Primary Lymphoproliferative Lung Diseases: Imaging and Multidisciplinary Approach
Abstract
:1. Introduction
2. Imaging Techniques and Multidisciplinary Approach
3. Imaging Patterns
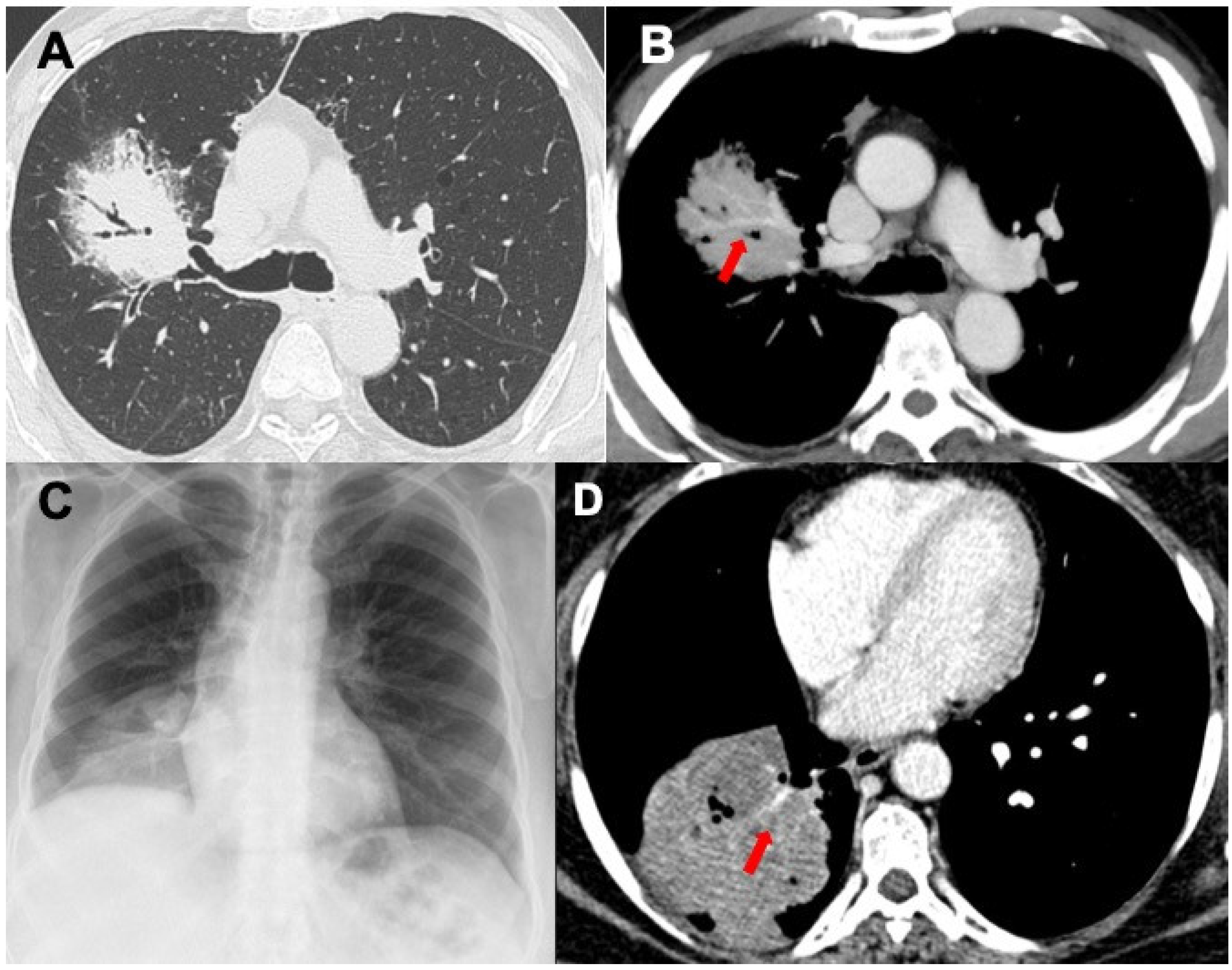
3.1. Consolidations
3.2. Nodules and Masses
3.3. Ground-Glass Opacities
3.4. Interstitial Involvement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hare, S.S.; Souza, C.A.; Bain, G.; Seely, J.M.; Frcpc Gomes, M.M.; Quigley, M. The radiological spectrum of pulmonary lymphoproliferative disease. Br. J. Radiol. 2012, 85, 848–864. [Google Scholar] [CrossRef] [Green Version]
- Cadranel, J.; Wislez, M.; Antoine, M. Primary pulmonary lymphoma. Eur. Respir. J 2002, 20, 750–762. [Google Scholar] [CrossRef]
- Sirajuddin, A.; Raparia, K.; Lewis, V.A.; Franks, T.J.; Dhand, S.; Galvin, J.R.; White, C.S. Primary pulmonary lymphoid lesions: Radiologic and pathologic findings. Radiographics 2016, 36, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koss, M.N. Malignant and benign lymphoid lesions of the lung. Ann. Diagn. Pathol. 2004, 8, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, Y.; Primack, S.L. Imaging of pulmonary lymphomas. AJR Am. J. Roentgenol. 1997, 168, 339-45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbo-Mussetto, A.; Saviolo, C.; Fornari, A.; Gottardi, D.; Petracchini, M.; Macera, A.; Lario, C.V.; Gallo, T.; Tarella, C.; Cirillo, S. Whole body MRI with qualitative and quantitative analysis of DWI for assessment of bone marrow involvement in lymphoma. Radiol. Med. 2017, 122, 623–632. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Travis, W.D. Lymphomatoid granulomatosis and lymphoproliferative disorders of the lung. In Immunologically Mediated Pulmonary Diseases; Lynch, J.P., De Remmee, R.A., Eds.; J. B. Lippincott Company: Philadelphia, PA, USA, 1991; pp. 274–301. [Google Scholar]
- Kennedy, J.L.; Nathwani, B.N.; Burke, J.S.; Hill, L.R.; Rappaport, H. Pulmonary lymphomas and other pulmonary lymphoid lesions. A clinicopathologic and immunologic study of 64 patients. Cancer 1985, 56, 539–552. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart, 4th ed.; IARC: Lyon, France, 2015. [Google Scholar]
- William, J.; Variakojis, D.; Yeldandi, A.; Raparia, K. Lymphoproliferative neoplasms of the lung: A review. Arch. Pathol. Lab. Med. 2013, 137, 382–391. [Google Scholar] [CrossRef]
- Restrepo, C.S.; Carrillo, J.; de Christenson, M.R.; Leon, P.O.; Rivera, A.L.; Koss, M.N. Lymphoproliferative Lung Disorders: A Radiologic-Pathologic Overview. Part II: Neoplastic Disorders. Semin. Ultrasound CT MRI 2013, 34, 535–549. [Google Scholar] [CrossRef]
- Le Tourneau, A.; Audouin, J.; Capron, F.; Servais, B.; Diebold, J.; Garbe, L.; Monges, G.; Payan, H. Primary pulmonary malignant lymphoma, clinical and patho- logical findings, immunocytochemical and ultra- structural studies in 15 cases. Hematol. Oncol. 1983, 1, 49–60. [Google Scholar] [CrossRef]
