Fabrication of a Low-Cost Microfluidic Device for High-Throughput Drug Testing on Static and Dynamic Cancer Spheroid Culture Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
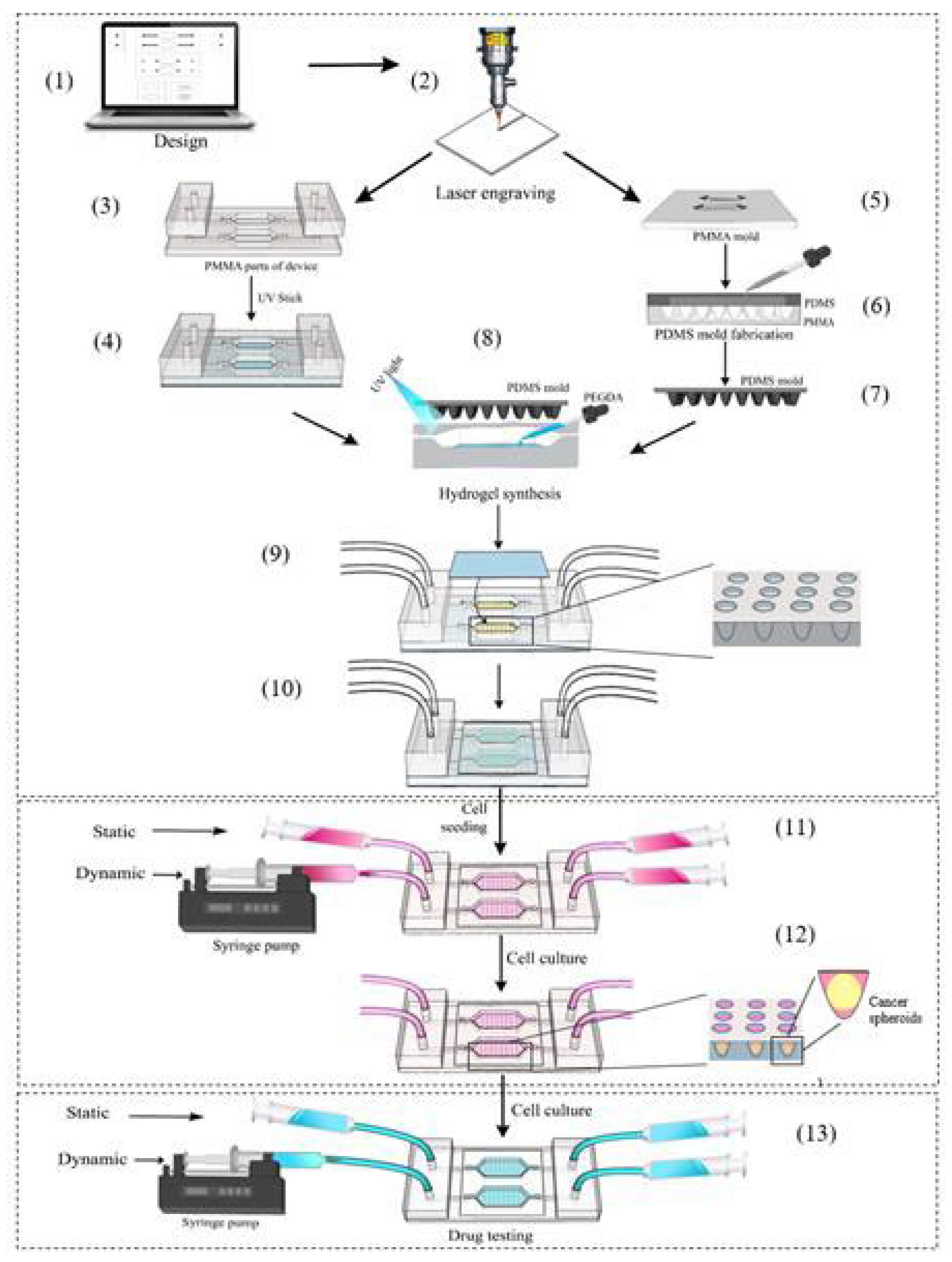
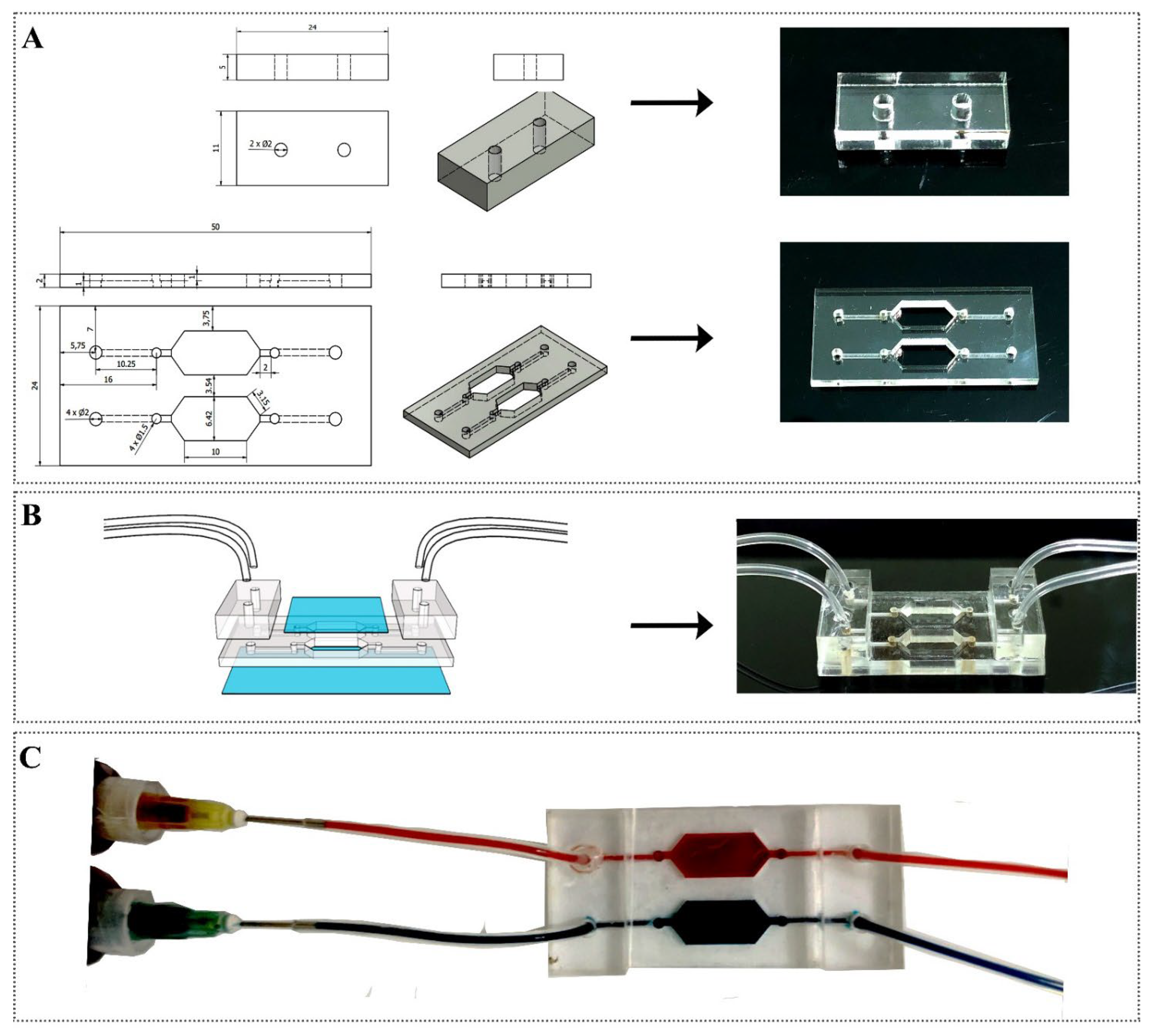
2.2.1. Fabrication of the Device
2.2.2. Leaking Test
2.2.3. Cell Seeding and Cancer Spheroid Formation
2.2.4. Drug Preparation and Testing
2.2.5. Cell Viability
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of Microfluidic Device
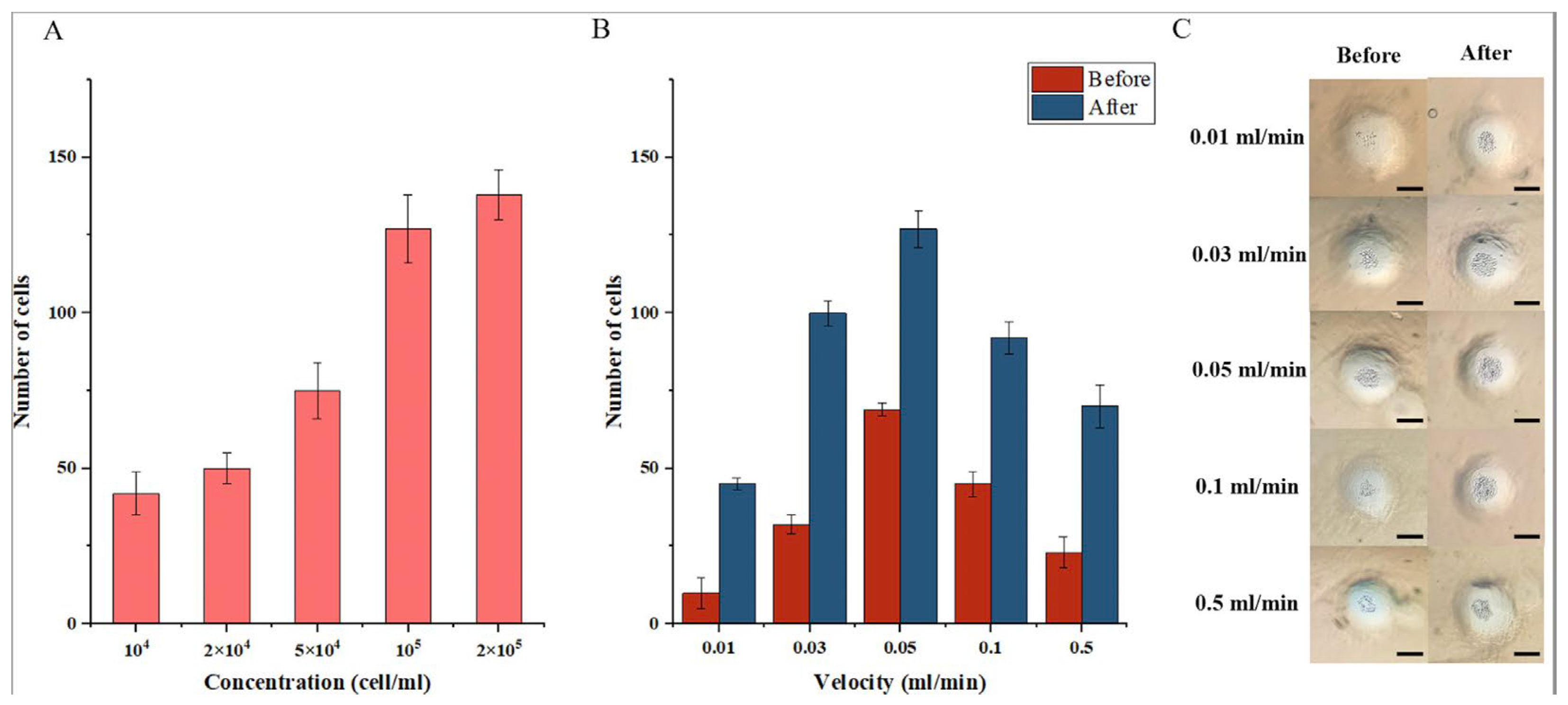
3.2. The Influence of Velocity on Cell Growth
3.3. The Influence of Velocity with Drug Treatment on Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlander, M.; Hernandez-Villafuerte, K.; Cheng, C.-Y.; Mestre-Ferrandiz, J.; Baumann, M. How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. PharmacoEconomics 2021, 39, 1243–1269. [Google Scholar] [CrossRef]
- Yildirim, O.; Gottwald, M.; Schüler, P.; Michel, M.C. Opportunities and Challenges for Drug Development: Public–Private Partnerships, Adaptive Designs and Big Data. Front. Pharmacol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Scannell, J.W.; Bosley, J.; Hickman, J.A.; Dawson, G.R.; Truebel, H.; Ferreira, G.S.; Richards, D.; Treherne, J.M. Predictive Validity in Drug Discovery: What It Is, Why It Matters and How to Improve It. Nat. Rev. Drug Discov. 2022, 21, 915–931. [Google Scholar] [CrossRef]
