Prognostic Value for Perioperative Serum Total Cholesterol Level on Postoperative Long-Term Prognosis of Pancreatic Cancer: A Retrospective Clinical Study
Abstract
:1. Introduction
2. Methods
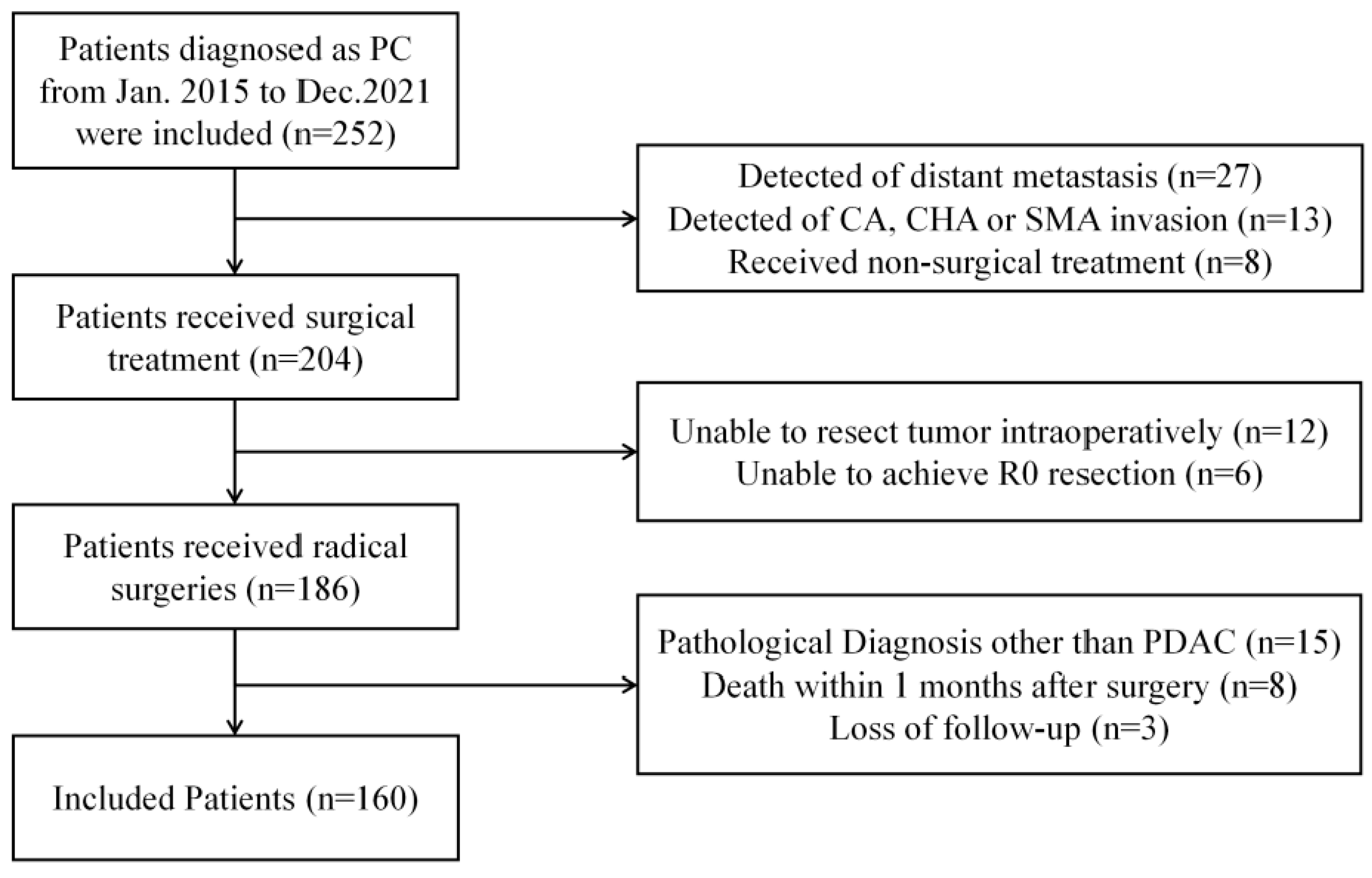
2.1. Patient Screening
2.2. Grouping
2.3. Data Analysis and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
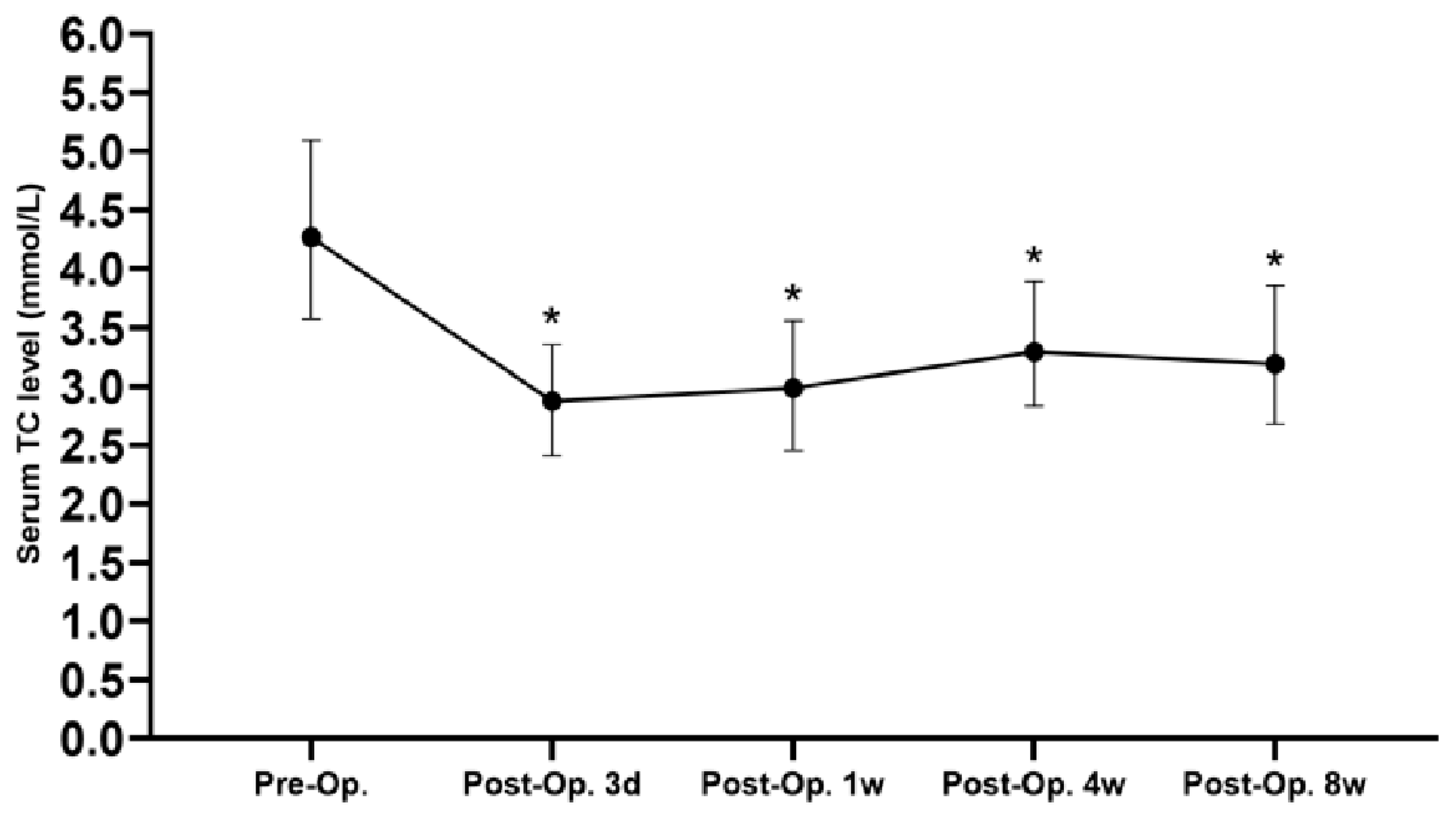
3.2. Perioperative Condition
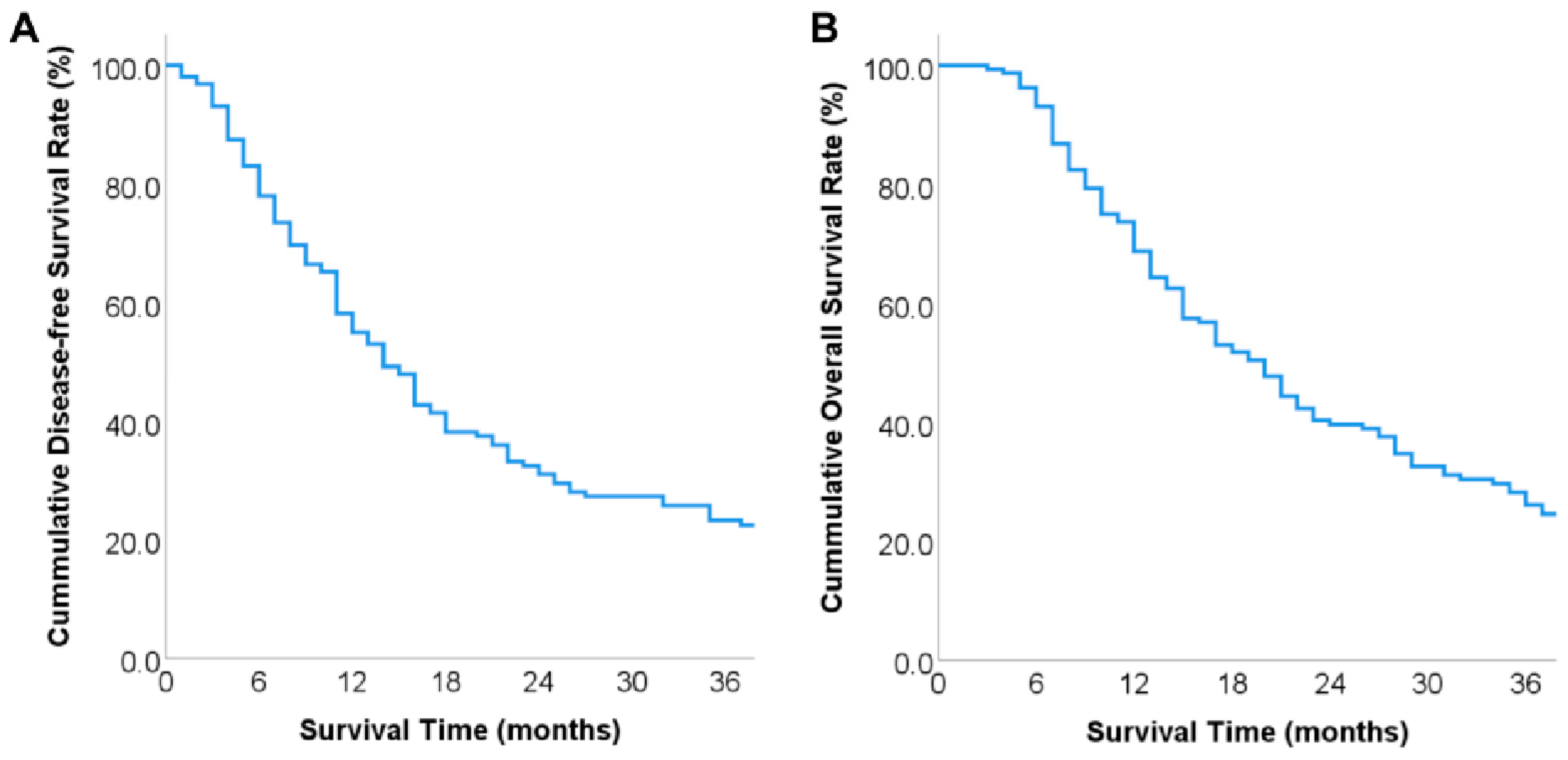
3.3. Overall Prognosis
3.4. Grouping Situation
3.5. Comparison of Perioperative and Long-Term Prognostic Data of Total Patients Grouped by Postoperative Serum TC Level
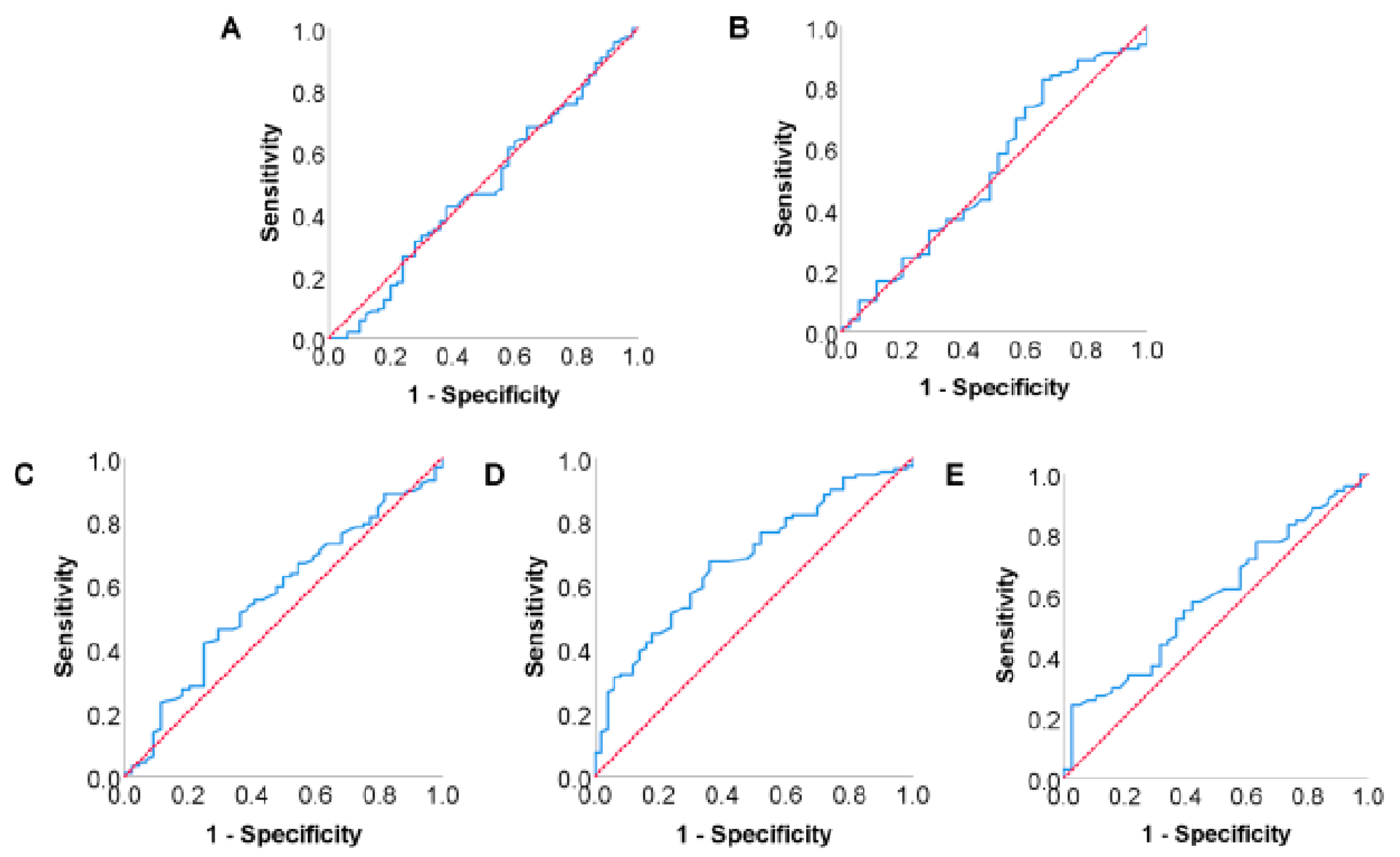
3.6. Analysis of Risk Factors for Postoperative Tumor Recurrence in Pancreatic Cancer Patients
3.7. Analysis of Risk Factors for Postoperative Long-Term Survival in Pancreatic
Cancer Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jager, D.; Buchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Donahue, T.R.; Reber, H.A. Surgical management of pancreatic cancer--pancreaticoduodenectomy. Semin. Oncol. 2015, 42, 98–109. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Cortes, V.A.; Busso, D.; Maiz, A.; Arteaga, A.; Nervi, F.; Rigotti, A. Physiological and pathological implications of cholesterol. Front. Biosci. 2014, 19, 416–428. [Google Scholar] [CrossRef]
- Chiarla, C.; Giovannini, I.; Giuliante, F.; Zadak, Z.; Vellone, M.; Ardito, F.; Clemente, G.; Murazio, M.; Nuzzo, G. Severe hypocholesterolemia in surgical patients, sepsis, and critical illness. J. Crit. Care 2010, 25, 361.e7–361.e12. [Google Scholar] [CrossRef]
- Shin, H.J.; Roh, C.K.; Son, S.Y.; Hoon, H.; Han, S.U. Prognostic value of hypocholesterolemia in patients with gastric cancer. Asian J. Surg. 2021, 44, 72–79. [Google Scholar] [CrossRef]
- Jung, S.M.; Kang, D.; Guallar, E.; Yu, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Cho, J.; Lee, S.K. Impact of Serum Lipid on Breast Cancer Recurrence. J. Clin. Med. 2020, 9, 2846. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, E.T.; Forsberg, C.G.; Chavin, K.D.; Baliga, P.K.; Taber, D.J. Incidence and risk factors of hepatocellular carcinoma after orthotopic liver transplantation. Surgery 2017, 161, 830–836. [Google Scholar] [CrossRef]