- Cordier, J.F.; Chailleux, E.; Lauque, D.; Reynaud-Gaubert, M.; Dietemann-Molard, A.; Dalphin, J.C.; Blanc-Jouvan, F.; Loire, R. Primary pulmonary lymphomas. A clinical study of 70 cases in nonimmunocompromised patients. Chest 1993, 103, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.A.; Lee, K.S.; Han, J.; Ko, Y.H.; Kim, B.T.; Chung, M.J.; Kim, T.S. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: Imaging findings in 21 patients. Chest 2008, 133, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Garcia, J.; Talwar, A.; Shah, R.; Fein, A. Update in primary pulmonary lymphomas. Curr. Opin. Pulm. Med. 2015, 21, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Fujita, N.; Morimoto, S.; Ikezoe, J.; Kozuka, T. MR Imaging of Primary Pulmonary Lymphoma. Australas. Radiol. 1990, 34, 353–355. [Google Scholar] [CrossRef]
- Ooi, G.C.; Chim, C.S.; Lie, A.K.W.; Tsang, K.W.T. Computed tomography features of primary pulmonary non-Hodgkin’s lymphoma. Clin. Radiol. 1999, 54, 438–443. [Google Scholar] [CrossRef]
- Jensen, L.J.; Kim, D.; Elgeti, T.; Steffen, I.G.; Hamm, B.; Nagel, S.N. Differentiation of Pulmonary Lymphoma Manifestations and Nonlymphoma Infiltrates in Possible Invasive Fungal Disease Using Fast T1-weighted Magnetic Resonance Imaging at 3 T Comparison of Texture Analysis, Mapping, and Signal Intensity Quotients. J. Thorac. Imaging 2022, 37, 80–89. [Google Scholar] [CrossRef]
- Kim, D.; Elgeti, T.; Penzkofer, T.; Steffen, I.G.; Jensen, L.J.; Schwartz, S.; Hamm, B.; Nagel, S.N. Enhancing the differentiation of pulmonary lymphoma and fungal pneumonia in hematological patients using texture analysis in 3-T MRI. Eur. Radiol. 2021, 31, 695–705. [Google Scholar] [CrossRef]
- Takamura, K.; Nasuhara, Y.; Mishina, T.; Matsuda, T.; Nishimura, M.; Kawakami, Y.; Fujita, M.; Mikuni, C.; Yamashiro, K. Intravascular lymphomatosis diagnosed by transbronchial lung biopsy. Eur. Respir. J. 1997, 10, 955–957. [Google Scholar] [CrossRef]
- Poletti, V.; Casoni, G.L.; Piciucchi, S.; Tomassetti, S.; Asioli, S.; Dubini, A.; Chilosi, M. Lymphoproliferative lung disorders. In Orphan Lung Diseases; Cottin, V., Cordier, J.F., Richeldi, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 493–515. [Google Scholar]
- Liu, C.; Lai, N.; Zhou, Y.; Li, S.; Chen, R.; Zhang, N. Intravascular large B-cell lymphoma confirmed by lung biopsy. Int. J. Clin. Exp. Pathol. 2014, 15, 6301–6306. [Google Scholar]
- Semenzato, G.; Poletti, V. Bronchoalveolar lavage in lung cancer. Respiration 1992, 59, 44–46. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Yatera, K.; Noguchi, S.; Sakurai, Y.; Nagata, S.; Kozaki, M.; Tokuyama, S.; Ogoshi, T.; Kawanami, T.; Yoshii, C.; et al. Detection of MALT1 gene rearrangements in BAL fluid cells for the diagnosis of pulmonary mucosa- associated lymphoid tissue lymphoma. Chest 2012, 141, 176–182. [Google Scholar] [CrossRef]
