Quantitative Electroencephalography Analysis for Improved Assessment of Consciousness Levels in Deep Coma Patients Using a Proposed Stimulus Stage
Abstract
1. Introduction
- The frequency analysis and machine learning methods used in this study may contribute to the detection of consciousness levels of patients in a deep coma and the development of BCI systems for objective determination of GCS.
- A new recording procedure including tactile and auditory stimuli has been proposed for this system, which was developed to examine changes in the EEG activity of different levels of consciousness and to measure the responses of patients to stimuli.
- Features extracted by power spectral density analysis from EEG signals could characterize the changes in brain function for a deep coma state. The results obtained may be valuable for future studies in predicting the prognosis of unconscious patients and are very important for further studies to show the difference in the levels of consciousness of patients in a deep coma.
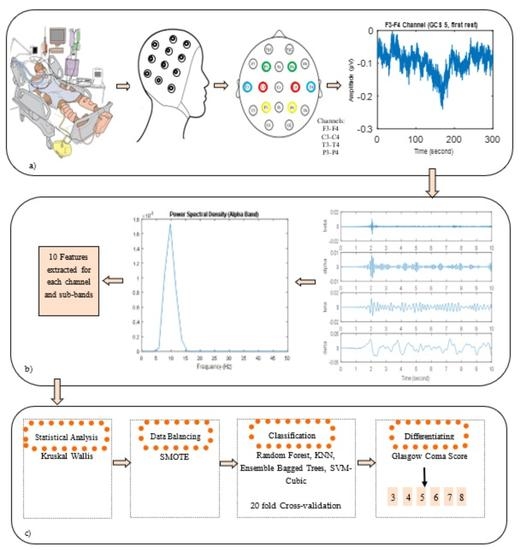
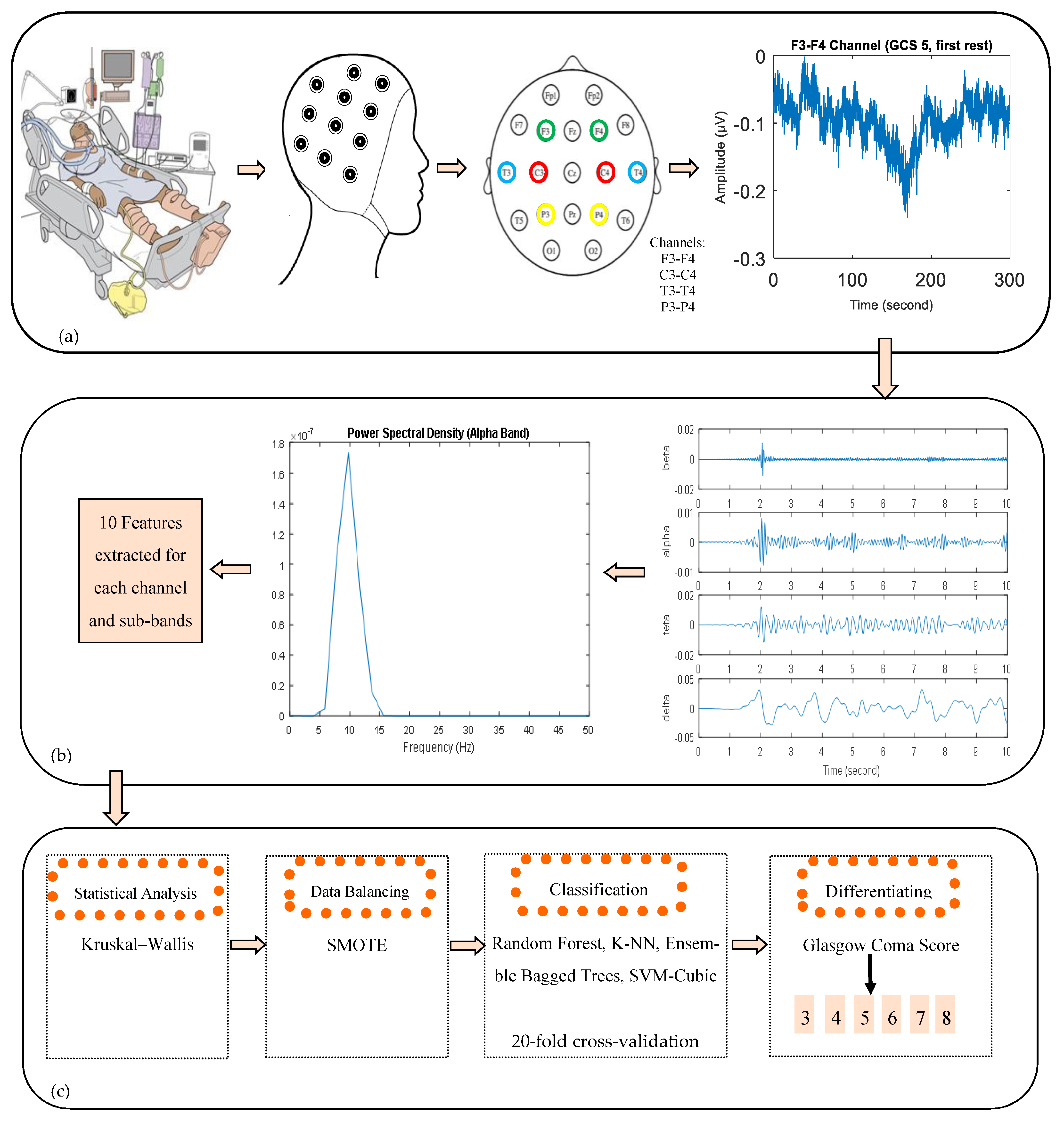