- Zhou, P.; Li, B.; Liu, B.; Chen, T.; Xiao, J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin. Chim. Acta 2018, 477, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Xu, R.; Song, J.; Ruze, R.; Chen, Y.; Wang, C.; Xu, Q. Lipid metabolism in pancreatic cancer: Emerging roles and potential targets. Cancer Commun. 2022, 42, 1234–1256. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Boursi, B.; Mamtani, R.; Yang, Y.X. Total Serum Cholesterol and Pancreatic Cancer: A Nested Case-Control Study. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 363–369. [Google Scholar] [CrossRef]
- Wang, Q.L.; Khil, J.; Hong, S.; Lee, D.H.; Ha, K.H.; Keum, N.; Kim, H.C.; Giovannucci, E.L. Temporal Association of Total Serum Cholesterol and Pancreatic Cancer Incidence. Nutrients 2022, 14, 4938. [Google Scholar] [CrossRef]
- Cerqueira, N.M.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.J.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Muka, T.; Kraja, B.; Ruiter, R.; de Keyser, C.E.; Hofman, A.; Stricker, B.H.; Kiefte-de, J.J.; Franco, O.H. Dietary polyunsaturated fatty acids intake modifies the positive association between serum total cholesterol and colorectal cancer risk: The Rotterdam Study. J. Epidemiol. Community Health 2016, 70, 881–887. [Google Scholar] [CrossRef]
- Lee, Y.L.; Li, W.C.; Tsai, T.H.; Chiang, H.Y.; Ting, C.T. Body mass index and cholesterol level predict surgical outcome in patients with hepatocellular carcinoma in Taiwan—A cohort study. Oncotarget 2016, 7, 22948–22959. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Seo, S.P.; Kim, W.T.; Yun, S.J.; Lee, S.C.; Kim, W.J.; Hwang, E.C.; Kang, S.H.; Hong, S.H.; Chung, J.; et al. Low preoperative serum cholesterol level is associated with aggressive pathologic features and poor cancer-specific survival in patients with surgically treated renal cell carcinoma. Int. J. Clin. Oncol. 2018, 23, 142–150. [Google Scholar] [CrossRef]
- Guillaumond, F.; Bidaut, G.; Ouaissi, M.; Servais, S.; Gouirand, V.; Olivares, O.; Lac, S.; Borge, L.; Roques, J.; Gayet, O.; et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 2473–2478. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Ignacio, D.U.J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran, C.H.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef]
- Terasaki, F.; Sugiura, T.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Uesaka, K. The preoperative controlling nutritional status (CONUT) score is an independent prognostic marker for pancreatic ductal adenocarcinoma. Updates Surg. 2021, 73, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Nuno-Lambarri, N.; Barbero-Becerra, V.J.; Uribe, M.; Chavez-Tapia, N.C. Elevated cholesterol levels have a poor prognosis in a cholestasis scenario. J. Biochem. Mol. Toxicol. 2017, 31, 1–6. [Google Scholar] [CrossRef]