- Colby, T.V.; Tomassetti, S.; Cavazza, A.; Dubini, A.; Poletti, V. Transbronchial cryobiopsy in diffuse lung disease: Update for the pathologist. Arch. Pathol. Lab. Med. 2017, 141, 891–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, R.; Dubini, A.; Asioli, S.; Ravaglia, C.; Tomassetti, S.; Puglisi, S.; Piciucchi, S.; Gurioli, C.; Gurioli, C.; Fiocca, R.; et al. Transbronchial cryobiopsy: An effective tool in the diagnosis of lymphoproliferative disorders of the lung. ERJ Open Res. 2020, 6, 00260–02019. [Google Scholar] [CrossRef] [PubMed]
- Juweid, M.E.; Stroobants, S.; Hoekstra, O.S.; Mottaghy, F.M.; Dietlein, M.; Guermazi, A.; Wiseman, G.A.; Kostakoglu, L.; Scheidhauer, K.; Buck, A.; et al. Imaging Subcommittee of International Harmonization Project in Lymphoma. Use of positron emission tomography for response assessment of lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J. Clin. Oncol. 2007, 25, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, K.; Hamada, K.; Inohara, H.; Higuchi, I.; Tomita, Y.; Kubo, T.; Hatazawa, J. Mucosa-associated lymphoid tissue lymphoma studied with FDG-PET: A comparison with CT and endoscopic findings. Ann. Nucl. Med. 2008, 22, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Albano, D.; Borghesi, A.; Bosio, G.; Bertoli, M.; Maroldi, R.; Giubbini, R.; Bertagna, F. Pulmonary mucosa-associated lymphoid tissue lymphoma: F-FDG PET/CT and CT findings in 28 patients. Br. J. Radiol. 2017, 90, 20170311. [Google Scholar] [CrossRef]
- Deng, W.; Wan, Y.; Yu, J.Q. Pulmonary MALT Lymphoma has variable features on CT. Sci. Rep. 2019, 9, 8657. [Google Scholar] [CrossRef] [Green Version]
- King, L.J.; Padley, S.P.; Wotherspoon, A.C.; Nicholson, A.G. Pulmonary MALT lymphoma: Imaging findings in 24 cases. Eur. Radiol. 2000, 10, 1932–1938. [Google Scholar] [CrossRef]
- Borie, R.; Wislez, M.; Antoine, M.; Copie-Bergman, C.; Thieblemont, C.; Cadranel, J. Pulmonary mucosa-associated lymphoid tissue lymphoma revisited. Eur. Respir. J 2016, 47, 1244–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wislez, M.; Cadranel, J.; Antoine, M.; Milleron, B.; Bazot, M.; Mayaud, C.; Carette, M.F. Lymphoma of pulmonary mucosa- associated lymphoid tissue: CT scan findings and patholo- gical correlations. Eur. Respir. J. 1999, 14, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, A.; Jiang, H.; Zhang, Y.; Zhu, L.; Xia, C.; Yu, H. HRCT in primary pulmonary lymphoma: Can CT imaging phenotypes differentiate histological subtypes between mucosa-associated lymphoid tissue (MALT) lymphoma and non-MALT lymphoma? J. Thorac. Dis. 2018, 10, 6040–6049. [Google Scholar] [CrossRef]
- McCulloch, G.L.; Sinnatamby, R.; Stewart, S.; Goddard, M.; Flower, C.D. High-resolution computed tomographic appearance of MALToma of the lung. Eur. Radiol. 1998, 8, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Sweetenham, J.W.; Williams, M.E. (Eds.) Lymphoma: Pathology, Diagnosis, and Treatment; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Sharma, A.; Fidias, P.; Hayman, L.A.; Loomis, S.L.; Taber, K.H.; Aquino, S.L. Patterns of lymphadenopathy in thoracic malignancies. Radiographics 2004, 24, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.R.; Myers, M.L.; Kirk, M.E. Incidental malignancy in internal thoracic artery lymph nodes. Ann. Thorac. Surg. 2001, 72, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Swigris, J.J.; Berry, G.J.; Raffin, T.A.; Kuschner, W.G. Lymphoid interstitial pneumonia: A narrative review. Chest 2002, 122, 2150–2164. [Google Scholar] [CrossRef] [Green Version]
