Eccrine Porocarcinoma: A Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology
4. Pathogenesis—Risk Factors
5. Diagnosis
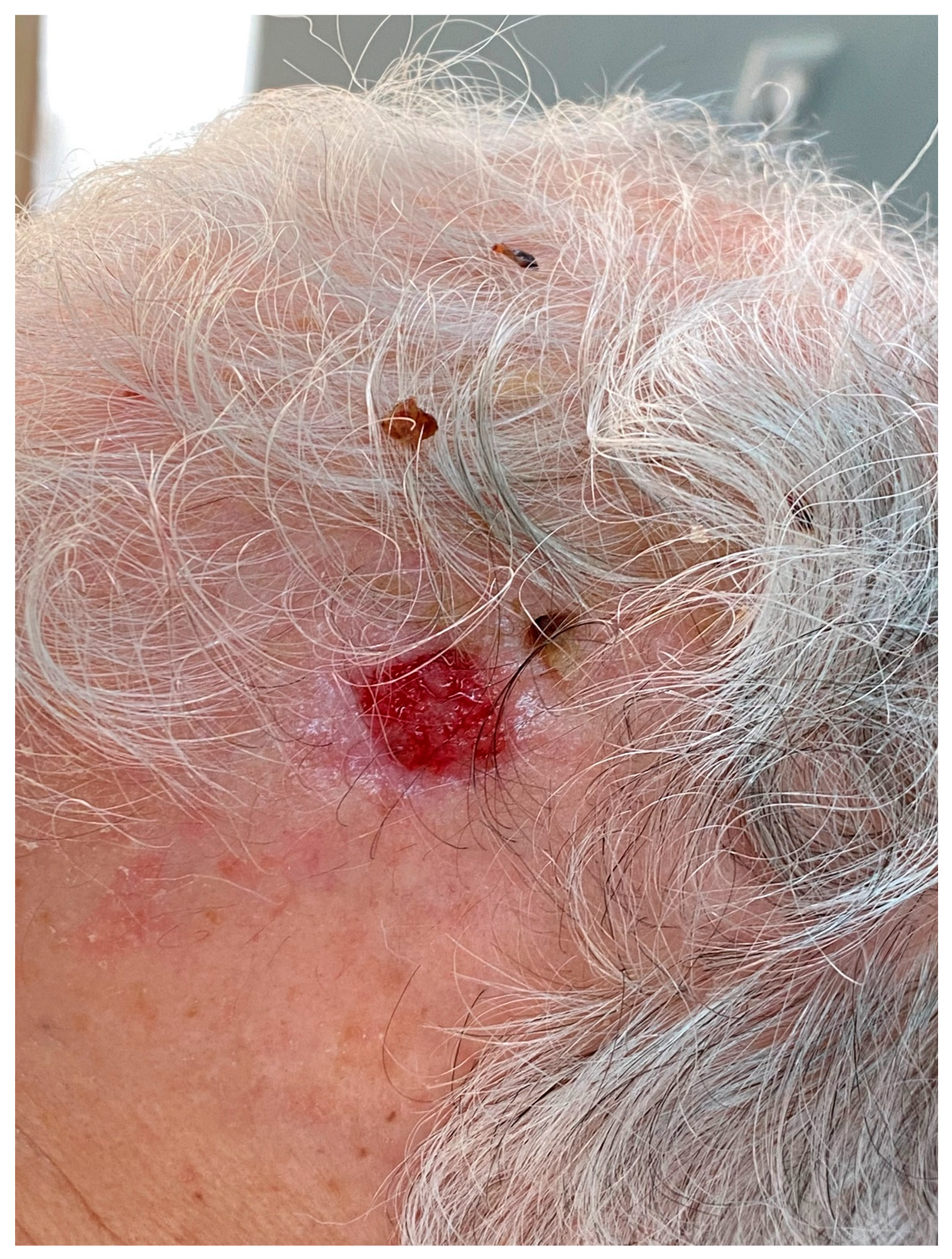
5.1. Clinical Presentation
5.2. Imaging
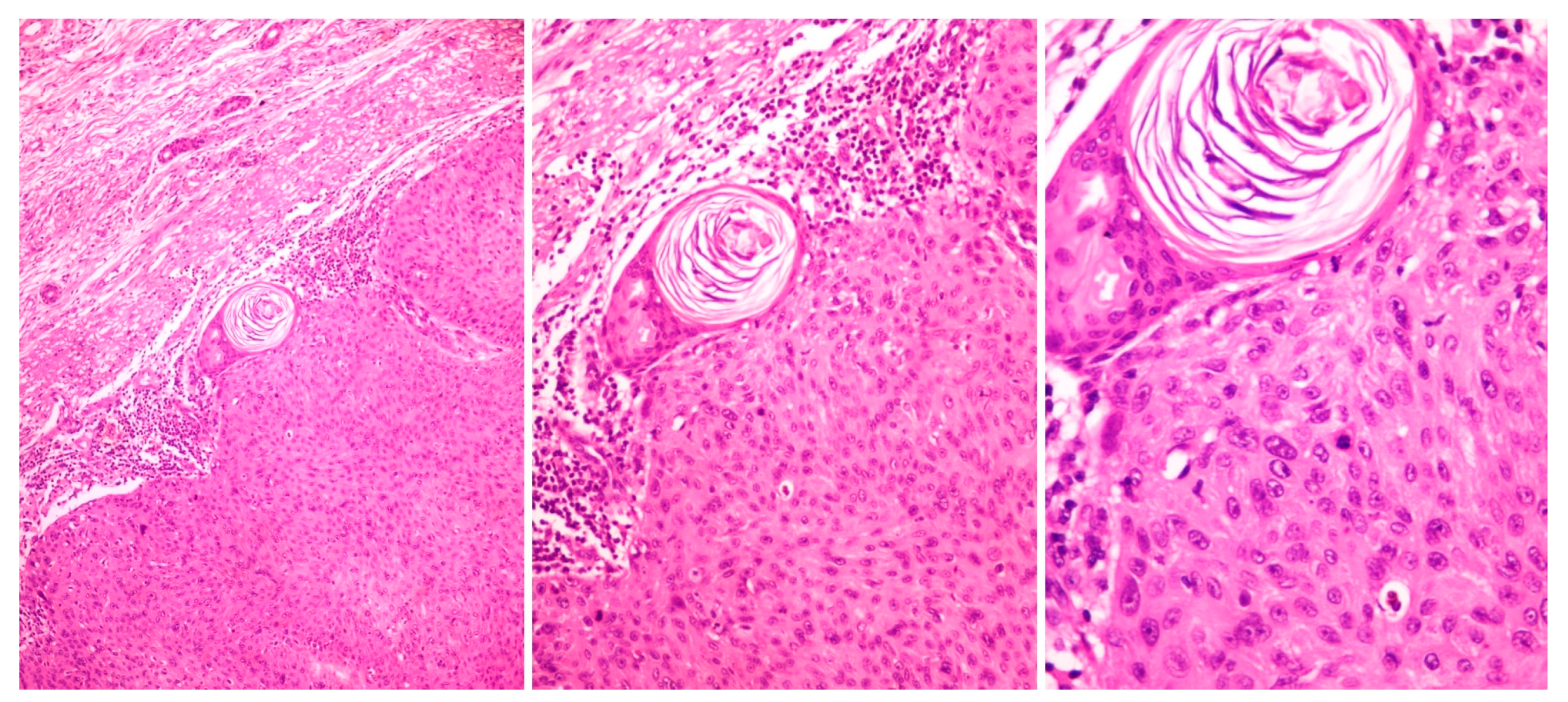
5.3. Histopathology
5.4. Immunohistochemistry
6. Disease Course and Prognosis
7. Treatment
7.1. Tumor Excision
7.2. Sentinel Lymph Node Biopsy
7.3. Radiotherapy
7.4. Systemic Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazemi, A.; Higgins, S.; Swift, R.; In, G.; Miller, K.; Wysong, A. Eccrine porocarcinoma: New insights and a systematic review of the literature. Derm. Surg. 2018, 44, 1247–1261. [Google Scholar] [CrossRef]
- Elder, D.E.M.D.; Scolyer, R.A.; Willemze, R. Appendageal tumours. In WHO Classification of Skin Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2018; Volume 11, p. 159. [Google Scholar]
- Pinkus, H.; Mehregan, A.H. Epidermotropic eccrine carcinoma. A case combining features of eccrine poroma and paget’s dermatosis. Arch. Derm. 1963, 88, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Morioka, S. Oncogenic differentiation of the intraepidermal eccrine sweat duct: Eccrine poroma, poroepithelioma and porocarcinoma. Dermatologica 1969, 138, 238–250. [Google Scholar] [CrossRef]