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Marsland, A.; Flory, J.D.; Rabin, B.S.; Whiteside, T.L.; Manuck, S.B. Immune system differences in men with hypo- or hypercholesterolemia. Clin. Immunol. Immunopathol. 1997, 84, 145–149. [Google Scholar] [CrossRef]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, I.; Boldrini, G.; Chiarla, C.; Giuliante, F.; Vellone, M.; Nuzzo, G. Pathophysiologic correlates of hypocholesterolemia in critically ill surgical patients. Intensive Care Med. 1999, 25, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Akgun, S.; Ertel, N.H.; Mosenthal, A.; Oser, W. Postsurgical reduction of serum lipoproteins: Interleukin-6 and the acute-phase response. J. Lab. Clin. Med. 1998, 131, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, N.; Uslar, V.N.; Obonyo, D.; Weyhe, D. Micronutritional status after pylorus preserving duodenopancreatectomy: Analysis of data from a randomized controlled trial. Sci. Rep. 2021, 11, 18475. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Oberlander, D.; Huang, J.; Weissman, C. Fluid resuscitation, nutritional support, and cholesterol in critically ill postsurgical patients. J. Clin. Anesth. 1998, 10, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, S.; Yan, X.; Fu, X.; Fan, Y.; Chen, D.; Qiu, Y.; Mao, L. Predictive Factors and Microbial Spectrum for Infectious Complications after Hepatectomy with Cholangiojejunostomy in Perihilar Cholangiocarcinoma. Surg. Infect. 2020, 21, 275–283. [Google Scholar] [CrossRef]
- Nuzzo, G.; Giovannini, I. Plasma cholesterol level after hepatopancreatobiliary surgery provides information on the postoperative clinical course. Updates Surg. 2010, 62, 131–133. [Google Scholar] [CrossRef]
- Giovannini, I.; Chiarla, C.; Greco, F.; Boldrini, G.; Nuzzo, G. Characterization of biochemical and clinical correlates of hypocholesterolemia after hepatectomy. Clin. Chem. 2003, 49, 317–319. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Shiozaki, H.; Shirai, Y.; Suka, M.; Hamura, R.; Horiuchi, T.; Yasuda, J.; Furukawa, K.; Onda, S.; Gocho, T.; Ikegami, T. Practical significance of pancreatectomy with lymphadenectomy around the superior mesenteric artery for pancreatic cancer: Comparison of prognosis after adjusting for major prognostic factors. Langenbecks Arch. Surg. 2021, 406, 703–711. [Google Scholar] [CrossRef]

| Characteristics | Overall (n = 160) |
|---|---|
| Gender, n (%) | |
