The Impact of Adding Digital Breast Tomosynthesis to BI-RADS Categorization of Mammographically Equivocal Breast Lesions
Abstract
:1. Introduction
2. Patients and Methods
2.1. Ethical Consent
2.2. Population and Study Design
2.3. The Technique of DM and DBT
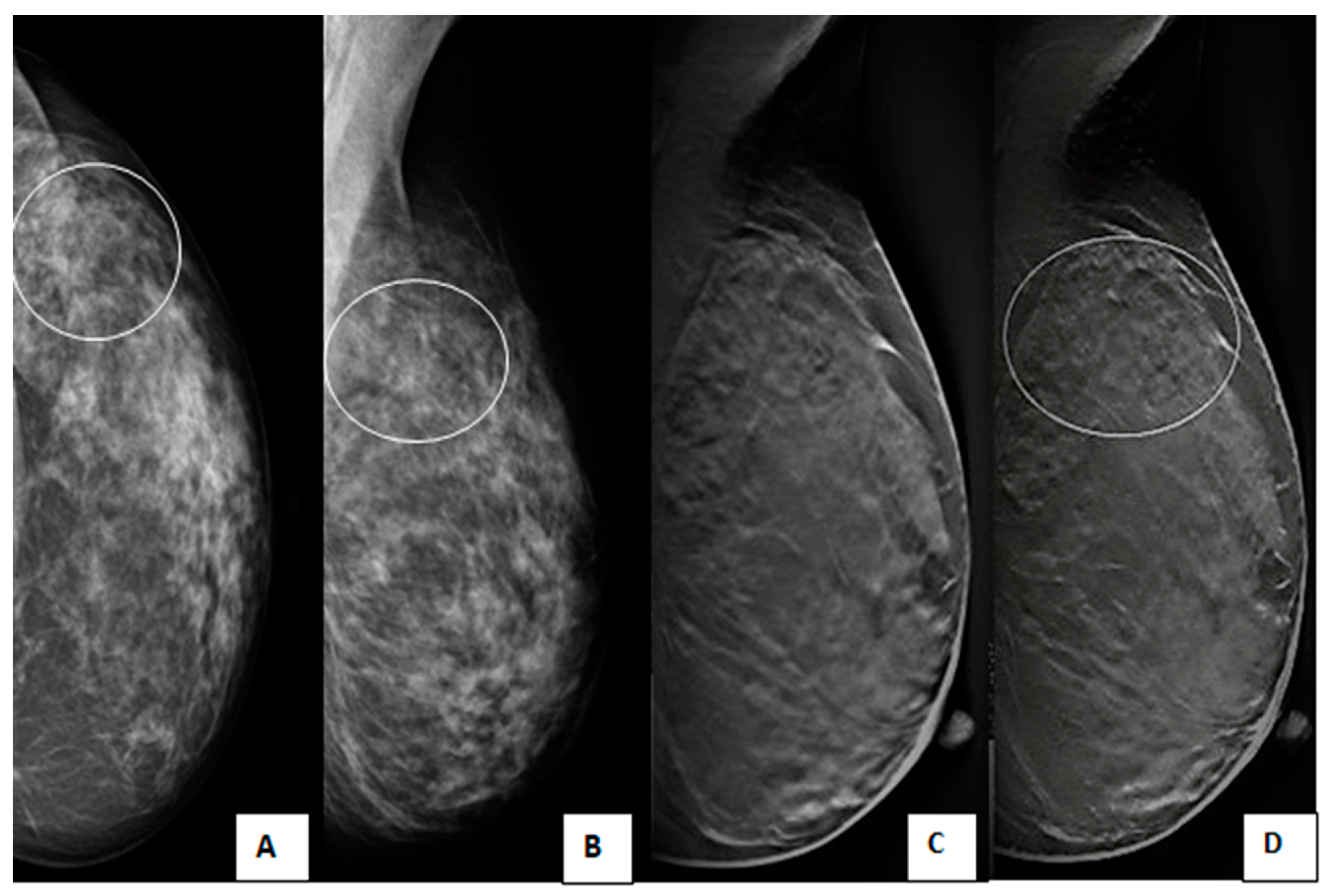
2.4. Interpretation and Data Analysis
2.5. Reference Standard
2.6. Statistical Analysis
3. Results
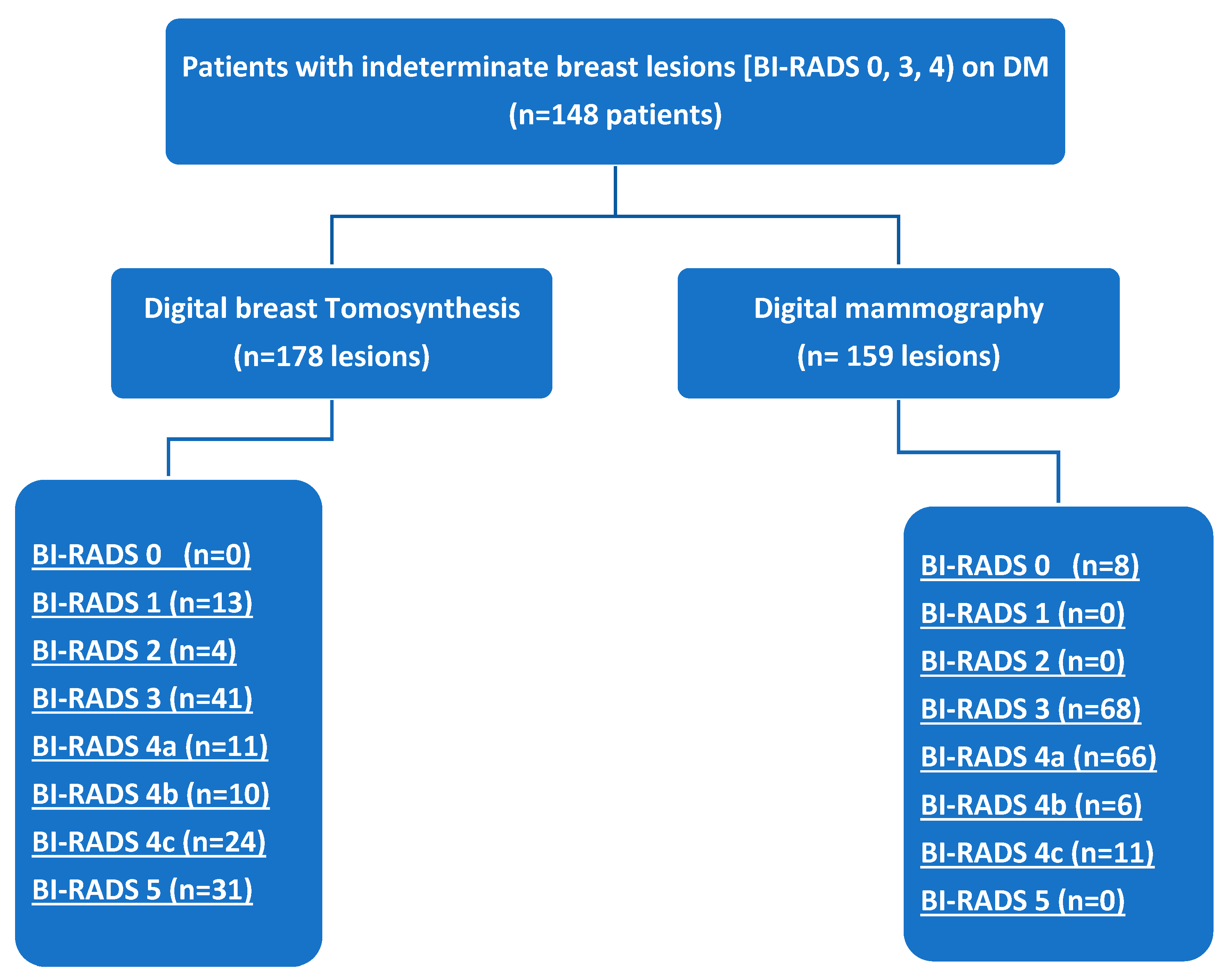
3.1. Research Sample
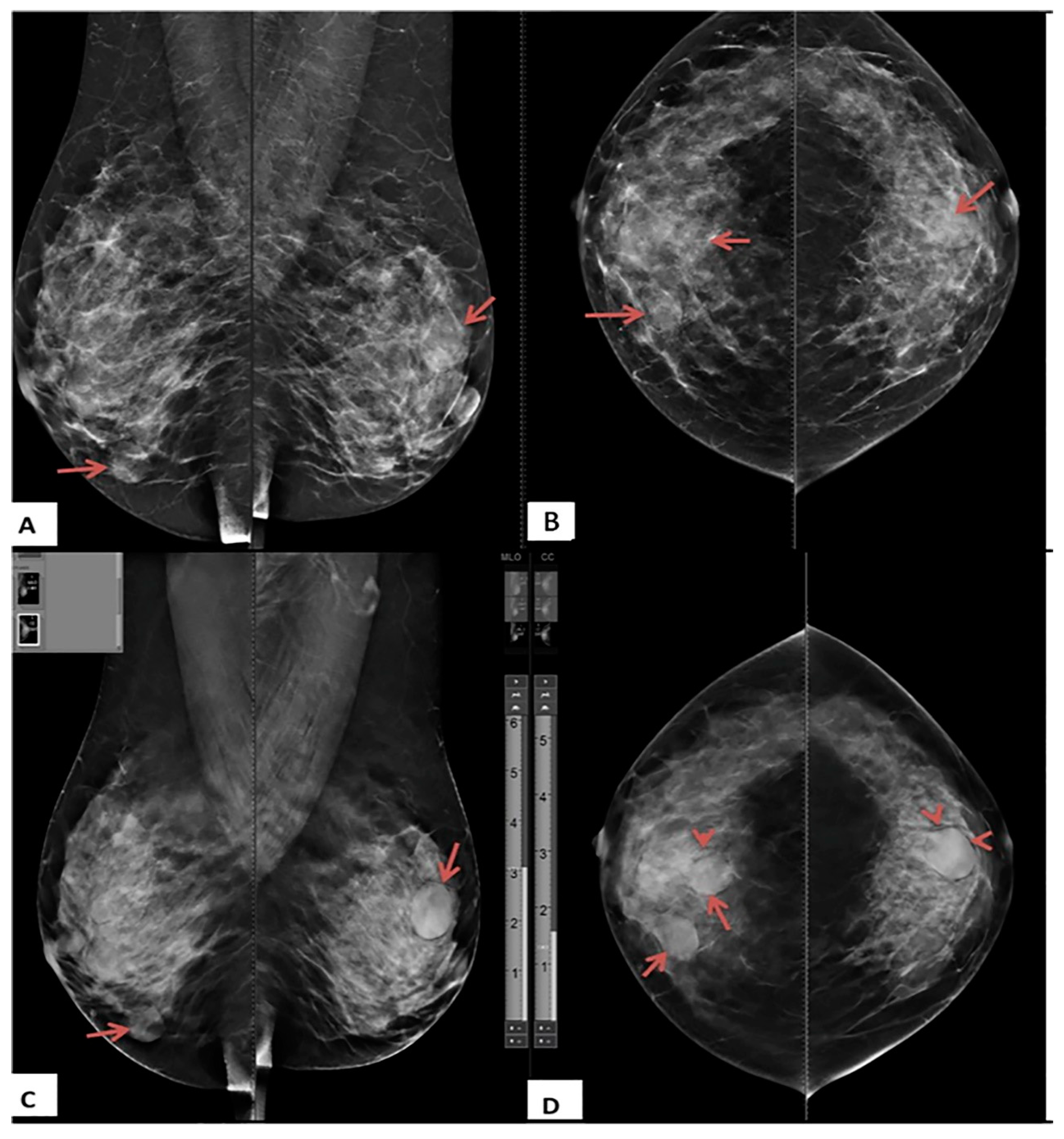
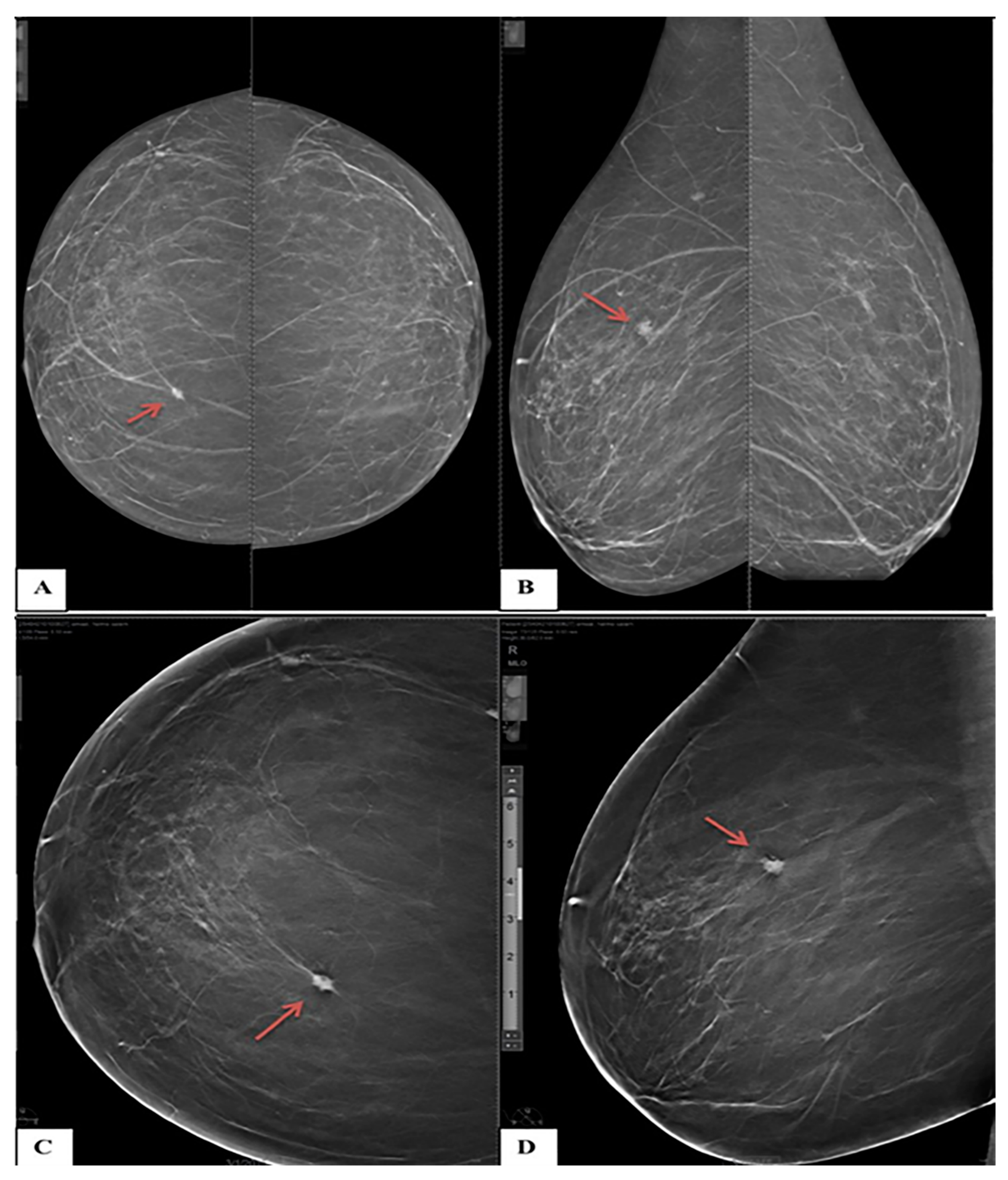
3.2. DBT and DM Results
3.3. Assignment of BI-RADS Categories by DBT and DM
3.4. Diagnostic Accuracy of DM, DBT and Integrated DBT and DM
3.5. Analyses of the ROC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S. Preventing breast cancer in LMICs via screening and/or early detection: The real and the surreal. World J. Clin. Oncol. 2014, 5, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Burhenne, H.J.; Burhenne, L.W.; Goldberg, F.; Hislop, T.G.; Worth, A.J.; Rebbeck, P.M.; Kan, L. Interval breast cancers in the Screening Mammography Program of British Columbia: Analysis and classification. Am. J. Roentgenol. 1994, 162, 1067–1071. [Google Scholar] [CrossRef]
- Choe, J.; Chikarmane, S.A.; Giess, C.S. Nonmass Findings at Breast US: Definition, Classifications, and Differential Diagnosis. RadioGraphics 2020, 40, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Nicholson, B.T.; Cohen, M.A. Finding Early Invasive Breast Cancers: A Practical Approach. Radiology 2008, 248, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casti, P.; Mencattini, A.; Salmeri, M.; Ancona, A.; Lorusso, M.; Pepe, M.; Di Natale, C.; Martinelli, E. Towards localization of malignant sites of asymmetry across bilateral mammograms. Comput. Methods Programs Biomed. 2017, 140, 11–18. [Google Scholar] [CrossRef]