2. Materials and Methods
2.1. Subjects
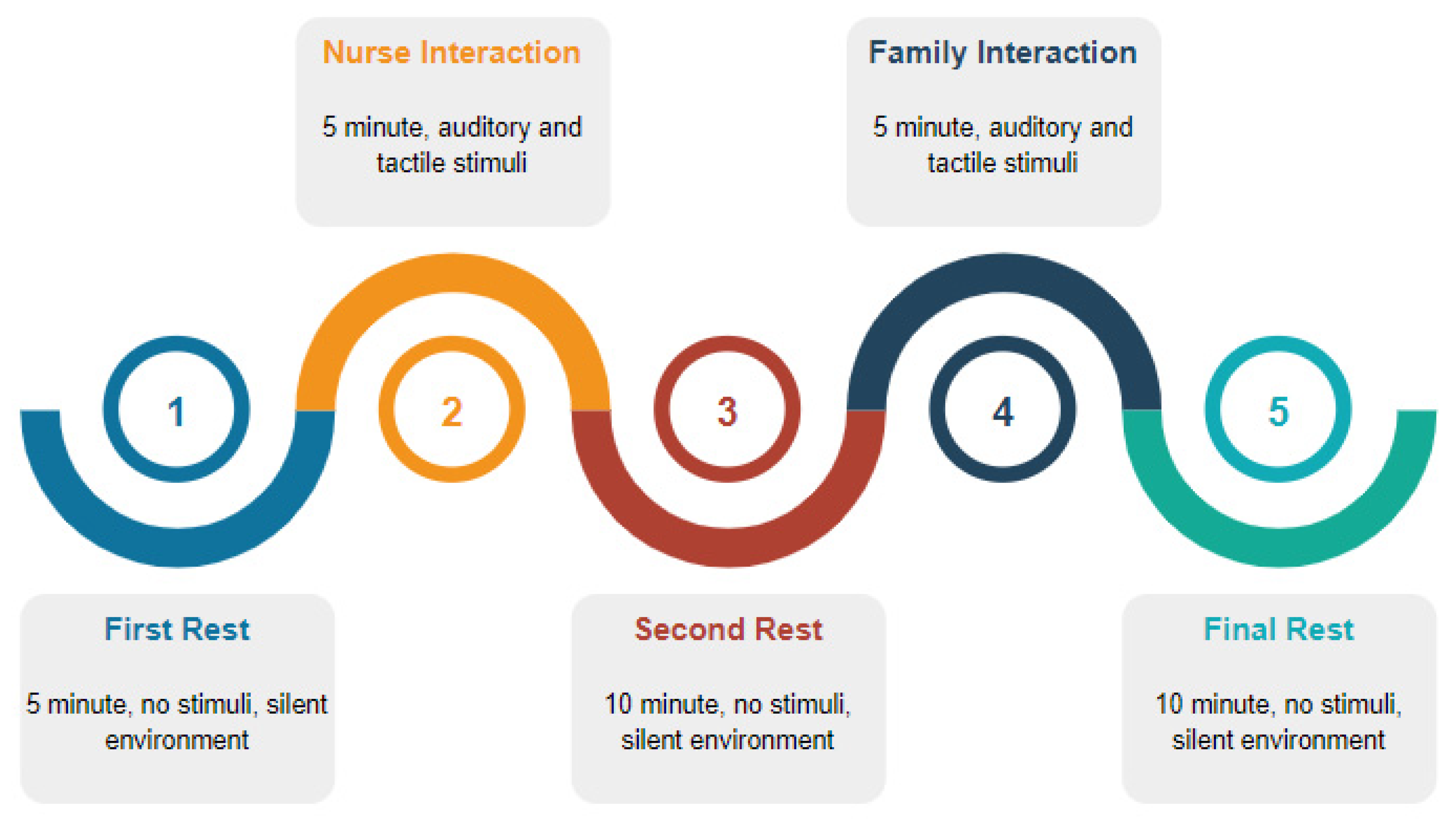
2.2. EEG Recordings and Pre-Processing
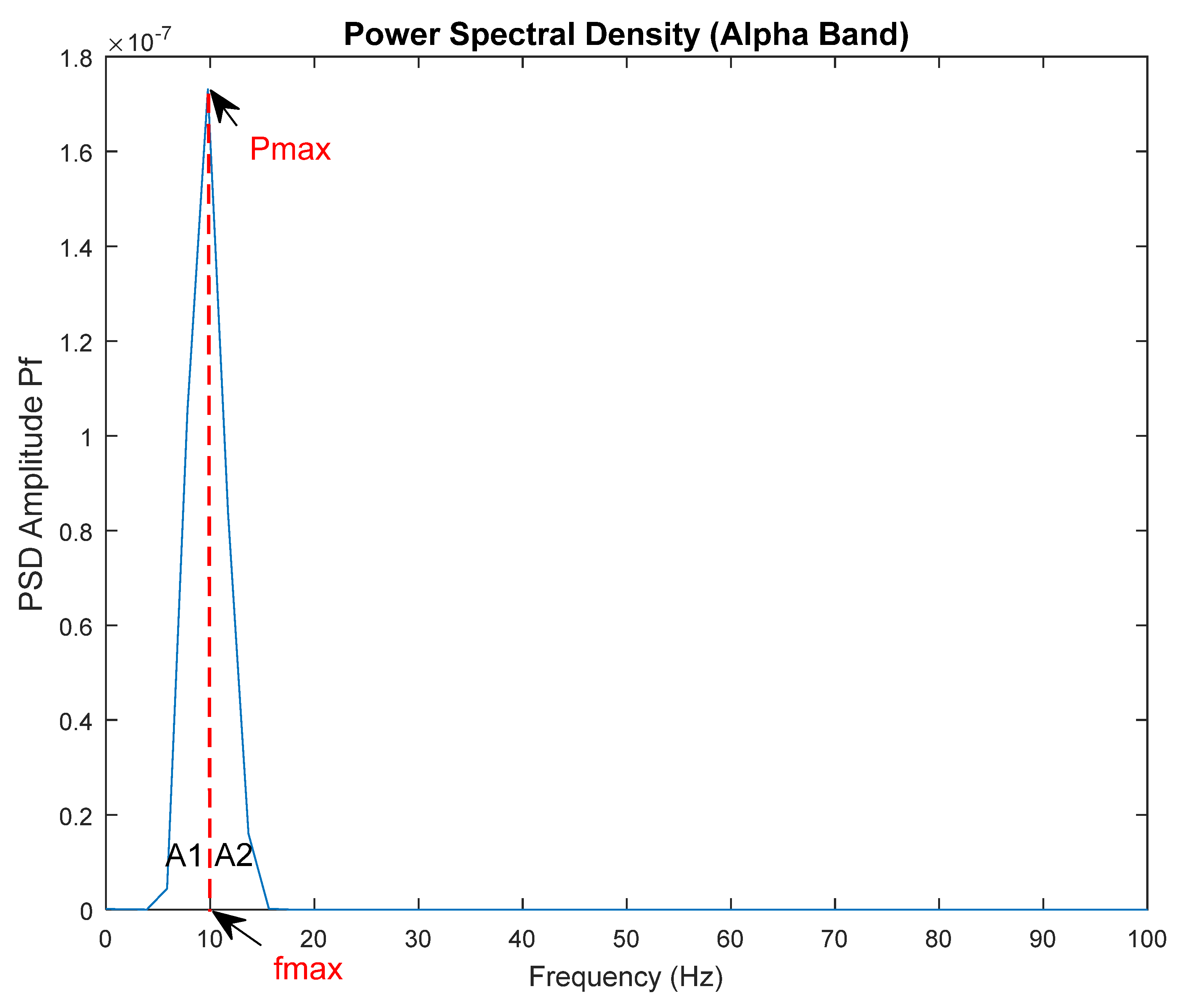
2.3. Power Spectral Density of EEG Signals and Feature Extraction
2.4. Statistical Analysis
2.5. Data Balancing
2.6. Classification
3. Results
3.1. Analysis of Energy Values
3.2. Statistical Analysis
3.3. Data Balance
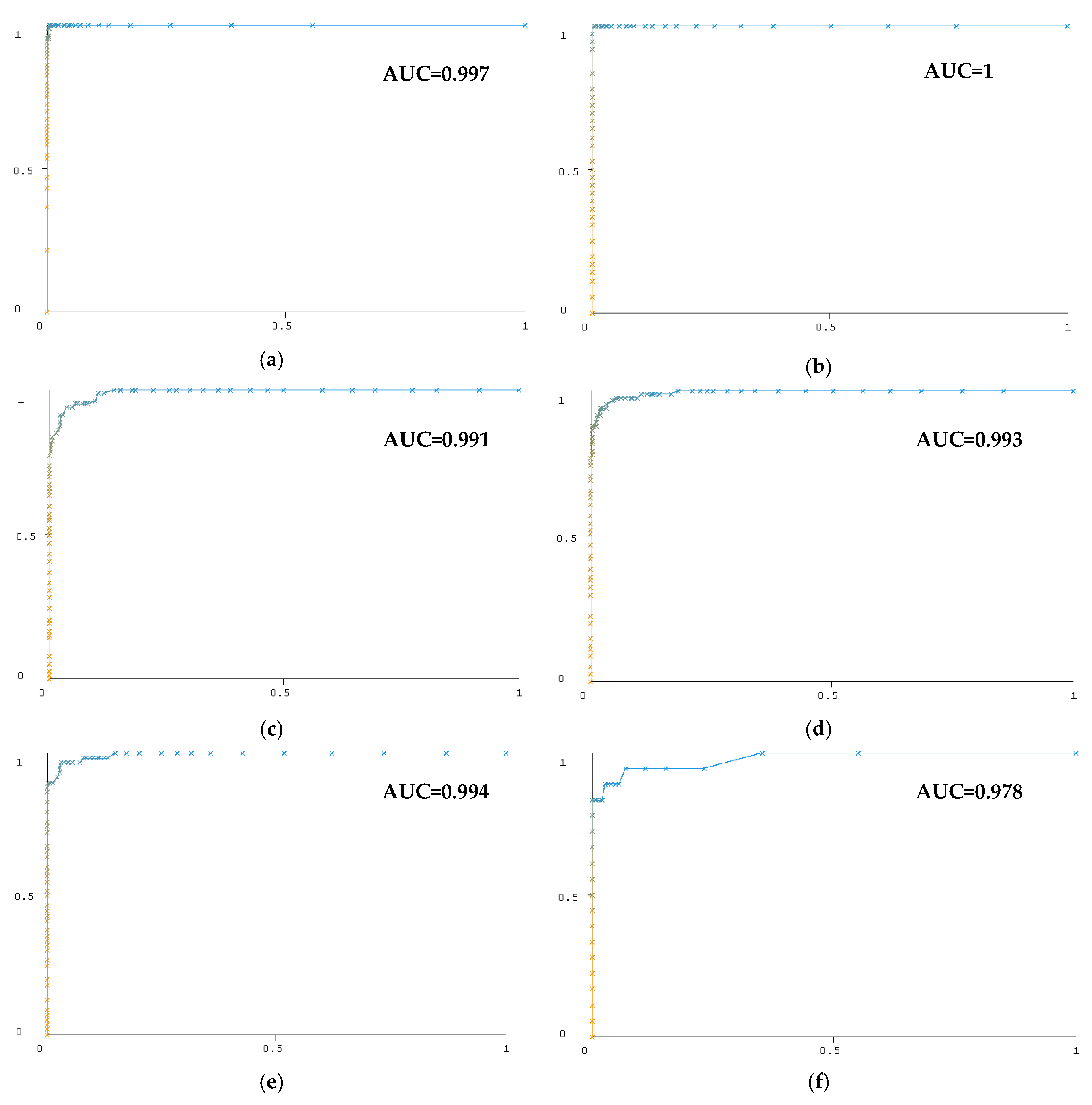
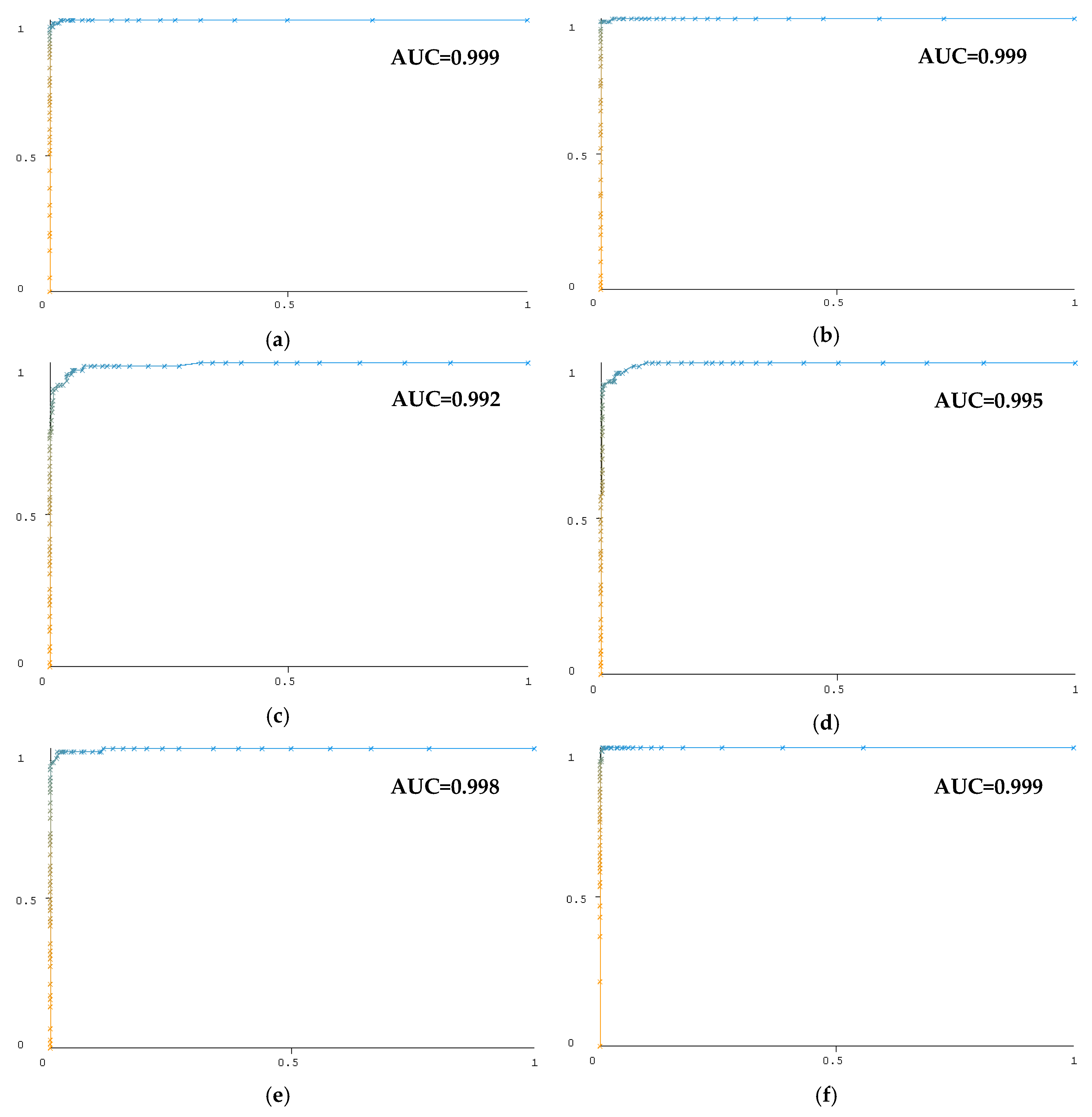
3.4. Classification Results
4. Discussion
4.1. Related Works
4.2. Limitations and Feature Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooksley, T.; Holland, M. The unconscious patient. Medicine 2013, 41, 146–150. [Google Scholar] [CrossRef]
- Campbell, S.; McCormick, W. Approach to the comatose patient. Can. J. CME 2002, 16, 77–84. [Google Scholar]
- Rapsang, A.G.; Shyam, D.C. Scoring systems in the intensive care unit: A compendium. Indian J. Crit. Care Med. 2014, 18, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Karabıyık, L. Yoğun Bakımda Skorlama Sistemleri. Yoğun Bakım Derg. 2010, 9, 129–143. [Google Scholar]