| Male | 89 (55.6%) |
| Female | 71 (44.4%) |
| Age (years) | 63.0 ± 10.1 |
| Initial symptoms, n (%) | |
| Jaundice | 73 (45.6%) |
| Abdominal pain | 61 (38.1%) |
| Atypical digestive symptoms | 8 (5.0%) |
| No relevant symptoms | 18 (11.3%) |
| Diabetes, n (%) | 53 (33.1%) |
| Preoperative jaundice reduction treatment, n (%) | |
| PTBD | 32 (20.0%) |
| ERCP | 4 (2.5%) |
| Characteristics | Overall (n = 160) |
|---|---|
| Neoadjuvant chemotherapy, n (%) | 12 (7.5%) |
| Portal vein system invasion, n (%) | 70 (43.8%) |
| Intraoperative blood loss (mL) | 500 (400–800) |
| Operation time (h) | 10 (8–12) |
| Intraoperative blood transfusion, n (%) | 62 (38.8%) |
| Tumor differentiation, n (%) | |
| Poor differentiation | 47 (29.4%) |
| Moderate differentiation | 101 (63.1%) |
| High differentiation | 12 (7.5%) |
| Tumor size (cm) | 3.5 (2.5, 4.5) |
| Lymph node metastasis, n (%) | 100 (62.5%) |
| Postoperative complications, n (%) | 48 (30.0%) |
| Delayed gastric emptying, n (%) | 15 (9.4%) |
| Abdominal infection, n (%) | 12 (7.5%) |
| Pancreatic fistula, n (%) | |
| Biochemical fistula | 9 (5.6%) |
| Grade B fistula | 7 (4.4%) |
| Grade C fistula | 4 (2.5%) |
| Gastrointestinal bleeding, n (%) | 3 (1.9%) |
| Abdominal hemorrhage, n (%) | 2 (1.3%) |
| Pulmonary infection, n (%) | 2 (1.3%) |
| Biliary fistula, n (%) | 1 (0.6%) |
| Portal venous embolism, n (%) | 1 (0.6%) |
| Pulmonary embolism, n (%) | 1 (0.6%) |
| Variables | Low-TC Group (n = 68) | High-TC Group (n = 92) | p Value |
|---|---|---|---|
| Gender (Male/Female) | 39/29 | 50/42 | 0.705 |
| Age (years) | 62.3 ± 10.4 | 63.7 ± 10.0 | 0.399 |
| Diabetes (Yes/No) | 25/43 | 28/64 | 0.400 |
| Preoperative jaundice reduction treatment (Yes/No) | 16/52 | 20/72 | 0.789 |
| Preoperative serum TB (μmol/L) | 47.2 (13.6, 144.3) | 30.3 (10.2, 127.8) | 0.187 |
| Preoperative serum GGT (U/L) | 224.0 (25.0, 496.3) | 134.0 (23.5, 547.0) | 0.466 |
| Preoperative serum CA19-9 (U/L) | 252.8 (56.0, 1127.0) | 175.6 (33.0, 634.0) | 0.114 |
| Preoperative serum ChE (U/L) | 7785.0 (5487.5, 12,040.0) | 8393.5 (4859.0, 11,250.3) | 0.691 |
| Preoperative serum albumin (g/L) | 37.0 ± 4.6 | 36.4 ± 5.4 | 0.439 |
| Postoperative 4-week serum ChE (U/L) | 8056.5 (5254.8, 10,656.5) | 9358.0 (5656.3, 11,006.5) | 0.385 |
| Postoperative 4-week serum albumin (g/L) | 34.9 ± 4.7 | 36.7 ± 5.4 | 0.029 |
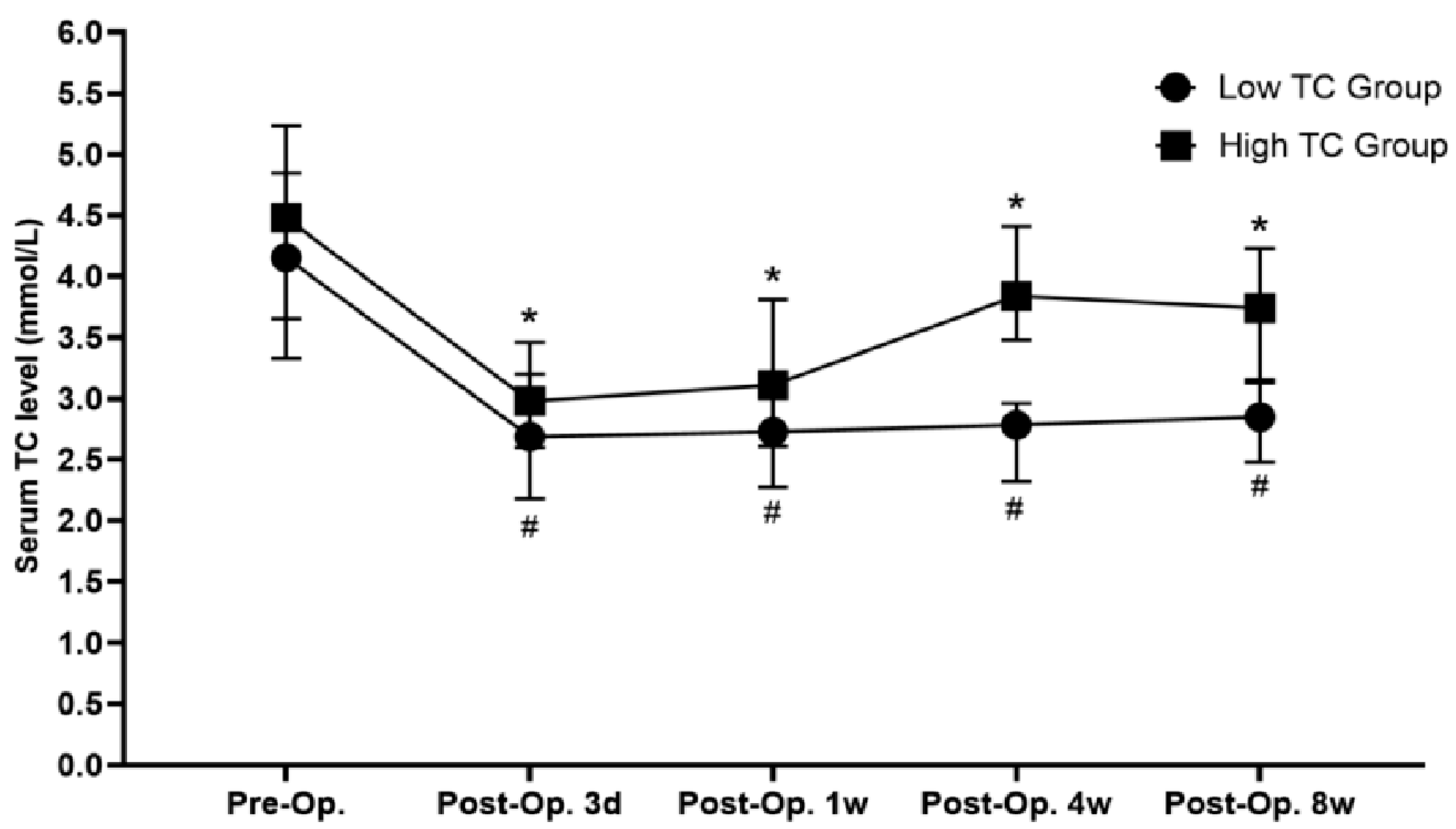
| Preoperative serum TC (mmol/L) | 4.16 (3.33, 4.85) | 4.51 (3.66, 5.23) | 0.033 |