- van den Biggelaar, F.J.; Kessels, A.G.; van Engelshoven, J.M.; Flobbe, K. Strategies for digital mammography interpretation in a clinical patient population. Int. J. Cancer 2009, 125, 2923–2929. [Google Scholar] [CrossRef]
- Park, J.M.; Franken, E.A., Jr.; Garg, M.; Fajardo, L.L.; Niklason, L.T. Breast Tomosynthesis: Present Considerations and Future Applications. RadioGraphics 2007, 27 (Suppl. 1), S231–S240. [Google Scholar] [CrossRef] [PubMed]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Engelken, F.; Krohn, M.; Singh, J.M.; Ingold-Heppner, B.; Winzer, K.J.; Bick, U.; Renz, D.M. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur. Radiol. 2014, 24, 256–264. [Google Scholar] [CrossRef]
- Gur, D. Tomosynthesis: Potential Clinical Role in Breast Imaging. Am. J. Roentgenol. 2007, 189, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Helvie, M.A. Digital Mammography Imaging: Breast Tomosynthesis and Advanced Applications. Radiol. Clin. North Am. 2010, 48, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Durand, M.A.; Wang, S.; Hooley, R.J.; Raghu, M.; Philpotts, L.E. Tomosynthesis-detected Architectural Distortion: Management Algorithm with Radiologic-Pathologic Correlation. RadioGraphics 2016, 36, 311–321. [Google Scholar] [CrossRef]
- Yaffe, M.J. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Chu, P.; Miglioretti, D.L.; Quale, C.; Rosenberg, R.D.; Cutter, G.; Geller, B.; Bacchetti, P.; Sickles, E.A.; Kerlikowske, K. Physician Predictors of Mammographic Accuracy. J. Natl. Cancer Inst. 2005, 97, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of Digital Mammography Alone and Digital Mammography Plus Tomosynthesis in a Population-based Screening