- Van, N.G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials. JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Franzen, N.; van Harten, W.H.; Retèl, V.P.; Loskill, P.; van den Eijnden-van Raaij, J.; IJzerman, M. Impact of Organ-on-a-Chip Technology on Pharmaceutical R&D Costs. Drug Discov. Today 2019, 24, 1720–1724. [Google Scholar] [CrossRef]
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an in Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D Cell Culture Models for Cell Growth, Gene Expression and Drug Resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, K.; Hsu, H.-Y. Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction. Bioengineering 2018, 5, 57. [Google Scholar] [CrossRef]
- Volkmer, E.; Drosse, I.; Otto, S.; Stangelmayer, A.; Stengele, M.; Kallukalam, B.C.; Mutschler, W.; Schieker, M. Hypoxia in Static and Dynamic 3D Culture Systems for Tissue Engineering of Bone. Tissue Eng. Part A 2008, 14, 1331–1340. [Google Scholar] [CrossRef]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-Chip and Body-on-a-Chip Systems for Drug Screening and Disease Modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Lee, J.; Choi, S.; Yang, J.H.; Sander, M.; Chung, S.; Lee, S.-H. In Vivo–Mimicking Microfluidic Perfusion Culture of Pancreatic Islet Spheroids. Sci. Adv. 2019, 5, eaax4520. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Makino, K.; Tabata, Y. Influence of Shaking Culture on the Biological Functions of Cell Aggregates Incorporating Gelatin Hydrogel Microspheres. J. Biosci. Bioeng. 2019, 128, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Niibe, K.; Ohori-Morita, Y.; Zhang, M.; Mabuchi, Y.; Matsuzaki, Y.; Egusa, H. A Shaking-Culture Method for Generating Bone Marrow Derived Mesenchymal Stromal/Stem Cell-Spheroids With Enhanced Multipotency in Vitro. Front. Bioeng. Biotechnol. 2020, 8, 590332. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Kotz, F.; Mader, M.; Dellen, N.; Risch, P.; Kick, A.; Helmer, D.; Rapp, B.E. Fused Deposition Modeling of Microfluidic Chips in Polymethylmethacrylate. Micromachines 2020, 11, 873. [Google Scholar] [CrossRef]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng. 2021, 7, 2880–2899. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Chung, P.-C.; Owaga, E.E.; Tsai, I.-J.; Wang, P.-Y.; Tsai, J.-I.; Yeh, T.-S.; Hsieh, R.