- Travis, W.D.; Galvin, J.R. Non-neoplastic pulmonary lymphoid lesions. Thorax 2001, 56, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Kim, J.S.; Bates, C.A.; Brown, K.K.; Cool, C.D.; Newell, J.D.; Lynch, D.A. Lung diseases in patients with common variable immunodeficiency: Chest radiographic and computed tomographic findings. J. Comput. Assist. Tomogr. 2006, 30, 828–838. [Google Scholar] [CrossRef]
- Do, K.H.; Lee, J.S.; Seo, J.B.; Song, J.W.; Chung, M.J.; Heo, J.N.; Song, K.S.; Lim, T.H. Pulmonary parenchymal involvement of low-grade lymphoproliferative disorders. J. Comput. Assist. Tomogr. 2005, 29, 825–830. [Google Scholar] [CrossRef]
- Cozzi, D.; Dini, C.; Mungai, F.; Puccini, B.; Rigacci, L.; Miele, V. Primary pulmonary lymphoma: Imaging findings in 30 cases. Radiol. Med. 2019, 124, 1262–1269. [Google Scholar] [CrossRef]
- Bligh, M.P.; Borgaonkar, J.N.; Burrell, S.C.; MacDonald, D.A.; Manos, D. Spectrum of CT findings in thoracic extranodal non-Hodgkin lymphoma. Radiographics 2017, 37, 439–461. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.M.; Ng, Y.Y.; Norton, A.J.; Armstrong, P. CT “angiogram sign” in primary pulmonary lymphoma. J. Comput. Assist. Tomogr. 1992, 16, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Diederich, S.; Link, T.; Zühlsdorf, H.; Steinmeyer, E.; Wormanns, D.; Heindel, W. Pulmonary manifestations of Hodgkin’s disease: Radiographic and CT findings. Eur. Radiol. 2001, 11, 2295–2305. [Google Scholar] [CrossRef]
- Angirish, B.; Sanghavi, P.; Jankharia, B. Pulmonary manifestations of lymphoma: A pictorial essay. Lung India 2020, 37, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.R.; Caskey, C.I.; Fishman, E.K. Lymphoma of the lung: CT findings in 31 patients. AJR Am. J. Roentgenol. 1991, 156, 711–714. [Google Scholar] [CrossRef]
- Howling, S.J.; Hansell, D.M.; Wells, A.U.; Nicholson, A.G.; Flint, J.D.; Müller, N.L. Follicular bronchiolitis: Thin-section CT and histologic findings. Radiology 1999, 212, 637–642. [Google Scholar] [CrossRef]
- Glickstein, M.; Kornstein, M.J.; Pietra, G.G.; Aronchick, J.M.; Gefter, W.B.; Epstein, D.M.; Miller, W. Nonlymphomatous lymphoid disorders of the lung. AJR Am. J. Roentgenol. 1986, 147, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Wu, C.C.; Gilman, M.D.; Palmer, E.L.; Hasserjian, R.P.; Shepard, J.A.O. Lymphomatoid granulomatosis: CT and FDG-PET findings. Korean J. Radiol. 2011, 12, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Tuder, R.; Lynch, D.A. Lymphomatoid granulomatosis: Radiologic features and pathologic correlations. AJR Am. J Roentgenol. 2000, 175, 1335–1339. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, K.S.; Ryu, Y.H.; Yoon, Y.C.; Choe, K.O.; Kim, T.S.; Sung, K.J. Reversed Halo Sign on High-Resolution CT of Cryptogenic Organizing Pneumonia: Diagnostic Implications. AJR Am. J. Roentgenol. 2003, 180, 1251–1254. [Google Scholar] [CrossRef]