| Postoperative 3 days serum TC (mmol/L) | 2.69 (2.18, 3.20) | 2.98 (2.60, 3.46) | 0.024 |
| Postoperative 1 week serum TC (mmol/L) | 2.73 (2.28, 3.20) | 3.11 (2.61, 3.81) | 0.003 |
| Postoperative 8-week serum TC (mmol/L) | 2.85 (2.48, 3.13) | 3.74 (3.16, 4.23) | 0.000 |
| Intraoperative blood loss (mL) | 500 (400, 800) | 500 (400, 775) | 0.242 |
| Intraoperative blood transfusion (Yes/No) | 31/37 | 31/61 | 0.127 |
| Operation time (hours) | 10.3 ± 3.2 | 9.6 ± 2.7 | 0.105 |
| pTNM stage (I&II/III) | 49/19 | 74/18 | 0.214 |
| Tumor location (pancreatic head/pancreatic neck and tail) | 55/13 | 64/28 | 0.105 |
| Peri-pancreatic invasion (Yes/No) | 65/3 | 89/3 | 0.705 |
| Tumor diameter (cm) | 3.5 (2.5, 4.7) | 3.5 (2.4, 4.2) | 0.684 |
| Tumor differentiation (Poor/moderate & high) | 20/48 | 27/65 | 0.993 |
| Portal vein system invasion (Yes/No) | 34/34 | 36/56 | 0.171 |
| Neoadjuvant chemotherapy (Yes/No) | 5/63 | 7/85 | 0.952 |
| Lymph nodes metastasis (Yes/No) | 46/22 | 54/38 | 0.248 |
| Postoperative chemotherapy (Yes/No) | 36/32 | 50/42 | 0.860 |
| Variables | Low-TC Group (n = 68) | High-TC Group (n = 92) | p Value |
|---|---|---|---|
| Postoperative hospital stay (days) | 19 (15, 28) | 19 (13, 25) | 0.265 |
| Postoperative complications | 19 | 29 | 0.722 |
| Biochemical fistula | 2 | 7 | 0.358 |
| Pancreatic fistula | |||
| Grade B | 3 | 4 | 1.000 |
| Grade C | 2 | 2 | 1.000 |
| Delayed gastric emptying | 6 | 9 | 0.837 |
| Abdominal infection | 6 | 6 | 0.585 |
| Pulmonary infection | 2 | 1 | 0.748 |
| Abdominal hemorrhage | 1 | 1 | 1.000 |
| Gastrointestinal bleeding | 0 | 3 | 0.614 |
| Variables | Number (n = 160) | 1-Year DFS Rate (%) | 3-Year DFS Rate (%) | χ2 Value | p Value |
|---|---|---|---|---|---|
| Gender | 0.660 | 0.416 | |||
| Male | 89 | 47.3 | 20.4 | ||
| Female | 71 | 64.5 | 25.2 | ||
| Age (years) | 0.015 | 0.904 | |||
| ≤60 | 60 | 59.5 | 18.5 | ||
| >60 | 100 | 52.3 | 25.1 | ||
| Diabetes | 0.045 | 0.833 | |||
| Yes | 53 | 49.4 | 22.2 | ||
| No | 107 | 57.7 | 22.8 | ||
| Preoperative jaundice reduction treatment | 0.000 | 0.994 | |||
| Yes | 36 | 47.2 | 18.3 | ||
| No | 124 | 57.3 | 22.7 | ||
| Preoperative serum TB (μmol/L) | 0.094 | 0.760 | |||
| ≤21 | 70 | 57.4 | 23.9 | ||
| >21 | 90 | 53.3 | 20.2 | ||
| Preoperative serum CA19-9 (U/mL) | 0.044 | 0.834 | |||
| ≤37 | 40 | 52.5 | 24.2 | ||
| >37 | 120 | 55.9 | 20.9 | ||
| Preoperative serum albumin (g/L) | 3.574 | 0.059 | |||
| ≤40 | 119 | 51.5 | 18.7 | ||
| >40 | 41 | 65.2 | 30.7 | ||