-H. Alpha-Mangostin from Mangosteen (Garcinia Mangostana Linn.) Pericarp Extract Reduces High Fat-Diet Induced Hepatic Steatosis in Rats by Regulating Mitochondria Function and Apoptosis. Nutr. Metab. 2016, 13, 88. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, W.; Ling, J.; Jiang, C. α-Mangostin Inhibits α-Synuclein-Induced Microglial Neuroinflammation and Neurotoxicity. Cell. Mol. Neurobiol. 2016, 36, 811–820. [Google Scholar] [CrossRef]
- Lei, J.; Huo, X.; Duan, W.; Xu, Q.; Li, R.; Ma, J.; Li, X.; Han, L.; Li, W.; Sun, H.; et al. α-Mangostin Inhibits Hypoxia-Driven ROS-Induced PSC Activation and Pancreatic Cancer Cell Invasion. Cancer Lett. 2014, 347, 129–138. [Google Scholar] [CrossRef]
- Benjakul, R.; Kongkaneramit, L.; Sarisuta, N.; Moongkarndi, P.; Müller-Goymann, C.C. Cytotoxic Effect and Mechanism Inducing Cell Death of α-Mangostin Liposomes in Various Human Carcinoma and Normal Cells. Anti-Cancer Drugs 2015, 26, 824–834. [Google Scholar] [CrossRef]
- Zhang, K.; Gu, Q.; Yang, K.; Ming, X.; Wang, J. Anticarcinogenic Effects of α-Mangostin: A Review. Planta Med. 2017, 83, 188–202. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for Drug Delivery. J. Biotechnol. Biomater. 2017, 7. [Google Scholar] [CrossRef]
- Liposomes as Nanomedical Devices—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4324542/ (accessed on 15 December 2022).