- Sakarya, M. Skorlama Sistemleri. Türk Yoğun Bakım Derneği Derg. 2006, 4, 66–73. [Google Scholar]
- Rosenfeld, J.V.; Lennarson, P.J. Coma and Brain Death. In Neurology and Clinical Neuroscience; Schapira, A.H.V., Byrne, E., Frackowiak, R.S.J., Mizuno, Y., Silberstein, S.D., Eds.; Mosby: Maryland Heights, MI, USA, 2007; Chapter 8; pp. 97–116. [Google Scholar]
- Zheng, W.B.; Liu, G.R.; Kong, K.M.; Wu, R.H. Coma Duration Prediction in Diffuse Axonal Injury: Analyses of Apparent Diffusion Coefficient and Clinical Prognostic Factors. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York City, NY, USA, 30 August–3 September 2006; pp. 1052–1055. [Google Scholar]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Namiki, J.; Yamazaki, M.; Funabiki, T.; Hori, S. Inaccuracy and misjudged factors of Glasgow Coma Scale scores when assessed by inexperienced physicians. Clin. Neurol. Neurosurg. 2011, 113, 393–398. [Google Scholar] [CrossRef]
- Brain Trauma Foundation and American Association of Neurological Surgeons, Early indicators of prognosis in severe traumatic brain injury, Glasgow Coma Scale score. J. Neurotrauma 2000, 17, 563–571.