Program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuley, M.L.; Bandos, A.I.; Ganott, M.A.; Sumkin, J.H.; Kelly, A.E.; Catullo, V.J.; Rathfon, G.Y.; Lu, A.H.; Gur, D. Digital Breast Tomosynthesis versus Supplemental Diagnostic Mammographic Views for Evaluation of Noncalcified Breast Lesions. Radiology 2013, 266, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Raghu, M.; Durand, M.A.; Andrejeva, L.; Goehler, A.; Michalski, M.H.; Geisel, J.L.; Hooley, R.J.; Horvath, L.J.; Butler, R.; Forman, H.P.; et al. Tomosynthesis in the Diagnostic Setting: Changing Rates of BI-RADS Final Assessment over Time. Radiology 2016, 281, 54–61. [Google Scholar] [CrossRef]
- Andersson, I.; Ikeda, D.M.; Zackrisson, S.; Ruschin, M.; Svahn, T.M.; Timberg, P.; Tingberg, A. Breast tomosynthesis and digital mammography: A comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur. Radiol. 2008, 18, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Chung, J.; Cha, E.-S.; Lee, J.E.; Kim, J.H. Digital breast tomosynthesis and breast ultrasound: Additional roles in dense breasts with category 0 at conventional digital mammography. Eur. J. Radiol. 2016, 85, 291–296. [Google Scholar] [CrossRef]
- Ray, K.M.; Turner, E.; Sickles, E.A.; Joe, B.N. Suspicious Findings at Digital Breast Tomosynthesis Occult to Conventional Digital Mammography: Imaging Features and Pathology Findings. Breast J. 2015, 21, 538–542. [Google Scholar] [CrossRef]
- Stepanek, T.; Constantinou, N.; Marshall, H.; Pham, R.; Thompson, C.; Dubchuk, C.; Plecha, D. Changes in the Utilization of the BI-RADS Category 3 Assessment in Recalled Patients Before and After the Implementation of Screening Digital Breast Tomosynthesis. Acad. Radiol. 2019, 26, 1515–1525. [Google Scholar] [CrossRef]