- Cai, Y.; Xu, Y.; Chan, H.F.; Fang, X.; He, C.; Chen, M. Glycyrrhetinic Acid Mediated Drug Delivery Carriers for Hepatocellular Carcinoma Therapy. Mol. Pharm. 2016, 13, 699–709. [Google Scholar] [CrossRef]
- Bithi, S.S.; Vanapalli, S.A. Microfluidic Cell Isolation Technology for Drug Testing of Single Tumor Cells and Their Clusters. Sci. Rep. 2017, 7, 41707. [Google Scholar] [CrossRef]
- Pham, D.T.; Nguyen, L.P.; Pham, Q.T.H.; Pham, C.K.; Pham, D.T.N.; Viet, N.T.; Nguyen, H.V.T.; Tran, T.Q.; Nguyen, D.T. A Low-Cost, Flexible Extruder for Liposomes Synthesis and Application for Murrayafoline A Delivery for Cancer Treatment. J. Biomater. Appl. 2022, 37, 872–880. [Google Scholar] [CrossRef]
- Thomsen, A.R.; Aldrian, C.; Bronsert, P.; Thomann, Y.; Nanko, N.; Melin, N.; Rücker, G.; Follo, M.; Grosu, A.L.; Niedermann, G.; et al. A Deep Conical Agarose Microwell Array for Adhesion Independent Three-Dimensional Cell Culture and Dynamic Volume Measurement. Lab Chip 2018, 18, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-D.; Wang, Y.-T.; Wang, J.-R.; Wu, J.-L.; Meng, X.-S.; Hu, P.; Mu, X.; Liang, Q.-L.; Luo, G.-A. Design and Fabrication of a Liver-on-a-Chip Platform for Convenient, Highly Efficient, and Safe in Situ Perfusion Culture of 3D Hepatic Spheroids. Lab Chip 2018, 18, 2547–2562. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, B.; Qin, J. Carcinoma-Associated Fibroblasts Promoted Tumor Spheroid Invasion on a Microfluidic 3D Co-Culture Device. Lab Chip 2010, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, Z.; Feng, Z.; Li, H.; Zhang, M.; Zhang, H.; Cui, X. Fabrication of Concave Microwells and Their Applications in Micro-Tissue Engineering: A Review. Micromachines 2022, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated Microfluidic Platform for Dynamic and Combinatorial Drug Screening of Tumor Organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef]
- Kayaku Advanced Materials Inc. SU-8 3025 500ML. Available online: https://www.fishersci.com/shop/products/NC0057282/NC0057282 (accessed on 15 December 2022).
- Caprio, N.D.; Burdick, J.A. Engineered Biomaterials to Guide Spheroid Formation, Function, and Fabrication into 3D Tissue Constructs. Acta Biomater. 2022, S1742706122006201. [Google Scholar] [CrossRef]
- Khot, M.I.; Levenstein, M.A.; de Boer, G.N.; Armstrong, G.; Maisey, T.; Svavarsdottir, H.S.; Andrew, H.; Perry, S.L.; Kapur, N.; Jayne, D.G. Characterising a PDMS Based 3D Cell Culturing Microfluidic Platform for Screening Chemotherapeutic Drug Cytotoxic Activity. Sci. Rep. 2020, 10, 15915. [Google Scholar] [CrossRef]
- Patra, B.; Peng, C.-C.; Liao, W.-H.; Lee, C.-H.; Tung, Y.-C. Drug Testing and Flow Cytometry Analysis on a Large Number of Uniform Sized Tumor Spheroids Using a Microfluidic Device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef]