- Marchiori, E.; Zanetti, G.; Irion, K.L.; Nobre, L.F.; Hochhegger, B.; Mançano, A.D.; Escuissato, D.L. Reversed Halo Sign in Active Pulmonary Tuberculosis: Criteria for Differentiation from Cryptogenic Organizing Pneumonia. AJR Am. J. Roentgenol. 2011, 197, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.C.B.; Viswanathan, C.; Marchiori, E.; Truong, M.T.; Benveniste, M.F.; Rossi, S.; Marom, E.M. The Reversed Halo Sign: Update and Differential Diagnosis. Br. J. Radiol. 2012, 85, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Bicci, E.; Bindi, A.; Cavigli, E.; Danti, G.; Galluzzo, M.; Granata, V.; Pradella, S.; Trinci, M.; Miele, V. Role of Chest Imaging in Viral Lung Diseases. Int. J. Environ. Res. Public Health 2021, 18, 6434. [Google Scholar] [CrossRef]
- Pinto, P. The CT Halo Sign. Radiology 2004, 230, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Choi, Y.W.; Lee, K.J.; Jeon, S.C.; Park, C.K.; Heo, J.N. CT Halo Sign: The Spectrum of Pulmonary Diseases. Br. J. Radiol. 2005, 78, 862–865. [Google Scholar] [CrossRef]
- Borhani, A.A.; Hosseinzadeh, K.; Almusa, O.; Furlan, A.; Nalesnik, M. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics 2009, 29, 981–1000. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Doxtader, E.; Narendra, S. Lymphomatoid granulomatosis: Insights gained over 4 decades. Am. J. Surg. Pathol 2010, 34, e35–e48. [Google Scholar] [CrossRef]
- Cadranel, J.; Naccache, J.; Wislez, M.; Mayaud, C. Pulmonary malignancies in the immunocompromised patient. Respiration 1999, 66, 289–309. [Google Scholar] [CrossRef]
- Gruden, J.F.; Webb, W.R.; Naidich, D.P.; McGuinness, G. Multinodular disease: Anatomic localization at thin-section CT-multireader evaluation of a simple algorithm. Radiology 1999, 210, 711–720. [Google Scholar] [CrossRef]
- Miller, B.H.; Rosado-de-Christenson, M.L.; McAdams, H.P.; Fishback, N.F. Thoracic sarcoidosis: Radiologic-pathologic correlation. Radiographics 1995, 15, 421–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, B.; Schneider, H.; Proto, A. Eggshell Calcification of Lymph Nodes: An Update. AJR Am. J. Roentgenol. 1980, 135, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-I.; Kim, C.W.; Lee, M.K.; Lee, K.S.; Park, C.-K.; Choi, S.J.; Kim, J.G. Imaging of occupational lung disease. Radiographics 2001, 21, 1371–1391. [Google Scholar] [CrossRef] [Green Version]
- Cox, C.W.; Rose, C.S.; Lynch, D.A. State of the art: Imaging of occupational lung disease. Radiology 2014, 270, 681–696. [Google Scholar] [CrossRef]
- Sugi, M.D.; Kawashima, A.; Salomao, M.A.; Bhalla, S.; Venkatesh, S.K.; Pickhardt, P.J. Amyloidosis: Multisystem spectrum of disease with pathologic correlation. Radiographics 2021, 41, 1454–1474. [Google Scholar] [CrossRef]