- Bahrs, S.D.; Otto, V.; Hattermann, V.; Klumpp, B.; Hahn, M.; Nikolaou, K.; Siegmann-Luz, K. Breast tomosynthesis for the clarification of mammographic BI-RADS 3 lesions can decrease follow-up examinations and enables immediate cancer diagnosis. Acta Radiol. 2018, 59, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Emlik, G.D.; Poyraz, N.; Altunkeser, A. Digital breast tomosynthesis and ultrasonography: Diagnostic performance and effect on recall rates versus digital mammography in category 0. Int. J. Clin. Exp. Med. 2017, 10, 10668–10675. [Google Scholar]
- Haas, B.M.; Kalra, V.; Geisel, J.; Raghu, M.; Durand, M.; Philpotts, L.E. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology 2013, 269, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Waldherr, C.; Cerny, P.; Altermatt, H.J.; Berclaz, G.; Ciriolo, M.; Buser, K.; Sonnenschein, M.J. Value of One-View Breast Tomosynthesis Versus Two-View Mammography in Diagnostic Workup of Women With Clinical Signs and Symptoms and in Women Recalled From Screening. Am. J. Roentgenol. 2013, 200, 226–231. [Google Scholar] [CrossRef]
- Gennaro, G.; Toledano, A.; Di Maggio, C.; Baldan, E.; Bezzon, E.; La Grassa, M.; Pescarini, L.; Polico, I.; Proietti, A.; Toffoli, A.; et al. Digital breast tomosynthesis versus digital mammography: A clinical performance study. Eur. Radiol. 2010, 20, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, E.A.; Park, J.M.; Philpotts, L.E.; Poplack, S.P.; Sumkin, J.H.; Halpern, E.F.; Niklason, L.T. Assessing Radiologist Performance Using Combined Digital Mammography and Breast Tomosynthesis Compared with Digital Mammography Alone: Results of a Multicenter, Multireader Trial. Radiology 2013, 266, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Basha, M.A.A.; Safwat, H.K.; Alaa Eldin, A.M.; Dawoud, H.A.; Hassanin, A.M. The added value of digital breast tomosynthesis in improving diagnostic performance of