- Crossman, J.; Bankes, M.; Bhan, A.; Crockard, H.A. The Glasgow Coma Score: Reliable evidence? Injury 1998, 29, 435–437. [Google Scholar] [CrossRef]
- Gill, M.R.; Reiley, D.G.; Green, S.M. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann. Emerg. Med. 2004, 43, 215–223. [Google Scholar] [CrossRef]
- Riechers, R.G.; Ramage, A.; Brown, W.; Kalehua, A.; Rhee, P.; Ecklund, J.M.; Ling, G.S.; Reith, F.C.; Brennan, P.M.; Maas, A.I.; et al. Physician Knowledge of the Glasgow Coma Scale. J. Neurotrauma 2005, 22, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Rowley, G.; Fielding, K. Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users. Lancet 1991, 337, 535–538. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Wieser, M.; Koenig, B.A.; Riener, R. Quantitative Description of the State of Awareness of Patients in Vegetative and Minimally Conscious State. In Proceedings of the 32nd Annual International Conference of the IEEE EMBS, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5533–5536. [Google Scholar]
- Goldberg, S.A.; Rojanasarntikul, D.; Jagoda, A. The prehospital management of traumatic brain injury. In Handbook of Clinical Neurology; Grafman, J., Andres Salazar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 23; Volume 127, pp. 367–378. [Google Scholar]
- Laureys, S. The neural correlate of (un)awareness: Lessons from the vegetative state. Trends Cogn. Sci. 2005, 9, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Tarassenko, L.; Clifton, D.; Pinksky, M.; Hravnak, M.; Woods, J.; Watkinson, P. Centile-based early warning scores derived from Statistical distributions of vital signals. Resuscitation 2011, 82, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Tarassenko, L.; Hann, A.; Young, D. Integrated monitoring and analysis for early warning of patient deterioration. Brit. J. Anaesthesia 2006, 97, 64–68. [Google Scholar] [CrossRef]
- Lin, M.A.; Chan, H.L.; Fang, S.C. Linear and Nonlinear EEG Indexes in Relation to the Severity of Coma. In Proceedings of the 27th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Shanghai, China, 1–4 September 2005; pp. 4580–4583. [Google Scholar]
- Van Gils, M.; Rosenfalck, A.; White, S.; Prior, P.; Gade, J.; Senhadji, L.; Thomsen, C.; Ghosh, I.; Longford, R.; Jensen, K. Signal processing in prolonged EEG recordings during intensive care. IEEE Eng. Med. Biol. Mag. 1997, 16, 56–63. [Google Scholar] [CrossRef]
- Shah, A.K.; Agarwal, R.; Carhuapoma, J.; Loeb, J.A. Compressed EEG Pattern Analysis for Critically Ill Neurological-Neurosurgical Patients. Neurocrit. Care 2006, 5, 124–133. [Google Scholar] [CrossRef]
- Flower, L. Literature Survey on Biomedical Signal Processing Methods. Int. J. Innov. Res. Comput. Commun. Eng. 2016, 4, 50–54. [Google Scholar]
- Li, L.; Witon, A.; Marcora, S.; Bowman, H.; Mandic, D.P. EEG-Based Brain Connectivity Analysis of States of Unawareness. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1002–1005. [Google Scholar]
- Ahmed, B.; Tafreshi, R.; Langari, R. The Future of Automatic EEG Monitoring in the Intensive Care. In Proceedings of the International Conference on BioMedical Engineering and Informatics, Sanya, China, 27–30 May 2008; pp. 520–524. [Google Scholar]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.-J. Quantitative Evaluation of Task-Induced Neurological Outcome after Stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Hussain, I.; Rahman, M.; Park, S.J.; Hossain, A. Explainable Artificial Intelligence Model for Stroke Prediction Using EEG Signal. Sensors 2022, 22, 9859. [Google Scholar] [CrossRef] [PubMed]
- Kotchoubey, B. Evoked and event-related potentials in disorders of consciousness: A quantitative review. Conscious. Cogn. 2017, 54, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mikola, A.; Särkelä, M.O.; Walsh, T.S.; Lipping, T. Power Spectrum and Cross Power Spectral Density Based EEG Correlates of Intensive Care Delirium. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4562–4565. [Google Scholar]
- Lechinger, J.; Bothe, K.; Pichler, G.; Michitsch, G.; Donis, J.; Klimesch, W.; Schabus, M. CRS-R score in disorders of consciousness is strongly related to spectral EEG at rest. J. Neurol. 2013, 260, 2348–2356. [Google Scholar] [CrossRef]
- Piarulli, A.; Bergamasco, M.; Thibaut, A.; Cologan, V.; Gosseries, O.; Laureys, S. EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J. Neurol. 2016, 263, 1746–1760. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, P.; Leo, A.; Cacciola, A.; Bramanti, A.; Manuli, A.; Calabrò, R.S. Towards a method to differentiate chronic disorder of consciousness patients’ awareness: The Low-Resolution Brain Electromagnetic Tomography Analysis. J. Neurol. Sci. 2016, 368, 178–183. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Lang, S.; Mezger, G.; Schmalohr, D.; Schneck, M.; Semmler, A.; Bostanov, V.; Birbaumer, N. Information processing in severe disorders of consciousness: Vegetative state and minimally conscious state. Clin. Neurophysiol. 2005, 116, 2441–2453. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Laurido-Soto, O.; Eisenman, L.N.; Kummer, T.T.; Ching, S. Intrinsic network reactivity differentiates levels of consciousness in comatose patients. Clin. Neurophysiol. 2018, 129, 2296–2305. [Google Scholar] [CrossRef]
- Serafmi, G.; Acra, W.; Scuteri, F.; Palmieri, A.M.R.; Simoncelli, C.; Serafini, G. Auditory Evoked Potentials at 40 Hz (SSR40Hz) in Post-Trauma Coma Patients. Laryngoscope 1994, 101, 182–184. [Google Scholar] [CrossRef]