- Klimek, M. Pulmonary Lymphangitis Carcinomatosis: Systematic Review and Meta-Analysis of Case Reports, 1970–2018. Postgrad Med. 2019, 131, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E. High-Resolution CT of the Lungs. AJR Am. J. Roentgenol. 2001, 177, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Tokuyasu, H.; Harada, T.; Watanabe, E.; Touge, H.; Kawasaki, Y.; Endo, A.; Maeda, R.; Isowa, N.; Ohnuma, H.; Miura, H.; et al. Non-Hodgkin’s lymphoma accompanied by pulmonary involvement with diffuse ground-glass opacity on chest CT: A report of 2 cases. Intern. Med. 2009, 48, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Stainer, A.; Faverio, P.; Bono, F.; Pesci, A. An unusual presentation of pulmonary lymphoma: When diffuse ground glass opacities can mean anything. Monaldi. Arch. Chest. Dis. 2018, 88, 907. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Jin, Y.; Li, Y.; Ye, J. Primary pulmonary lymphoma manifesting as diffuse ground glass opacities: A case report and literature review. Int. J. Clin. Exp. Pathol. 2020, 13, 2181–2186. [Google Scholar]
- Nishii-Ito, S.; Izumi, H.; Touge, H.; Takeda, K.; Hosoda, Y.; Yamasaki, A.; Kuwamoto, S.; Shimizu, E.; Motokura, T. Pulmonary intravascular large B-cell lymphoma successfully treated with rituximab, cyclophosphamide, vincristine, doxorubicin and prednisolone immunochemotherapy: Report of a patient surviving for over 1 year. Mol. Clin. Oncol. 2016, 5, 689–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaty, H.; Artiles, C.; Yadav, R.; Garcha, P.; Mukhopadhyay, S.; Sahoo, D. Diffuse Large B-Cell Lymphoma Presenting as Diffuse Bilateral Ground-Glass Opacities and Diagnosed on Transbronchial Lung Biopsy. Ann. Am. Thorac. Soc. 2017, 14, 605–607. [Google Scholar] [CrossRef]
- Higashiyama, R.I.; Makita, S.; Maeshima, A.M.; Maruyama, D. Atypical radiological presentation of pulmonary invasion of diffuse large B-cell lymphoma mimicking Pneumocystis jiroveci pneumonia. Jpn. J. Clin. Oncol. 2018, 48, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Ogata-Suetsugu, S.; Maeyama, T.; Takeshita, M.; Hamada, N.; Takayama, K.; Inoue, H.; Nakanishi, Y. A case of diffuse large B-cell lymphoma of the lung demonstrating diffuse ground-glass shadows. Ann. Thorac. Cardiovasc. Surg. 2011, 17, 591–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patricelli, G.; Galeone, C.; Rapicetta, C.; Valli, R.; Mengoli, M.C.; Carbonelli, C.; Paci, M.; Lococo, F. Primary pulmonary MALT-lymphoma mimicking pulmonary infection: A case report and overview on the pertinent literature. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 260–263. [Google Scholar] [CrossRef]
- Fujita, M.; Uchino, J.; Nabeshima, K.; Watanabe, K. Pulmonary MALT lymphoma demonstrating a crazy-paving appearance on imaging. Intern. Med. 2015, 54, 2705–2706. [Google Scholar] [CrossRef] [Green Version]
