous pyelonephritis: A case report. Urol. Case Rep. 2018, 19, 65–66. [Google Scholar] [CrossRef]
- Noyola, A.; Gil, J.F.; Lujano, H.; Pinon, O.; Munoz, G.; Michel, J.M.; Garcia, J.; Valdez, J.; Morales, O. Xanthogranulomatous Prostatitis, a Rare Prostatic Entity. Urol. Case Rep. 2017, 10, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Sanguesa Nebot, C.; Pico Aliaga, S.; Serrano Durba, A.; Roca, M.J. Xantogranulomatous pyeloneprhritis in children. Insights Imaging 2018, 9, 643–651. [Google Scholar] [CrossRef]
- Siddappa, S.; Ramprasad, K.; Muddegowda, M.K. Xanthogranulomatous pyelonephritis: A retrospective review of 16 cases. Korean J. Urol. 2011, 52, 421–424. [Google Scholar] [CrossRef]
- Rachidi, S.A.; Zeriouel, A. Renal tumor or pseudotumoral xanthogranulomatous pyelonephritis. Pan Afr. Med. J. 2018, 29, 67. [Google Scholar] [CrossRef]
- Chandanwale, S.S. Xanthogranulomatous Pyelonephritis: Unusual Clinical Presentation: A Case Report with Literature Review. J. Fam. Med. Prim. Care 2013, 2, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Zorzos, I.; Moutzouris, V.; Korakianitis, G.; Katsou, G. Analysis of 39 cases of xanthogranulomatous pyelonephritis with emphasis on CT findings. Scand. J. Urol. Nephrol. 2003, 37, 342–347. [Google Scholar] [CrossRef]
- Proca, E.; Lucan, M.; Alexianu, M. Xanthogranulomatous pyelonephritis. Comments apropos 145 clinical cases. Rev. Chir. Oncol. Radiol. O R L Oftalmol. Stomatol. Chir. 1988, 37, 451–466. [Google Scholar]
- Montelongo-Rodriguez, F.A.; Pallares-Mendez, R.; Robles-Torres, J.I.; Romero-Mata, R.; Cervantes-Miranda, D.E.; Gutierrez-Gonzalez, A.; Garcia-Arreola, J.A. Perioperative predictors for complications in patients with Xanthogranulomatous Pyelonephritis treated with nephrectomy. Urologia 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.T. Bear paw sign: Classic presentation of xanthogranulomatous pyelonephritis. QJM 2019, 112, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Sureka, B.; Singh, V.P.; Rajesh, S.R.; Laroia, S.; Bansal, K.; Rastogi, A.; Bihari, C.; Bhadoria, A.S.; Agrawal, N.; Arora, A. Computed Tomography (CT) and Magnetic Resonance (MR) Findings in Xanthogranulomatous Cholecystitis: Retrospective Analysis of Pathologically Proven 30 Cases—Tertiary Care Experience. Pol. J. Radiol. 2017, 82, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, L.; Mena, E.; Choyke, P.L.; Bouchelouche, K. PET imaging in renal cancer. Curr. Opin. Oncol. 2019, 31, 216–221. [Google Scholar] [CrossRef]