BI-RADS categorization of mammographically indeterminate breast lesions. Insights Imaging 2020, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.A.; Adel, L. Study of role of digital breast tomosynthesis over digital mammography in the assessment of BIRADS 3 breast lesions. Egypt. J. Radiol. Nucl. Med. 2019, 50, 48. [Google Scholar] [CrossRef]
- Mansour, S.; Adel, L.; Mokhtar, O.; Omar, O.S. Comparative study between breast tomosynthesis and classic digital mammography in the evaluation of different breast lesions. Egypt. J. Radiol. Nucl. Med. 2014, 45, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Galati, F.; Marzocca, F.; Bassetti, E.; Luciani, M.L.; Tan, S.; Catalano, C.; Pediconi, F. Added Value of Digital Breast Tomosynthesis Combined with Digital Mammography According to Reader Agreement: Changes in BI-RADS Rate and Follow-Up Management. Breast Care 2017, 12, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-Q.; Yan, J.-X.; Chen, Q.-S.; Huang, M.-L.; Cai, D.-L. Significance and Application of Digital Breast Tomosynthesis for the BI-RADS Classification of Breast Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4109–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomassin-Naggara, I.; Perrot, N.; Dechoux, S.; Ribeiro, C.; Chopier, J.; de Bazelaire, C. Added value of one-view breast tomosynthesis combined with digital mammography according to reader experience. Eur. J. Radiol. 2015, 84, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, K.; Uematsu, T.; Itoh, T.; Takahashi, K.; Nishimura, S.; Hayashi, T.; Sugino, T. Comparison of visibility of circumscribed masses on Digital Breast Tomosynthesis (DBT) and 2D mammography: Are circumscribed masses better visualized and assured of being benign on DBT? Eur. Radiol. 2017, 27, 570–577. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value |