- Firsching, R.; Luther, J.; Eidelberg, E.; Brown, W.E., Jr.; Story, J.L.; Boop, F.A. 40 Hz—middle latency auditory evoked response in comatose patients. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 213–216. [Google Scholar] [CrossRef]
- Binder, M.; Górska, U.; Griskova-Bulanova, I. 40 Hz auditory steady-state responses in patients with disorders of consciousness: Correlation between phase-locking index and Coma Recovery Scale-Revised score. Clin. Neurophysiol. 2017, 128, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lu, S.; Qian, P.; Chen, G.; Hu, N. An automatic detection method for 40-Hz auditory steady state response and its application in prognosis of comatose patients. Clin. Neurophysiol. 2020, 131, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Kailath, T. Modern Signal Processing; CRC Press: Boca Raton, FL, USA, 1986; 445p. [Google Scholar]
- Proakis, J.G.; Manolakis, D.G. Digital Signal Processing: Principles, Algorithms, and Applications, 4th ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Ariananda, D.D.; Lakshmanan, M.K.; Nikookar, H. A survey on spectrum sensing techniques for cognitive radio. In Proceedings of the 2009 Second International Workshop on Cognitive Radio and Advanced Spectrum Management, Aalborg, Denmark, 18–20 May 2009; pp. 74–79. [Google Scholar]
- Zhao, H.; Gui, L. Nonparametric and parametric methods of spectral analysis. MATEC Web Conf. 2019, 283, 07002. [Google Scholar] [CrossRef]
- Shiman, F.; Safavi, S.H.; Vaneghi, F.M.; Oladazimi, M.; Safari, M.J.; Ibrahim, F. EEG feature extraction using parametric and non-parametric models. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 66–70. [Google Scholar]
- Welch, P.D. The use of fast Fourier transform for the estimation ofpower spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Stoica, P.; Moses, R. Spectral Analysis of Signals; Prentice Hall: Upper Saddle River, NJ, USA, 2005; 447p. [Google Scholar]
- Chaparro, L. Frequency Analysis: The Fourier Series. In Signals and Systems Using MATLAB, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 263–332. [Google Scholar]
- Kara, S.; Latifoğlu, F. Analysis of internal carotid artery and ophthalmic artery Doppler signals using discrete wavelet transformation. In Proceedings of the European Symposium on Biomedical Engineering, Patras, Greece, 25 August 2006. [Google Scholar]
- Batbat, T.; Güven, A.; Dolu, N. Evaluation of divided attention using different stimulation models in event-related potentials. Med Biol. Eng. Comput. 2019, 57, 2069–2079. [Google Scholar] [CrossRef]
- Güven, A.; Altınkaynak, M.; Dolu, N.; Ünlühızarcı, K. Advanced analysis of auditory evoked potentials in hyperthyroid patients: The effect of filtering. J. Med. Syst. 2015, 39, 13. [Google Scholar] [CrossRef]
- Anand, A.; Pugalenthi, G.; Fogel, G.B.; Suganthan, P.N. An approach for classification of highly imbalanced data using weighting and undersampling. Amino Acids 2010, 39, 1385–1391. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, R.; Ding, Z.; Du, X.; Chen, J.; Zhang, Y. A Parameter-Free Cleaning Method for SMOTE in Imbalanced Classification. IEEE Access 2019, 7, 23537–23548. [Google Scholar] [CrossRef]
- Esteves, V.M.S. Techniques to Deal with Imbalanced Data in Multi-Class Problems: A Review of Existing Methods. Master’s Thesis, Universidade do Porto, Porto, Portugal, 2020. [Google Scholar]
- Shelke, M.S.; Deshmukh, P.R.; Shandilya, V.K. A Review on Imbalanced Data Handling Using Undersampling and Oversampling Technique. Int. J. Recent Trends Eng. Res. 2017, 3, 444–449. [Google Scholar]
- Wang, Y.; Simon, M.; Bonde, P.; Harris, B.U.; Teuteberg, J.J.; Kormos, R.L.; Antaki, J.F. Prognosis of Right Ventricular Failure in Patients With Left Ventricular Assist Device Based on Decision Tree With SMOTE. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 383–390. [Google Scholar] [CrossRef]
- Lijun, L.; Tingting, L.; Meiya, H. Feature identification from imbalanced data sets for diagnosis of Cardiac Arrhythmia. In Proceedings of the 11th International Symposium on Computational Intelligence and Design (ISCID), Hangzhou, China, 8–9 December 2018; pp. 52–55. [Google Scholar]
- Shree, B.; Sheshadri, B.S. An approach to preprocess data in the diagnosis of Alzheimer’s Disease. In Proceedings of the 2014 International Conference on Cloud Computing and Internet of Things, Barcelona, Spain, 13–14 December 2014; pp. 135–139. [Google Scholar]
- Gao, R.; Peng, J.; Nguyen, L.; Liang, Y.; Thng, S.; Lin, Z. Classification of Non-Tumorous Facial Pigmentation Disorders using Deep Learning and SMOTE. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Sapporo, Japan, 26–29 May 2019; pp. 1–5. [Google Scholar]
- Abdoh, S.F.; Rizka, M.A.; Maghraby, F.A. Cervical Cancer Diagnosis Using Random Forest Classifier With SMOTE and Feature Reduction Techniques. IEEE Access 2018, 6, 59475–59485. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Bou Rjeily, C.; Badr, G.; Hajjarm El Hassani, A.; Andres, E. Medical Data Mining for Heart Diseases and the Future of Sequential Mining in Medical Field. In Machine Learning Paradigms. Intelligent Systems Reference Library; Tsihrintzis, G., Sotiropoulos, D., Jain, L., Eds.; Springer: Cham, Switzerland, 2018; Volume 149. [Google Scholar]