- Liu, Y. The Place of FDG PET/CT in Renal Cell Carcinoma: Value and Limitations. Front. Oncol. 2016, 6, 201. [Google Scholar] [CrossRef]
| XGP | p-Value | |||
|---|---|---|---|---|
| NO (n = 23) | YES (n = 18) | TOTAL n = (41) | ||
| Demographic data | ||||
| Age (years), mean (SD) | 54 (20) | 67 (11) | 60 (17) | 0.011 |
| Sex (female), n (%) | 13 (56.5) | 13 (72.2) | 26 (63.4) | 0.300 |
| Symptoms | ||||
| Flank pain, n (%) | 15 (65.2) | 13 (72.2) | 28 (68.3) | 0.742 |
| Fever, n (%) | 4 (17.4) | 6 (33.3) | 10 (24.4) | 0.289 |
| Palpable mass, n (%) | 0 (0.0) | 2 (11.1) | 2 (4.5) | 0.187 |
| Comorbidities | ||||
| Diabetes, n (%) | 9 (39.1) | 13 (72.2) | 22 (53.7) | 0.035 |
| Hypertension, n (%) | 0 (0.0) | 3 (16.7) | 3 (7.3) | 0.077 |
| Hematuria, n (%) | 6 (26.1) | 3 (16.7) | 9 (22.0) | 0.706 |
| Laboratory analysis | ||||
| Hemoglobin (g/dL), median (IQ–3Q) | 12.6 (9.4–14.6) | 9.6 (8.6–12.2) | 10.9 (8.9–13.4) | 0.020 |
| Leucocytes (103 mm3), median (IQ–3Q) | 9.6 (5.5–12.2) | 11.3 (9.1–16.2) | 10.7 (7.13–12.9) | 0.033 |
| Cholinesterase (U/L), mean (SD) | 6837 (2554) | 4166 (2442) | 5704 (2809) | 0.005 |
| Creatinine (md/dL), median (IQ–3Q) | 1.2 (1.0–1.9) | 1.4 (1.0–2.2) | 1.3 (1.0–2.0) | 0.627 |
| Urea (mg/dL), median (IQ–3Q) | 44 (31–61) | 45 (38–87) | 44 (33–62) | 0.415 |
| Procalcitonin (mg/dL), median (IQ–3Q) | 7.1 (1.9–25.5) | 16.7 (7.8–23.3) | 14.6 (4.4–25.2) | 0.304 |
| CT Findings | ||||
| Staghorn calculi, n (%) | 1 (4.4) | 10 (55.6) | 11 (26.8) | <0.001 |
| Stone disease, n (%) | 14 (70.9) | 15 (83.3) | 29 (70.7) | 0.171 |
| Bear paw sign, n (%) | 0 (0.0) | 13 (72.2) | 13 (31.7) | <0.001 |
| Xantoma, n (%) | 0 (0.0) | 1 (5.6) | 1 (2.4) | 0.439 |
| Hydronephrosis, n (%) | 21 (91.3) | 18 (100.0) | 39 (95.1) | 0.495 |
| Pyonephrosis, n (%) | 5 (21.7) | 16 (88.9) | 21 (51.2) | <0.001 |
| Stent, n (%) | 4 (17.4) | 7 (38.9) | 11 (26.8) | 0.164 |
| Air in the collecting system, n (%) | 1 (4.4) | 6 (33.3) | 7 (17.1) | 0.031 |
| Blood in the collecting system, n (%) | 3 (13.0) | 5 (27.8) | 8 (19.5) | 0.267 |
| Fat stranding, n (%) | 7 (30.4) | 16 (88.9) | 23 (56.1) | <0.001 |
| Renal abscess, n (%) | 4 (17.4) | 11 (61.1) | 15 (36.6) | 0.008 |
| Perirenal abscess, n (%) | 4 (17.4) | 13 (72.2) | 17 (41.5) | 0.001 |
| Pararenal abscess, n (%) | 3 (13.0) | 10 (55.6) | 13 (31.7) | 0.006 |
| Spleen abscess, n (%) | 0 (0.0) | 4 (22.2) | 4 (9.8) | 0.030 |
| Psoas abscess, n (%) | 2 (8.7) | 9 (50.0) | 11 (26.8) | 0.005 |
| Liver abscess, n (%) | 0 (0.0) | 2 (11.1) | 2 (4.9) | 0.187 |
| Fistulization, n (%) | 2 (8.7) | 6 (33.3) | 8 (19.5) | 0.109 |