| Postoperative 4-week serum albumin (g/L) | 3.367 | 0.067 | |||
| ≤40 | 124 | 50.7 | 19.7 | ||
| >40 | 36 | 69.4 | 32.2 | ||
| Preoperative serum ChE (U/L) | 0.106 | 0.745 | |||
| ≤4900 | 38 | 57.9 | 23.0 | ||
| >4900 | 122 | 52.4 | 21.3 | ||
| Postoperative 4-week serum ChE (U/L) | 0.002 | 0.965 | |||
| ≤4900 | 33 | 45.5 | 22.5 | ||
| >4900 | 127 | 57.6 | 22.4 | ||
| Preoperative serum TC (mmol/L) | 0.189 | 0.663 | |||
| ≤4.535 | 91 | 56.4 | 24.7 | ||
| >4.535 | 69 | 53.1 | 19.6 | ||
| Postoperative 3 days serum TC (mmol/L) | 1.140 | 0.286 | |||
| ≤2.375 | 60 | 46.2 | 20.6 | ||
| >2.375 | 100 | 59.3 | 23.8 | ||
| Postoperative 1 week serum TC (mmol/L) | 0.039 | 0.843 | |||
| ≤3.145 | 89 | 51.3 | 23.2 | ||
| >3.145 | 71 | 59.7 | 21.7 | ||
| Postoperative 4-week serum TC (mmol/L) | 4.172 | 0.041 | |||
| ≤3.095 | 68 | 46.1 | 14.7 | ||
| >3.095 | 92 | 61.6 | 27.9 | ||
| Postoperative 8-week serum TC (mmol/L) | 0.339 | 0.560 | |||
| ≤4.185 | 119 | 53.2 | 22.8 | ||
| >4.185 | 41 | 60.3 | 21.4 | ||
| Operation time (hours) | 1.373 | 0.241 | |||
| ≤8 | 58 | 57.5 | 33.7 | ||
| >8 | 102 | 53.5 | 18.1 | ||
| Intraoperative blood loss (mL) | 1.058 | 0.304 | |||
| ≤800 | 90 | 58.9 | 25.2 | ||
| >800 | 70 | 49.9 | 19.0 | ||
| Intraoperative blood transfusion | 1.829 | 0.176 | |||
| Yes | 62 | 47.4 | 16.0 | ||
| No | 98 | 59.8 | 27.5 | ||
| Tumor differentiation | 18.268 | 0.000 | |||
| Poor | 47 | 29.8 | 7.2 | ||
| Moderate and high | 113 | 65.7 | 29.1 | ||
| pTNM stage | 16.692 | 0.000 | |||
| I&II | 123 | 61.1 | 29.6 | ||
| III | 37 | 35.1 | 0.0 | ||
| Peripancreatic invasion | 0.951 | 0.329 | |||
| Yes | 6 | 33.3 | 16.7 | ||
| No | 154 | 55.9 | 22.0 | ||
| Tumor location | 0.015 | 0.902 | |||
| Pancreatic head | 119 | 55.5 | 21.4 | ||
| Pancreatic neck and tail | 41 | 53.6 | 23.0 | ||
| Tumor diameter (cm) | 7.440 | 0.006 | |||
| ≤4 | 112 | 58.5 | 27.5 | ||
| >4 | 48 | 44.3 | 6.6 | ||
| Lymph node metastasis | 12.105 | 0.001 | |||
| Yes | 100 | 44.6 | 14.8 | ||
| No | 60 | 72.7 | 35.5 | ||
| Portal vein system invasion | 1.889 | 0.169 | |||
| Yes | 70 | 51.4 | 17.3 | ||
| No | 90 | 57.8 | 26.9 | ||
| Postoperative complications | 0.793 | 0.373 | |||
| Yes | 48 | 44.8 | 21.5 | ||
| No | 112 | 59.4 | 22.9 | ||
| Postoperative chemotherapy | 0.005 | 0.945 | |||
| Yes | 86 | 59.8 | 20.0 | ||
| No | 74 | 49.4 | 23.2 |
| Variables | RR Value | 95% CI | p Value |
|---|---|---|---|
| Postoperative 4-week serum TC | 0.782 | 0.549–1.115 | 0.175 |
| Tumor differentiation | 2.177 | 1.478–3.209 | 0.000 |