- Fraser, T.; Nagarur, A. Pulmonary involvement of peripheral T-cell lymphoma manifesting as crazy paving pattern. Proc. (Bayl. Univ. Med. Cent.) 2015, 28, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Gomatou, G.; Tzilas, V.; Kourti, G.; Lagou, S.; Bouros, D.; Syrigos, K. Crazy paving pattern as a rare radiological manifestation of peripheral T-cell lymphoma (PTCL) with lung involvement: A case report. Respir. Med. Case. Rep. 2018, 25, 253–256. [Google Scholar] [CrossRef]
- Raderer, M.; Kiesewetter, B.; Ferreri, A.J.M. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA A Cancer J. Clin. 2016, 66, 152–171. [Google Scholar] [CrossRef]
- Suzuki, T.; Akizawa, T.; Suzuki, H.; Kitazume, K.; Omine, M.; Mitsuya, T. Primary tracheal mucosa-associated lymphoid tissue lymphoma accompanying lung cancer. Jpn. J. Thorac. Cardiovasc. Surg. 2000, 48, 817–819. [Google Scholar] [CrossRef]
- Touil, I.; Amor, H.I.H.; Brahem, Y.; Bouchareb, S.; Ayeb, J.; Boussoffara, L.; Boudawara, N.K.; Knani, J. Pulmonary MALT lymphoma associated with interstitial pulmonary disease. Respir. Med. Case Rep. 2022, 36, 101598. [Google Scholar] [CrossRef] [PubMed]
- Mirili, C.; Paydas, S.; Ogul, A.; Gokcay, S.; Buyuksimsek, M.; Yetisir, E.; Hanta, I.; Ergin, M.; Kılıç, E.B.; Guney, I.B. Pulmonary MALT lymphoma with underlying interstitial lung disease: A case report and rewiev of the literature. J. Oncol. Sci. 2018, 4, 56–59. [Google Scholar] [CrossRef]
- Khojeini, E.V.; Song, J.Y. Intravascular large B-cell lymphoma presenting as interstitial lung disease. Case Rep. Pathol. 2014, 2014, 928065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, H.; Suzuki, A.; Takahashi, Y.; Kubota, K.; Kano, T.; Mimori, A. Intravascular large B-cell lymphoma with diffuse FDG uptake in the lung by 18FDG-PET/CT without chest CT findings. Ann. Nucl. Med. 2012, 26, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Hemmaway, C.J.; Danga, A.; Igbokwe, U.; Radunovic, A. FDG-PET guided diagnosis of vaginal intravascular diffuse large B-cell lymphoma. Br. J. Haematol. 2012, 158, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johkoh, T.; Müller, N.L.; Pickford, H.A. Lymphocytic interstitial pneumonia: Thin-section CT findings in 22 patients. Radiology 1999, 212, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Nomura, K.; Shimizu, D.; Okuda, T.; Yoshida, N.; Matsumoto, Y.; Taki, T.; Horiike, S.; Taniwaki, M. Pulmonary relapse of non-Hodgkin’s lymphoma in bilateral upper lobes. Intern. Med. 2006, 45, 971–973. [Google Scholar] [CrossRef] [Green Version]
- Honda, O.; Johkoh, T.; Ichikado, K.; Yoshida, S.; Mihara, N.; Higashi, M.; Tomiyama, N.; Maeda, M.; Hamada, S.; Naito, H.; et al. Comparison of high- resolution CT findings of sarcoidosis, lymphoma, and lymphang- itic carcinoma: Is there any difference of involved interstitium? J. Comput. Assist. Tomogr. 1999, 23, 374–379. [Google Scholar] [CrossRef]