- Sabhachandani, P.; Motwani, V.; Cohen, N.; Sarkar, S.; Torchilin, V.; Konry, T. Generation and Functional Assessment of 3D Multicellular Spheroids in Droplet Based Microfluidics Platform. Lab Chip 2016, 16, 497–505. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Lu, B.; Yan, M.; Han, K.; Wang, J. A Microfluidic Platform for Multi-Size 3D Tumor Culture, Monitoring and Drug Resistance Testing. Sens. Actuators B Chem. 2019, 292, 111–120. [Google Scholar] [CrossRef]
- Clancy, A.; Chen, D.; Bruns, J.; Nadella, J.; Stealey, S.; Zhang, Y.; Timperman, A.; Zustiak, S.P. Hydrogel-Based Microfluidic Device with Multiplexed 3D in Vitro Cell Culture. Sci. Rep. 2022, 12, 17781. [Google Scholar] [CrossRef]
- Fridman, I.B.; Ugolini, G.S.; VanDelinder, V.; Cohen, S.; Konry, T. High Throughput Microfluidic System with Multiple Oxygen Levels for the Study of Hypoxia in Tumor Spheroids. Biofabrication 2021, 13, 035037. [Google Scholar] [CrossRef]
- Belgorosky, D.; Fernández-Cabada, T.; Peñaherrera-Pazmiño, A.B.; Langle, Y.; Booth, R.; Bhansali, S.; Pérez, M.S.; Eiján, A.M.; Lerner, B. Analysis of Tumoral Spheres Growing in a Multichamber Microfluidic Device. J. Cell. Physiol. 2018, 233, 6327–6336. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.K.; Jeong, G.S.; Hyun, J.K.; Lee, C.J.; Lee, S.H. Three-Dimensional Brain-on-a-Chip with an Interstitial Level of Flow and Its Application as an in Vitro Model of Alzheimer’s Disease. Lab A Chip 2015, 15, 141–150. [Google Scholar] [CrossRef]


| Product Name | Price (USD) | Number of Products | Amount (USD) | |
|---|---|---|---|---|
| 1 | PMMA sheet | 0.35 | 3 | 1.05 |
| 2 | Medical tube | 0.1 | 4 | 0.40 |
| 3 | UV Glue 5 mL | 0.5 | 1 | 0.50 |
| 4 | Glass | 0.05 | 2 | 0.10 |
| 5 | Syringe 5 mL | 0.2 | 4 | 0.80 |
| Total | USD 2.85 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.D.; Pham, U.T.; Nguyen, L.P.; Nguyen, T.M.; Bui, C.N.; Oliver, S.; Pham, P.; Tran, T.Q.; Hoang, B.T.; Pham, M.T.H.; et al. Fabrication of a Low-Cost Microfluidic Device for High-Throughput Drug Testing on Static and Dynamic Cancer Spheroid Culture Models. Diagnostics 2023, 13, 1394. https://doi.org/10.3390/diagnostics13081394
Do TD, Pham UT, Nguyen LP, Nguyen TM, Bui CN, Oliver S, Pham P, Tran TQ, Hoang BT, Pham MTH, et al. Fabrication of a Low-Cost Microfluidic Device for High-Throughput Drug Testing on Static and Dynamic Cancer Spheroid Culture Models. Diagnostics. 2023; 13(8):1394. https://doi.org/10.3390/diagnostics13081394
Chicago/Turabian StyleDo, Tung Dinh, Uyen Thu Pham, Linh Phuong Nguyen, Trang Minh Nguyen, Cuong Nguyen Bui, Susan Oliver, Phuong Pham, Toan Quoc Tran, Bich Thi Hoang, Minh Thi Hong Pham, and et al. 2023. "Fabrication of a Low-Cost Microfluidic Device for High-Throughput Drug Testing on Static and Dynamic Cancer Spheroid Culture Models" Diagnostics 13, no. 8: 1394. https://doi.org/10.3390/diagnostics13081394
APA StyleDo, T. D., Pham, U. T., Nguyen, L. P., Nguyen, T. M., Bui, C. N., Oliver, S., Pham, P., Tran, T. Q., Hoang, B. T., Pham, M. T. H., Pham, D. T. N., & Nguyen, D. T. (2023). Fabrication of a Low-Cost Microfluidic Device for High-Throughput Drug Testing on Static and Dynamic Cancer Spheroid Culture Models. Diagnostics, 13(8), 1394. https://doi.org/10.3390/diagnostics13081394