|---|---|
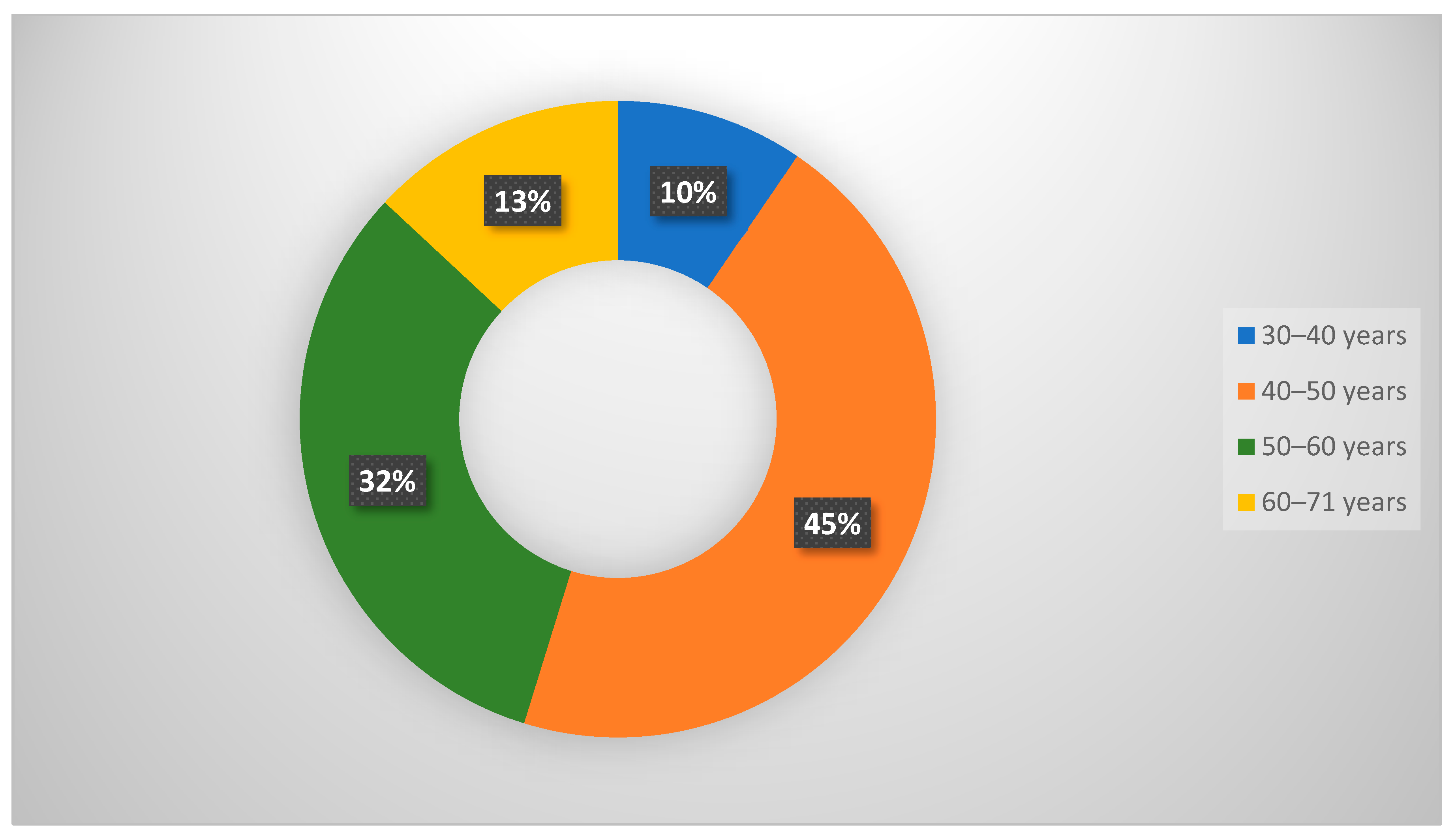
| Age, years, mean S D (range) | 44.2 7.3 (30–71) |
| Family history | |
| Negative family history | 74 (50) |
| 1st degree relative | 44 (29.7) |
| 2nd degree relative | 30 (20.2) |
| Site of lesions | |
| Both breasts | 4 (2.7) |
| Right breast | 85 (57.4) |
| Left breast | 59 (39.9) |
| Clinical presentations | |
| Asymptomatic | 42 (28.3) |
| Breast mass only | 91 (61.4) |
| Breast mass and edema | 7 (4.7) |
| Breast mass and retracted nipple | 8 (5.4) |
| ACR BI-RADS density | |
| A | 7 (4.7) |
| B | 50 (33.8) |
| C | 78 (52.7) |
| D | 13 (8.8) |
| Final diagnosis of 178 lesions | |
| Benign | 104 (58.4) |
| Fibrocystic changes | 43 (41.3) |
| Fibroadenomas | 32 (30.8) |
| Normal | 13 (12.5) |
| Postoperative scar | 5 (4.8) |
| Duct ectasia | 4 (3.9) |
| Granulomatous mastitis | 3 (2.9) |
| Abscess | 2 (1.9) |
| Benign phylloides | 2 (1.9) |
| Malignant | 74 (41.6) |
| Invasive duct carcinoma | 52 (70.3) |
| Invasive lobular carcinoma | 13 (17.6) |
| Ductal carcinoma in situ | 6 (8.1) |
| Mucinous carcinoma | 3 (4) |
| Findings | DM (n = 159) | DBT (n = 178) |
|---|---|---|
| Site of lesion | ||
| Both breasts | 7 (4.4) | 8 (4.5) |
| Right breast | 91 (57.2) | 102 (57.3) |
| Left breast | 61 (38.4) | 68 (38.2) |
| Type of lesion | ||
| Mass | 74 (46.5) | 140 (78.7) |
| Asymmetry | 55 (34.6) | - |
| Architecture distortion | 13 (8.2) | 8 (4.5) |
| Clusters of microcalcification with no underlying mass | 9 (5.7) | 4 (2.2) |
| Dense breasts (BI-RADS 0) | 8 (5) | - |
| Dilated ducts | - | 5 (2.8) |
| Asymmetric densities | - | 8 (4.5) |
| Overlapped glandular tissue | - | 13 (7.3%) |
| Characters of mass | ||
| Mass margin | ||
| Obscured on mammography, but speculated on tomosynthesis | 35 (47.3) | 55 (39.3) |