- Salih, A.M.; Kakamad, F.H.; Baba, H.O.; Salih, R.Q.; Hawbash, M.R.; Mohammed, S.H.; Othman, S.; Saeed, Y.A.; Habibullah, I.J.; Muhialdeen, A.S.; et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann. Med. Surg. 2017, 20, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Le, N.S.; Janik, S.; Liu, D.T.; Grasl, S.; Faisal, M.; Pammer, J.; Schickinger-Fischer, B.; Hamzavi, J.; Seemann, R.; Erovic, B.M. Eccrine porocarcinoma of the head and neck: Meta-analysis of 120 cases. Head Neck 2020, 42, 2644–2659. [Google Scholar] [CrossRef]
- Behbahani, S.; Malerba, S.; Karanfilian, K.M.; Warren, C.J.; Alhatem, A.; Samie, F.H. Demographics and outcomes of eccrine porocarcinoma: Results from the National Cancer Database. Br. J. Dermatol. 2020, 183, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.W.; Bradford, P.T.; Devesa, S.S.; Toro, J.R. Cutaneous Appendageal Carcinoma Incidence and Survival Patterns in the United States. Arch. Dermatol. 2010, 146, 625–632. [Google Scholar] [CrossRef]
- Gibbs, D.C.; Yeung, H.; Blalock, T.W. Incidence and trends of cutaneous adnexal tumors in the United States in 2000–2018: A population-based study. J. Am. Acad. Dermatol. 2023, 88, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Tolkachjov, S.N.; Schmitt, A.R.; Muzic, J.G.; Weaver, A.L.; Baum, C.L. Incidence and Clinical Features of Rare Cutaneous Malignancies in Olmsted County, Minnesota, 2000–2010. Dermatol. Surg. 2018, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, A.S.; Pukkala, E.; Böhling, T.; Koljonen, V. Malignant eccrine porocarcinoma in finland during 2007 to 2017. Acta Derm. Venereol. 2021, 101, dv00363. [Google Scholar] [CrossRef]