| Pleural effusion, n (%) | 4 (17.4) | 10 (55.6) | 14 (34.2) | 0.010 |
| Lung atelectasis/disventilation, n (%) | 6 (26.1) | 12 (66.7) | 18 (43.9) | 0.009 |
| Cases with Available Data | |||
|---|---|---|---|
| OR (95%CI) | p-Value | ||
| Demographic data | |||
| Age > 55 | 41 | 12.4 (2.3–67.6) | 0.003 |
| Sex (female) | 41 | 2.0 (0.5–7.5) | 0.304 |
| Symptoms | |||
| Flank pain | 41 | 1.4 (0.4–5.3) | 0.633 |
| Fever | 41 | 2.4 (0.6–10.2) | 0.245 |
| Palpable mass | 41 | - | - |
| Comorbidities | |||
| Diabetes | 41 | 4.0 (1.1–15.3) | 0.039 |
| Hypertension | 41 | - | - |
| Hematuria | 30 | 0.4 (0.1–1.9) | 0.239 |
| Laboratory analysis | |||
| Hemoglobin < 11.5 g/dL | 40 | 2.9 (0.8–10.6) | 0.109 |
| Leucocytes > 10.5 103/ mm3 | 41 | 4.0 (1.1–15.3) | 0.039 |
| Cholinesterasis (<4000 U/L for female and <5000 U/L for male) | 16 | 6.8 (1.4–31.9) | 0.016 |
| Creatinine > 0.95 md/dL | 41 | 0.5 (0.1–2.4) | 0.429 |
| Urea > 50 mg/dL | 41 | 1.5 (0.4–5.3) | 0.530 |
| Procalcitonin > 0.5 mg/dL | 26 | 2.6 (0.2–32.9) | 0.461 |
| CT Findings | |||
| Staghorn calculi | 41 | 27.5 (3.0–250.5) | 0.003 |
| Lithiasis | 41 | 3.2 (0.7–14.3) | 0.126 |
| Hydronephrosis | 41 | - | - |
| Bear paw sign | 41 | - | - |
| Xantoma | 41 | - | - |
| Pyonephrosis | 41 | 28.8 (4.9–169.5) | <0.001 |
| Stent | 41 | 3.0 (0.7–12.7) | 0.131 |
| Air in the collecting system | 41 | 11.0 (1.2–102.4) | 0.035 |
| Blood in the collecting system | 41 | 2.6 (0.5–12.6) | 0.247 |
| Fat stranding | 41 | 18.3 (3.3–101.9) | 0.001 |
| Renal abscess | 41 | 7.5 (1.8–31.4) | 0.006 |
| Perirenal abscess | 41 | 12.4 (2.8–54.9) | 0.001 |
| Pararenal abscess | 41 | 8.7 (1.8–38.4) | 0.003 |
| Spleen abscess | 41 | - | - |
| Psoas abscess | 41 | 10.5 (1.9–58.6) | 0.007 |
| Liver abscess | 41 | - | - |
| Fistulization | 41 | 5.3 (0.9–30.2) | 0.063 |
| Pleural effusion | 41 | 5.9 (1.4–24.7) | 0.014 |
| Lung atelectasis/disventilation | 41 | 5.7 (1.5–21.9) | 0.012 |
| OR (95%CI) | p-Value | |
|---|---|---|
| Age | ||
| ≤55 | Reference | |
| >55 years | 41.5 (1.8–979.3) | 0.021 |
| Staghorn calculi | ||
| No | Reference | |
| Yes | 61.8 (1.5–2496.2) | 0.029 |
| Pyonephrosis | ||
| No | Reference | |
| Yes | 19.0 (0.99–364.8) | 0.051 |
| Pleural effusion | ||
| No | Reference | |
| Yes | 10.3 (0.5–206.9) | 0.129 |
| Stage I (n = 5) | Stage II (n = 2) | Stage III (n = 11) | Total (n = 18) | p-Value | |
|---|---|---|---|---|---|
| Staghorn calculi, n (%) | 2 (40.0) | 2 (100.0) | 6 (54.5) | 10 (55.6) | 0.638 |
| Stone disease, n (%) | 4 (80.0) | 2 (100.0) | 9 (81.8) | 15 (82.3) | 0.999 |