| Ill-defined | 28 (37.8) | 22 (15.7) |
| Well-defined | 11 (14.9) | 63 (45) |
| Mass Shape | ||
| Irregular | 33 (44.6) | 65 (46.4) |
| Round | 22 (29.7) | 46 (32.9) |
| Oval | 10 (13.5) | 19 (13.6) |
| Macrolobulated | 9 (12.2) | 10 (7.1) |
| ACR BI-RADS density | ||
| A | 8 (5) | 8 (4.5) |
| B | 53 (33.3) | 55 (30.9) |
| C | 85 (53.5) | 95 (53.4) |
| D | 13 (8.2) | 20 (11.2) |
| DM | DBT | |||||
|---|---|---|---|---|---|---|
| Malignant | Benign | Total | Malignant | Benign | Total | |
| Not Seen | 7 (3.9) | 11 (6.2) | 18 (10.1) | 0 | 0 | 0 |
| BI-RADS 0 | 2 (1.1) | 6 (3.4) | 8 (4.5) | 0 | 0 | 0 |
| BI-RADS 1 | 0 | 0 | 0 | 0 | 13 (7.3) | 13 (7.3) |
| BI-RADS 2 | 0 | 0 | 0 | 0 | 48 (27) | 48 (27) |
| BI-RADS 3 | 15 (8.4) | 54 (30.3) | 69 (38.7) | 8 (4.5) | 33 (18.5) | 41 (23) |
| BI-RADS 4a | 33 (18.5) | 33 (18.5) | 66 (37.1) | 8 (4.5) | 3 (1.7) | 11 (6.2) |
| BI-RADS 4b | 6 (3.4) | 0 | 6 (3.4) | 10 (5.6) | 0 | 10 (5.6) |
| BI-RADS 4c | 11 (6.2) | 0 | 11 (6.2) | 17 (9.5) | 7 (3.9) | 24 (13.5) |
| BI-RADS 5 | 0 | 0 | 0 | 31 (17.4) | 0 | 31 (17.4) |
| Total | 74 (41.6) | 104 (58.4) | 178 | 74 (41.6) | 104 (58.4) | 178 (100) |
| DM | DBT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BI-RADS 0 | BI-RADS 1 | BI-RADS 2 | BI-RADS 3 | BI-RADS 4a | BI-RADS 4b | BI-RADS 4c | BI-RADS 5 | Total | |
| Not seen | 10 (5.6) | 2(1.1) | 4 (2.2) | 2 (1.1) | 1(0.6) | 19 (10.6) | |||
| BI-RADS 0 | 6 (3.4) | 2 (1.1) | 8 (4.5) | ||||||
| BI-RADS 1 | 0 | ||||||||
| BI-RADS 2 | 0 | ||||||||
| BI-RADS 3 | 13 (7.3) | 20 (11.2) | 22 (12.3) | 2 (1.1) | 6 (3.4) | 5 (2.8) | 68 (38.2) | ||
| BI-RADS 4a | 18 (10.1) | 11 (6.2) | 9 (5.1) | 6 (3.4) | 22 (12.3) | 66 (37.1) | |||
| BI-RADS 4b | 6 (3.4) | 6 (3.4) | |||||||
| BI-RADS 4c | 9 (5.1) | 2 (1.1) | 11 (6.2) | ||||||