- Sverzellati, N.; Odone, A.; Silva, M.; Polverosi, R.; Florio, C.; Cardinale, L.; Cortese, G.; Addonisio, G.; Zompatori, M.; Dalpiaz, G.; et al. Structured reperting for fibrosing lung disease: A model shared by radiologist and pulmonologist. Radiol. Med 2018, 123, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozzi, D.; Bargagli, E.; Calabrò, A.G.; Torricelli, E.; Giannelli, F.; Cavigli, E.; Miele, V. Atypical HRCT manifestations of pulmonary sarcoidosis. Radiol. Med. 2018, 123, 174–184. [Google Scholar] [CrossRef]




| Main Radiological Findings | Main Differential Diagnoses | |
|---|---|---|
| MALT | Consolidations, bronchovascular distribution without topographic predominance, lobar-like pneumonia (rare), ±bulging fissure sign, ±angiogram sign, ±air bronchogram; less frequently nodules, masses and GGO; interstitial involvement (very rare) | Neoplasms, lobar or focal atelectasis, infections (Klebsiella pneumoniae), OP, LIP, NLH, sarcoidosis |
| DLBCL | Masses and nodules, mediastinal nodes enlargement, GGO | Neoplasms, LYG, metastasis |
| LIP | Air-filled cysts, “halo sign”, masses and nodules with centrilobular appearance, GGO, patchy interstitial involvement | Infections, metastasis, neoplasm, MALT, sarcoidosis, amiloidosis |
| NHL | Nodule (usually single) < 2 cm; less frequently mass or consolidation, air bronchogram± | Infections, neoplasm, metastasis, MALT |
| LYG | Masses and nodules with peribronchovascolar distribution, basal predominance, GGO, “halo sign”, “reverse halo sign” | Vasculitis, sarcoidosis, neoplasms, metastasis, PTLD, angioinvasive aspergillosi, DLBCL |
| PTLD | Nodules, masses or consolidations (rare), ±air bronchogram, “halo sign”, perilymphatic distribution; mediastinal nodes enlargement; interstitial thickening | Angioinvasive aspergillosis, LYG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozzi, L.; Cozzi, D.; Cavigli, E.; Moroni, C.; Giannessi, C.; Zantonelli, G.; Smorchkova, O.; Ruzga, R.; Danti, G.; Bertelli, E.; et al. Primary Lymphoproliferative Lung Diseases: Imaging and Multidisciplinary Approach. Diagnostics 2023, 13, 1360. https://doi.org/10.3390/diagnostics13071360
Gozzi L, Cozzi D, Cavigli E, Moroni C, Giannessi C, Zantonelli G, Smorchkova O, Ruzga R, Danti G, Bertelli E, et al. Primary Lymphoproliferative Lung Diseases: Imaging and Multidisciplinary Approach. Diagnostics. 2023; 13(7):1360. https://doi.org/10.3390/diagnostics13071360
Chicago/Turabian StyleGozzi, Luca, Diletta Cozzi, Edoardo Cavigli, Chiara Moroni, Caterina Giannessi, Giulia Zantonelli, Olga Smorchkova, Ron Ruzga, Ginevra Danti, Elena Bertelli, and et al. 2023. "Primary Lymphoproliferative Lung Diseases: Imaging and Multidisciplinary Approach" Diagnostics 13, no. 7: 1360. https://doi.org/10.3390/diagnostics13071360
APA StyleGozzi, L., Cozzi, D., Cavigli, E., Moroni, C., Giannessi, C., Zantonelli, G., Smorchkova, O., Ruzga, R., Danti, G., Bertelli, E., Luzzi, V., Pasini, V., & Miele, V. (2023). Primary Lymphoproliferative Lung Diseases: Imaging and Multidisciplinary Approach. Diagnostics, 13(7), 1360. https://doi.org/10.3390/diagnostics13071360