| pTNM stage | 1.609 | 1.023–2.529 | 0.040 |
| Tumor diameter | 1.189 | 0.775–1.825 | 0.429 |
| Lymph node metastasis | 1.637 | 1.087–2.465 | 0.018 |
| Variables | Number (n = 160) | 1-Year OS Rate (%) | 3-Year OS Rate (%) | χ2 Value | p Value |
|---|---|---|---|---|---|
| Gender | 0.335 | 0.563 | |||
| Male | 89 | 66.3 | 25.4 | ||
| Female | 71 | 71.8 | 26.9 | ||
| Age (years) | 0.070 | 0.792 | |||
| ≤60 | 60 | 71.7 | 19.5 | ||
| >60 | 100 | 67.0 | 30.5 | ||
| Diabetes | 0.361 | 0.548 | |||
| Yes | 53 | 67.9 | 28.8 | ||
| No | 107 | 69.2 | 24.9 | ||
| Preoperative jaundice reduction treatment | 0.497 | 0.481 | |||
| Yes | 36 | 55.6 | 20.7 | ||
| No | 124 | 72.6 | 26.6 | ||
| Preoperative serum TB (μmol/L) | 0.020 | 0.887 | |||
| ≤21 | 70 | 72.9 | 31.4 | ||
| >21 | 90 | 65.6 | 26.0 | ||
| Preoperative serum CA19-9 (U/mL) | 0.268 | 0.605 | |||
| ≤37 | 40 | 70.0 | 32.1 | ||
| >37 | 120 | 68.3 | 22.4 | ||
| Preoperative serum albumin (g/L) | 2.255 | 0.133 | |||
| ≤40 | 119 | 67.2 | 23.5 | ||
| >40 | 41 | 73.2 | 34.5 | ||
| Postoperative 4-week serum albumin (g/L) | 2.031 | 0.154 | |||
| ≤40 | 124 | 66.1 | 23.8 | ||
| >40 | 36 | 77.8 | 25.5 | ||
| Preoperative serum ChE (U/L) | 0.045 | 0.832 | |||
| ≤4900 | 38 | 65.8 | 24.7 | ||
| >4900 | 122 | 67.2 | 26.5 | ||
| Postoperative 4-week serum ChE (U/L) | 0.068 | 0.794 | |||
| ≤4900 | 33 | 60.6 | 22.8 | ||
| >4900 | 127 | 70.9 | 26.9 | ||
| Preoperative serum TC (mmol/L) | 0.000 | 0.990 | |||
| ≤4.535 | 91 | 67.0 | 28.3 | ||
| >4.535 | 69 | 71.0 | 23.5 | ||
| Postoperative 3 days serum TC (mmol/L) | 1.467 | 0.226 | |||
| ≤2.375 | 60 | 58.3 | 19.9 | ||
| >2.375 | 100 | 75.0 | 28.2 | ||
| Postoperative 1 week serum TC (mmol/L) | 0.421 | 0.517 | |||
| ≤3.145 | 89 | 62.9 | 26.0 | ||
| >3.145 | 71 | 76.1 | 24.6 | ||
| Postoperative 4-week serum TC (mmol/L) | 7.817 | 0.005 | |||
| ≤3.095 | 68 | 52.9 | 15.6 | ||
| >3.095 | 92 | 80.4 | 33.8 | ||
| Postoperative 8-week serum TC (mmol/L) | 2.304 | 0.129 | |||
| ≤4.185 | 119 | 63.9 | 25.6 | ||
| >4.185 | 41 | 82.9 | 43.1 | ||
| Operation time (hours) | 1.932 | 0.165 | |||
| ≤8 | 58 | 75.9 | 36.6 | ||
| >8 | 102 | 64.9 | 20.8 | ||
| Intraoperative blood loss (mL) | 0.912 | 0.340 | |||
| ≤500 | 90 | 74.4 | 29.6 | ||
| >500 | 70 | 61.4 | 21.6 | ||
| Intraoperative blood transfusion | 1.997 | 0.158 | |||
| Yes | 62 | 62.9 | 17.9 | ||
| No | 98 | 72.4 | 30.9 | ||
| Tumor differentiation | 14.154 | 0.000 | |||
| Poor | 47 | 48.9 | 11.4 | ||
| Moderate & High | 113 | 77.0 | 32.4 | ||