- Prichep, L.; John, E.; Ferris, S.; Rausch, L.; Fang, Z.; Cancro, R.; Torossian, C.; Reisberg, B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol. Aging 2006, 27, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.W.; North, D.M.; Curtin, R.T.; Walker, R.A.; Biver, C.J.; Gomez, J.F.; Salazar, A.M. An EEG severity index of traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 77–81. [Google Scholar] [CrossRef]
- Cao, C.; Tutwiler, R.L.; Slobounov, S. Automatic Classification of Athletes With Residual Functional Deficits Following Concussion by Means of EEG Signal Using Support Vector Machine. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 327–335. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Podgorelec, V.; Kokol, P.; Stiglic, B.; Rozman, I. Decision trees: An overview andtheir use in medicine. J. Med. Syst. 2002, 26, 445–463. [Google Scholar] [CrossRef]
- Gou, J.; Ma, H.; Ou, W.; Zeng, S.; Rao, Y.; Yang, H. A generalized mean distance-based k-nearest neighbor classifier. Expert Syst. Appl. 2019, 115, 356–372. [Google Scholar] [CrossRef]
- Zhou, Z.H. Ensemble Methods: Foundations and Algorithms. In Bookshelf; Chapman & Hall/CRC: Horsham Township, PA, USA, 2012. [Google Scholar]
- Gosain, A.; Sardana, S. Handling Class Imbalance Problem using Oversampling Techniques: A Review. In Proceedings of the 2017 ICACCI, Udupi, India, 13–16 September 2017; pp. 79–85. [Google Scholar]
- Michal, T. Fundamental of EEG Measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar]
- Nunez, P.L. Neocortical Dynamics and Human EEG Rhythms; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Blakemore, S.-J.; Frith, U. The Learning Brain; Blackwell Publishing: Hoboken, NJ, USA, 2005. [Google Scholar]
- Schacter, D.L.; Gilbert, D.T.; Wegner, D.M. Psychology, 2nd ed.; Worth Publishers: New York, NY, USA, 2010. [Google Scholar]
- Niedermeyer, E.; da Silva, F.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; 1309p. [Google Scholar]
- Scher, M. Pediatric Neurophysiologic Evaluation, Swaiman’s Pediatric Neurology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–96. [Google Scholar]
- Mari-Acevedo, J.; Yelvington, K.; Tatum, W.O. Normal EEG variants. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 9; Volume 160, pp. 143–160. [Google Scholar]
- Kustermann, T.; Nguissi, N.A.N.; Pfeiffer, C.; Haenggi, M.; Kurmann, R.; Zubler, F.; Oddo, M.; Rossetti, A.O.; De Lucia, M. Electroencephalography-based power spectra allow coma outcome prediction within 24 h of cardiac arrest. Resuscitation 2019, 142, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, J.; Cao, J.; Tanaka, T.; Wang, R.; Zhu, H. EEG data analysis based on EMD for coma and quasi-brain-death patients. J. Exp. Theor. Artif. Intell. 2011, 23, 97–110. [Google Scholar] [CrossRef]
- Zhu, L.; Cui, G.; Cao, J.; Cichocki, A.; Zhang, J.; Zhou, C. A Hybrid System for Distinguishing between Brain Death and Coma Using Diverse EEG Features. Sensors 2019, 19, 1342. [Google Scholar] [CrossRef]
- Claassen, J.; Velazquez, A.; Meyers, E.; Witsch, J.; Falo, M.C.; Park, S.; Agarwal, S.; Schmidt, J.M.; Schiff, N.D.; Sitt, J.D.; et al. Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Ann. Neurol. 2016, 80, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Lehembre, R.; Marie-Aurélie, B.; Vanhaudenhuyse, A.; Chatelle, C.; Cologan, V.; Leclercq, Y.; Soddu, A.; Macq, B.; Laureys, S.; Noirhomme, Q. Resting-state EEG study of comatose patients: A connectivity and frequency analysis to find differences between vegetative and minimally conscious states. Funct. Neurol. 2012, 27, 41–47. [Google Scholar]
- Miao, Y.; Cao, J. Descriptive statistical analysis based on patients EEG energy in coma and quasi-brain-death state. Int. J. Comput. Technol. 2018, 17, 7140–7145. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Li, X.; Wang, Y.; Yang, Y.; Liu, Y.; Liang, Z.; He, J. Spinal cord stimulation modulates frontal delta and gamma in patients of minimally consciousness state. Neuroscience 2017, 346, 247–254. [Google Scholar] [CrossRef]
- Stefan, S.; Schorr, B.; Lopez-Rolon, A.; Kolassa, I.-T.; Shock, J.P.; Rosenfelder, M.; Heck, S.; Bender, A. Consciousness Indexing and Outcome Prediction with Resting-State EEG in Severe Disorders of Consciousness. Brain Topogr. 2018, 31, 848–862. [Google Scholar] [CrossRef]
- Bagnato, S.; Boccagni, C.; Sant’Angelo, A.; Prestandrea, C.; Mazzilli, R.; Galardi, G. EEG predictors of outcome in patients with disorders of consciousness admitted for intensive rehabilitation. Clin. Neurophysiol. 2015, 126, 959–966. [Google Scholar] [CrossRef]
- Estraneo, A.; Loreto, V.; Guarino, I.; Boemia, V.; Paone, G.; Moretta, P.; Trojano, L. Standard EEG in diagnostic process of prolonged disorders of consciousness. Clin. Neurophysiol. 2016, 127, 2379–2385. [Google Scholar] [CrossRef]
- Corchs, S.; Chioma, G.; Dondi, R.; Gasparini, F.; Manzoni, S.; Markowska-Kacznar, U.; Mauri, G.; Zoppis, I.; Morreale, A. Computational Methods for Resting-State EEG of Patients With Disorders of Consciousness. Front. Neurosci. 2019, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.; Playford, E.D.; Leff, A. Patients with a severe prolonged Disorder of Consciousness can show classical EEG responses to their own name compared with others’ names. NeuroImage Clin. 2018, 19, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Maggio, M.G.; Leo, A.; Calabrò, R.S. Multiplex and Multilayer Network EEG Analyses: A Novel Strategy in the Differential Diagnosis of Patients with Chronic Disorders of Consciousness. Int. J. Neural Syst. 2021, 31, 2050052. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Lin, M.A.; Fang, S.C. Linear and Nonlinear Analysis of Electroencephalogram of the Coma. In Proceedings of the 26th Annual International Conference of the IEEE EMBS, San Francisco, CA, USA, 1–5 September 2004; pp. 593–595. [Google Scholar]