| Hydronephrosis, n (%) | 5 (100.0) | 2 (100.0) | 11 (100.0) | 18 (100.0) | - |
| Bear paw sign, n (%) | 3 (60.0) | 2 (100.0) | 8 (72.7) | 13 (72.2) | 0.999 |
| Xantoma, n (%) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | - |
| Pyonephrosis, n (%) | 3 (60.0) | 2 (100.0) | 11 (100.0) | 16 (88.9) | 0.137 |
| Stent, n (%) | 2 (40.0) | 1 (50.0) | 4 (36.4) | 7 (38.9) | 0.999 |
| Air in the collecting system, n (%) | 1 (20.0) | 1 (50.0) | 4 (36.4) | 6 (33.3) | 0.999 |
| Blood in the collecting system, n (%) | 1 (20.0) | 1 (50.0) | 3 (27.3) | 5 (27.8) | 0.999 |
| Fat stranding, n (%) | 3 (60.0) | 2 (100.0) | 11 (100.0) | 16 (88.9) | 0.137 |
| Renal abscess, n (%) | 0 (0.0) | 1 (50.0) | 10 (90.9) | 11 (61.1) | 0.001 |
| Perirenal abscess, n (%) | 0 (0.0) | 2 (100.0) | 11 (100.0) | 13 (72.2) | <0.001 |
| Pararenal abscess, n (%) | 0 (0.0) | 0 (0.0) | 10 (90.9) | 10 (55.6) | <0.001 |
| Extrarenal abscess, n (%) | 0 (0.0) | 0 (0.0) | 10 (90.9) | 10 (55.6) | <0.001 |
| Fistulization, n (%) | 0 (0.0) | 0 (0.0) | 6 (54.5) | 6 (33.3) | 0.070 |
| Pleural effusion, n (%) | 2 (40.0) | 1 (50.0) | 7 (63.6) | 10 (55.6) | 0.789 |
| Lung atelectasis/disventilation, n (%) | 2 (40.0) | 1 (50.0) | 9 (81.8) | 12 (66.7) | 0.253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburrini, S.; Comune, R.; Lassandro, G.; Pezzullo, F.; Liguori, C.; Fiorini, V.; Picchi, S.G.; Lugarà, M.; Del Biondo, D.; Masala, S.; et al. MDCT Diagnosis and Staging of Xanthogranulomatous Pyelonephritis. Diagnostics 2023, 13, 1340. https://doi.org/10.3390/diagnostics13071340
Tamburrini S, Comune R, Lassandro G, Pezzullo F, Liguori C, Fiorini V, Picchi SG, Lugarà M, Del Biondo D, Masala S, et al. MDCT Diagnosis and Staging of Xanthogranulomatous Pyelonephritis. Diagnostics. 2023; 13(7):1340. https://doi.org/10.3390/diagnostics13071340
Chicago/Turabian StyleTamburrini, Stefania, Rosita Comune, Giulia Lassandro, Filomena Pezzullo, Carlo Liguori, Valeria Fiorini, Stefano Giusto Picchi, Marina Lugarà, Dario Del Biondo, Salvatore Masala, and et al. 2023. "MDCT Diagnosis and Staging of Xanthogranulomatous Pyelonephritis" Diagnostics 13, no. 7: 1340. https://doi.org/10.3390/diagnostics13071340
APA StyleTamburrini, S., Comune, R., Lassandro, G., Pezzullo, F., Liguori, C., Fiorini, V., Picchi, S. G., Lugarà, M., Del Biondo, D., Masala, S., Tamburro, F., & Scaglione, M. (2023). MDCT Diagnosis and Staging of Xanthogranulomatous Pyelonephritis. Diagnostics, 13(7), 1340. https://doi.org/10.3390/diagnostics13071340