| pTNM stage | 16.079 | 0.000 | |||
| I&II | 123 | 71.5 | 31.3 | ||
| III | 37 | 59.5 | 5.9 | ||
| Extrapancreatic invasion | 0.751 | 0.386 | |||
| Yes | 6 | 50.0 | 16.7 | ||
| No | 154 | 68.8 | 26.5 | ||
| Tumor location | 0.255 | 0.613 | |||
| Pancreatic head | 119 | 65.5 | 24.9 | ||
| Pancreatic neck and tail | 41 | 70.7 | 30.2 | ||
| Tumor diameter (cm) | 7.028 | 0.008 | |||
| ≤4 | 112 | 72.0 | 32.6 | ||
| >4 | 48 | 59.5 | 8.1 | ||
| Lymph node metastasis | 14.425 | 0.000 | |||
| Yes | 100 | 62.0 | 17.6 | ||
| No | 60 | 80.0 | 40.3 | ||
| Portal vein system invasion | 3.255 | 0.071 | |||
| Yes | 70 | 61.4 | 20.7 | ||
| No | 90 | 74.4 | 30.4 | ||
| Postoperative complications | 0.230 | 0.631 | |||
| Yes | 48 | 64.6 | 27.1 | ||
| No | 112 | 70.5 | 25.5 | ||
| Postoperative chemotherapy | 0.173 | 0.677 | |||
| Yes | 86 | 75.6 | 22.4 | ||
| No | 74 | 60.8 | 31.3 |
| Variables | RR Value | 95% CI | p Value |
|---|---|---|---|
| Postoperative 4-week serum TC | 0.663 | 0.466–0.944 | 0.023 |
| Tumor differentiation | 2.054 | 1.396–3.025 | 0.000 |
| pTNM stage | 1.595 | 1.020–2.494 | 0.041 |
| Tumor diameter | 1.259 | 0.844–1.878 | 0.260 |
| Lymph node metastasis | 1.693 | 1.127–2.544 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-X.; Ding, C.; Huang, J.-C.; Ma, Y.-W.; Lyu, S.-C.; Lang, R. Prognostic Value for Perioperative Serum Total Cholesterol Level on Postoperative Long-Term Prognosis of Pancreatic Cancer: A Retrospective Clinical Study. Diagnostics 2023, 13, 1402. https://doi.org/10.3390/diagnostics13081402
Wang H-X, Ding C, Huang J-C, Ma Y-W, Lyu S-C, Lang R. Prognostic Value for Perioperative Serum Total Cholesterol Level on Postoperative Long-Term Prognosis of Pancreatic Cancer: A Retrospective Clinical Study. Diagnostics. 2023; 13(8):1402. https://doi.org/10.3390/diagnostics13081402
Chicago/Turabian StyleWang, Han-Xuan, Cheng Ding, Jin-Can Huang, You-Wei Ma, Shao-Cheng Lyu, and Ren Lang. 2023. "Prognostic Value for Perioperative Serum Total Cholesterol Level on Postoperative Long-Term Prognosis of Pancreatic Cancer: A Retrospective Clinical Study" Diagnostics 13, no. 8: 1402. https://doi.org/10.3390/diagnostics13081402
APA StyleWang, H.-X., Ding, C., Huang, J.-C., Ma, Y.-W., Lyu, S.-C., & Lang, R. (2023). Prognostic Value for Perioperative Serum Total Cholesterol Level on Postoperative Long-Term Prognosis of Pancreatic Cancer: A Retrospective Clinical Study. Diagnostics, 13(8), 1402. https://doi.org/10.3390/diagnostics13081402