- Cui, G.; Yin, Y.; Zhao, Q.; Cichocki, A.; Cao, J. Patients’ consciousness analysis using Dynamic Approximate Entropy and MEMD method. In Proceedings of the 2013 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference, Kaohsiung, Taiwan, 29 October–1 November 2013; pp. 1–5. [Google Scholar]
- Kotchoubey, B.; Daltrozzo, J.; Wioland, N.; Mutschler, V.; Lutun, P.; Birbaumer, N.; Jaeger, A. Semantic processing in a coma patient. Grand Rounds 2005, 5, 37–41. [Google Scholar]
- Armanfard, N.; Komeili, M.; Reilly, J.P.; Connolly, J.F. A Machine Learning Framework for Automatic and Continuous MMN Detection With Preliminary Results for Coma Outcome Prediction. IEEE J. Biomed. Health Inform. 2019, 23, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Prichep, L.S.; Jacquin, A.; Filipenko, J.; Dastidar, S.G.; Zabele, S.; Vodencarevic, A.; Rothman, N.S. Classification of Traumatic Brain Injury Severity Using Informed Data Reduction in a Series of Binary Classifier Algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, G.; Latifoğlu, F.; Akın, A.K.; Çetin, B. A novel approach for detection of consciousness level in comatose patients from EEG signals with 1-D convolutional neural network. Biocybern. Biomed. Eng. 2022, 42, 16–26. [Google Scholar] [CrossRef]
- Altıntop, Ç.G. Determination of Consciousness Levels by Analysis and Classification of Physiological Signals of Deep Coma Patients in Intensive Care Units. Ph.D. Thesis, Erciyes University Turkey, Kayseri, Turkey, 2021. [Google Scholar]
- Altıntop, Ç.G.; Latifoğlu, F.; Akın, A.K.; Bayram, A.; Çiftçi, M. Classification of Depth of Coma Using Complexity Measures and Nonlinear Features of Electroencephalogram Signals. Int. J. Neural Syst. 2022, 52, 2250018. [Google Scholar] [CrossRef]




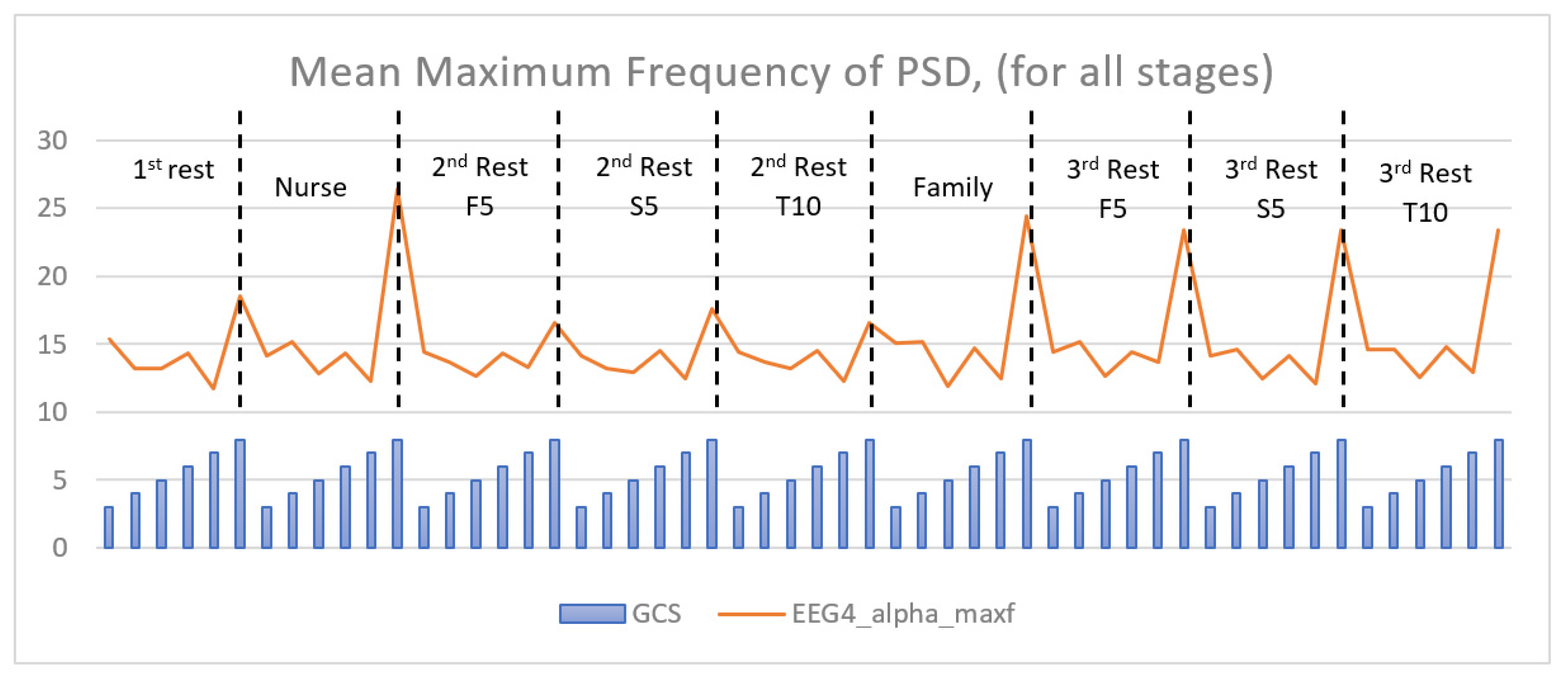
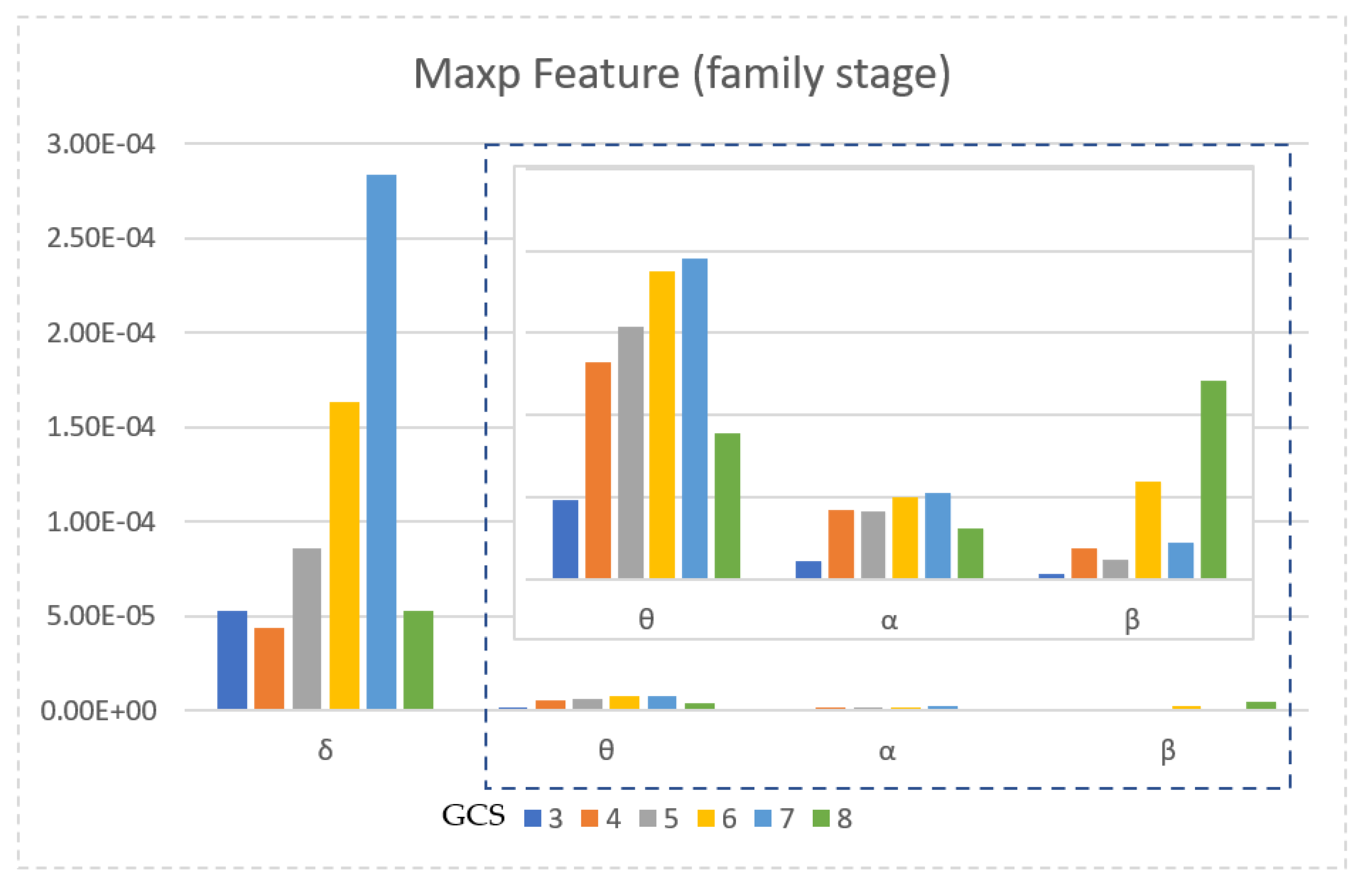
| Glasgow Coma Scale | Number of Patients | Gender | Age | Time Since Onset (Days) | |
|---|---|---|---|---|---|
| Female | Male | Mean + SD | Mean + SD | ||
| 3 | 8 | 4 | 4 | 71 ± 14 | 7 ± 4 |
| 4 | 4 | 2 | 2 | 76 ± 7 | 25 ± 18 |
| 5 | 9 | 6 | 3 | 74 ± 12 | 24 ± 19 |
| 6 | 9 | 5 | 4 | 64 ± 19 | 116 ± 140 |
| 7 | 7 | 3 | 4 | 62 ± 25 | 74 ± 108 |
| 8 | 2 | - | 2 | 34 ± 21 | 27 ± 21 |
| Total | 39 | 20 | 19 | 67 ± 19 | 51 ± 19 |
| Feature | Explanation |
|---|---|
| Energy | The signal’s energy value; see Equation (3). |
| Maxf | Frequency value corresponding to the peak power in the PSD curve; see Figure 4. |
| Maxp | PSD’s maximum power value |
| AUC1 | A1 area under the PSD curve, up to the peak power of PSD (as seen in Figure 4). |
| AUC2 | A2 area of the curve after the PSD’s peak power (as seen in Figure 4). |
| Rate1 | A1/(A1 + A2) |
| Rate2 | A1/A2 |
| Rate3 | A2/(A1 + A2) |
| NPower | The entire power value, normalized Pf; see Equation (4). |
| TPower | Total power; see Equation (5). |
| Predicted Class | |||||||
|---|---|---|---|---|---|---|---|
| GCS3 | GCS4 | GCS5 | GCS6 | GCS7 | GCS8 | ||
| Actual Class | GCS3 | TP (True Positive) | FN (False Negatives) | FN | FN | FN | FN |
| GCS4 | FP (False Positive) | TN (True Negatives) | TN | TN | TN | TN | |
| GCS5 | FP | TN | TN | TN | TN | TN | |
| GCS6 | FP | TN | TN | TN | TN | TN | |
| GCS7 | FP | TN | TN | TN | TN | TN | |
| GCS8 | FP | TN | TN | TN | TN | TN | |
| Average Energy of Signal ± Standard Error of Mean | |||||||
|---|---|---|---|---|---|---|---|
| Channel | GCS3 | GCS4 | GCS5 | GCS6 | GCS7 | GCS8 | |
| Alpha | F3-F4 | 0.2599 ± 0.0673 | 1.3997 ± 0.1812 | 1.2945 ± 0.1649 | 1.2653 ± 0.1623 | 1.5436 ± 0.4026 | 0.9883 ± 0.2727 |
| C3-C4 | 0.3754 ± 0.0497 | 2.3023 ± 0.3188 | 3.0098 ± 0.3541 | 2.0656 ± 0.2398 | 1.9960 ± 0.2122 | 0.5827 ± 0.1410 | |
| T3-T4 | 0.4883 ± 0.0598 | 4.4359 ± 1.2724 | 2.1588 ± 0.3276 | 3.4919 ± 0.5847 | 2.5317 ± 0.4696 | 0.4524 ± 0.1500 | |
| P3-P4 | 0.6420 ± 0.0829 | 4.5651 ± 1.0438 | 1.9746 ± 0.3957 | 1.6648 ± 0.1857 | 1.7292 ± 0.2369 | 0.7800 ± 0.2712 | |
| Beta | F3-F4 | 0.1297 ± 0.0318 | 0.6304 ± 0.0728 | 0.7827 ± 0.959 | 0.8539 ± 0.1751 | 0.9186 ± 0.2275 | 1.5938 ± 0.4470 |
| C3-C4 | 0.1760 ± 0.0213 | 1.4150 ± 0.2359 | 1.9921 ± 0.2959 | 1.3428 ± 0.2029 | 1.2878 ± 0.1959 | 2.2744 ± 0.8734 | |
| T3-T4 | 0.3047 ± 0.0363 | 4.5623 ± 1.3450 | 1.7158 ± 0.2593 | 2.5749 ± 0.4866 | 1.7147 ± 0.3258 | 0.4410 ± 0.1489 | |
| P3-P4 | 0.3432 ± 0.0446 | 4.0443 ± 1.0795 | 1.1305 ± 0.2628 | 0.9953 ± 0.1606 | 0.9440 ± 0.1509 | 1.4213 ± 0.3480 | |
| Theta | F3-F4 | 0.8103 ± 0.2217 | 4.1358 ± 0.5382 | 3.6714 ± 0.5268 | 3.8572 ± 0.4296 | 3.9233 ± 0.8821 | 2.1754 ± 0.7269 |
| C3-C4 | 1.2475 ± 0.2041 | 5.4085 ± 0.7598 | 7.5364 ± 0.8815 | 5.6074 ± 0.5775 | 5.5979 ± 0.6504 | 0.7547 ± 0.2462 | |
| T3-T4 | 1.2003 ± 0.1863 | 7.2399 ± 2.0547 | 5.7090 ± 0.8948 | 9.7930 ± 1.4021 | 7.2743 ± 1.4515 | 1.1654 ± 0.3935 | |
| P3-P4 | 1.5104 ± 0.1897 | 8.5885 ± 1.8550 | 5.4061 ± 1.0263 | 4.4837 ± 0.4578 | 5.0909 ± 0.6817 | 1.4382 ± 0.5709 | |
| Delta | F3-F4 | 11.0369 ± 3.0512 | 25.2814 ± 2.0644 | 26.6152 ± 3.9481 | 36.7705 ± 4.5119 | 19.4978 ± 2.6905 | 18.8845 ± 7.1786 |
| C3-C4 | 19.3139 ± 3.9593 | 28.9473 ± 2.8899 | 41.5905 ± 4.4525 | 43.1349 ± 6.9944 | 64.8723 ± 13.2537 | 7.2981 ± 2.5011 | |
| T3-T4 | 11.1114 ± 1.6397 | 22.0236 ± 5.8738 | 50.7514 ± 6.1570 | 110.7027 ± 15.9699 | 116.4803 ± 32.1824 | 12.5969 ± 4.1904 | |