| BI-RADS 5 | 0 | ||||||||
| Total | 13 (7.3) | 48 (27) | 41(23) | 11 (6.2) | 10 (5.6) | 24 (13.5) | 31(17.4) | 178 (100) | |
| Criterion | DM | DBT | DM + DBT | |||
|---|---|---|---|---|---|---|
| % | 95%CI | % | 95%CI | % | 95%CI | |
| Accuracy | 67.3 | 90 | 97.5 | |||
| Specificity | 67.6 | 60.8–74 | 90.3 | 85.5–94 | 96.6 | 93.2–98.6 |
| Sensitivity | 66.9 | 58.7–74.4 | 89.2 | 83–93.7 | 98.7 | 95.2–99.8 |
| Positive likelihood ratio | 2.1 | 1.7–2.6 | 9.23 | 6.1–14.1 | 29.2 | 14.1–60.4 |
| Negative likelihood ratio | 0.49 | 0.38–0.36 | 0.12 | 0.08–0.19 | 0.01 | 0–0.06 |
| PPV | 59.6 | 51.8–67.2 | 86.8 | 80.4–91.8 | 95.4 | 90.8–98.1 |
| NPV | 74.1 | 67.2–80.2 | 92.1 | 87.5–95 | 99 | 96.5–99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, R.M.; Almalki, Y.E.; Basha, M.A.A.; Alduraibi, S.K.; Aboualkheir, M.; Almushayti, Z.A.; Aldhilan, A.S.; Aly, S.A.; Alshamy, A.A. The Impact of Adding Digital Breast Tomosynthesis to BI-RADS Categorization of Mammographically Equivocal Breast Lesions. Diagnostics 2023, 13, 1423. https://doi.org/10.3390/diagnostics13081423
Hassan RM, Almalki YE, Basha MAA, Alduraibi SK, Aboualkheir M, Almushayti ZA, Aldhilan AS, Aly SA, Alshamy AA. The Impact of Adding Digital Breast Tomosynthesis to BI-RADS Categorization of Mammographically Equivocal Breast Lesions. Diagnostics. 2023; 13(8):1423. https://doi.org/10.3390/diagnostics13081423
Chicago/Turabian StyleHassan, Rania Mostafa, Yassir Edrees Almalki, Mohammad Abd Alkhalik Basha, Sharifa Khalid Alduraibi, Mervat Aboualkheir, Ziyad A. Almushayti, Asim S. Aldhilan, Sameh Abdelaziz Aly, and Asmaa A. Alshamy. 2023. "The Impact of Adding Digital Breast Tomosynthesis to BI-RADS Categorization of Mammographically Equivocal Breast Lesions" Diagnostics 13, no. 8: 1423. https://doi.org/10.3390/diagnostics13081423