| P3-P4 | 13.0764 ± 2.8072 | 28.3919 ± 5.6813 | 28.6629 ± 5.2250 | 27.5718 ± 3.0316 | 51.9683 ± 9.7944 | 12.6481 ± 4.1413 | |
| Channel 1 (F3-F4) | Channel 2 (C3-C4) | Channel 3 (T3-T4) | Channel 4 (P3-P4) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feature/ EEG Band | Alpha | Beta | Theta | Delta | Alpha | Beta | Theta | Delta | Alpha | Beta | Theta | Delta | Alpha | Beta | Theta | Delta |
| Energy | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Maxf | 0.000 | 0.000 | 0.002 | 0.056 | 0.083 | 0.000 | 0.054 | 0.163 | 0.000 | 0.000 | 0.001 | 0.009 | 0.000 | 0.000 | 0.000 | 0.055 |
| Maxp | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| AUC1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| AUC2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Rate1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Rate2 | 0.021 | 0.000 | 0.000 | 0.337 | 0.005 | 0.000 | 0.004 | 0.057 | 0.000 | 0.000 | 0.163 | 0.002 | 0.003 | 0.000 | 0.000 | 0.176 |
| Rate3 | 0.021 | 0.000 | 0.000 | 0.336 | 0.005 | 0.000 | 0.004 | 0.057 | 0.000 | 0.000 | 0.163 | 0.002 | 0.003 | 0.000 | 0.000 | 0.176 |
| NTPower | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| TPower | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| GCS Score | Instance Number | Increase Amount (%) | Instance Number Using SMOTE |
|---|---|---|---|
| 3 | 74 | %8 | 79 |
| 4 | 36 | %120 | 79 |
| 5 | 79 | - | 79 |
| 6 | 81 | - | 81 |
| 7 | 57 | %40 | 81 |
| 8 | 18 | %340 | 79 |
| Total | 345 | %38.55 | 478 |
| Imbalance Method | Classification Method | Sensitivity | Specificity | Precision | F-Score | G-Mean | Overall Accuracy |
|---|---|---|---|---|---|---|---|
| No | Random Forest | 0.9343 | 0.9881 | 0.9605 | 0.9458 | 0.9604 | 0.9449 |
| K-NN | 0.9049 | 0.9846 | 0.9439 | 0.9204 | 0.9426 | 0.9275 | |
| Ensemble Bagged Trees | 0.9137 | 0.9840 | 0.9397 | 0.9252 | 0.9478 | 0.9246 | |
| SVM-Cubic | 0.8664 | 0.9769 | 0.8972 | 0.8781 | 0.9183 | 0.8899 | |
| SMOTE | Random Forest | 0.9644 | 0.9929 | 0.9653 | 0.9646 | 0.9785 | 0.9644 |
| K-NN | 0.9625 | 0.9925 | 0.9624 | 0.9624 | 0.9773 | 0.9623 | |
| Ensemble Bagged Trees | 0.9561 | 0.9912 | 0.9563 | 0.9560 | 0.9734 | 0.9561 | |
| SVM-Cubic | 0.9293 | 0.9858 | 0.9289 | 0.9290 | 0.9568 | 0.9289 |
| Data Balance | Algorithm | Alpha | Beta | Theta | Delta | All Band |
|---|---|---|---|---|---|---|
| Imbalance | RF | 0.8870 | 0.8928 | 0.9159 | 0.8812 | 0.9449 |
| Balance with SMOTE | 0.9121 | 0.9121 | 0.9310 | 0.9247 | 0.9644 |
| Data | Subjects | Recording Time/Scenario | Methods and Features | Statistical Analysis | Classification | Study |
|---|---|---|---|---|---|---|
| EEG (19 channels) | 20 coma patients | Not specified | Power spectral analysis and nonlinear analysis (Lempel-Ziv Complexity and entropy values) | Correlation p < 0.005 | - | [21] |
| EEG (6 channels) | 17 coma patients, 17 quasi-brain death patients | Not specified | Phase value with Shannon’s entopy | Independent sample t-test p < 0.0033 | - | [25] |
| EEG (19 channels) | 64 healthy subjects, 36 coma patients | Not specified | Lempel-Ziv complexity, approximate entropy, spectral entropy | p < 0.05 for two groups | - | [92] |
| EEG (6 channels) | 2 coma patients | Recording at rest, (1535 s and 1030 s record) | Multivariate Empirical Mode Decomposition and Approximate Entropy | - | - | [93] |
| EEG (evoked potentials, ERP) | 1 coma patient | Two experimental paradigms, word pairs and sentences | ANOVA, t-test, PCA-based t2 test, Wavelet | p ≤ 0.01 | - | [94] |
| EEG (32 channels) | 22 healthy subjects and 2 coma patients | Auditory odd-ball paradigm | Wavelet transform skewness, kurtosis, variance, maximum, minimum, and power values | - | Machine Learning Localized Feature Selection Method (%92.7 accuracy for healthy subjects) | [95] |
| EEG (5 channels) BT images | 633 patients (GCS above 8) | 10 min eyes closed resting | Fast Fourier transform, fractal analysis | - | Genetic Algorithm, Binary Classifier (96% sensitivity and 78% specificity for the structurally damaged group) | [96] |
| EEG (4 channels) | 39 coma patiens (GCS ≤ 8) | 35 min/tactile and auditory stimuli | Power spectral analysis | Kruskal–Wallis p < 0.000 in most features | Random Forest, SVM, Ensemble Bagged Trees, K-NN 96.44% accuracy | Proposed study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altıntop, Ç.G.; Latifoğlu, F.; Akın, A.K.; Ülgey, A. Quantitative Electroencephalography Analysis for Improved Assessment of Consciousness Levels in Deep Coma Patients Using a Proposed Stimulus Stage. Diagnostics 2023, 13, 1383. https://doi.org/10.3390/diagnostics13081383
Altıntop ÇG, Latifoğlu F, Akın AK, Ülgey A. Quantitative Electroencephalography Analysis for Improved Assessment of Consciousness Levels in Deep Coma Patients Using a Proposed Stimulus Stage. Diagnostics. 2023; 13(8):1383. https://doi.org/10.3390/diagnostics13081383
Chicago/Turabian StyleAltıntop, Çiğdem Gülüzar, Fatma Latifoğlu, Aynur Karayol Akın, and Ayşe Ülgey. 2023. "Quantitative Electroencephalography Analysis for Improved Assessment of Consciousness Levels in Deep Coma Patients Using a Proposed Stimulus Stage" Diagnostics 13, no. 8: 1383. https://doi.org/10.3390/diagnostics13081383
APA StyleAltıntop, Ç. G., Latifoğlu, F., Akın, A. K., & Ülgey, A. (2023). Quantitative Electroencephalography Analysis for Improved Assessment of Consciousness Levels in Deep Coma Patients Using a Proposed Stimulus Stage. Diagnostics, 13(8), 1383. https://doi.org/10.3390/diagnostics13081383