- Stam, H.; Lohuis, P.J.F.M.; Zupan-Kajcovski, B.; Wouters, M.W.J.M.; Van Der Hage, J.A.; Visser, O. Increasing incidence and survival of a rare skin cancer in the Netherlands. A population-based study of 2,220 cases of skin adnexal carcinoma. J. Surg. Oncol. 2013, 107, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Goon, P.K.C.; Gurung, P.; Levell, N.J.; Subramanian, P.; Yong, A.S.W.; Lee, K.Y.C.; Igali, L.; Greenberg, D.; Shah, S.; Tan, E. Eccrine Porocarcinoma of the Skin is Rising in Incidence in the East of England. Acta Derm. Venereol. 2018, 98, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Joshy, J.; Mistry, K.; Levell, N.J.; van Bodegraven, B.; Vernon, S.; Rajan, N.; Craig, P.; Venables, Z.C. Porocarcinoma: A review. Clin. Exp. Dermatol. 2022, 47, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.; Greene, J.; Ansari, N.; Kim, B.; Seed, P.T.; McKee, P.H.; Calonje, E. Eccrine porocarcinoma (malignant eccrine poroma): A clinicopathologic study of 69 cases. Am. J. Surg. Pathol. 2001, 25, 710–720. [Google Scholar] [CrossRef]
- Shaw, M.; McKee, P.H.; Lowe, D.; Black, M.M. Malignant eccrine poroma: A study of twenty-seven cases. Br. J. Dermatol. 1982, 107, 675–680. [Google Scholar] [CrossRef]
- Prieto-Granada, C.; Morlote, D.; Pavlidakey, P.; Rodriguez-Waitkus, P.; Ramirez, C.; Florento, E.; Swensen, J.; Gatalica, Z.; Stevens, T.M. Poroid adnexal skin tumors with YAP1 fusions exhibit similar histopathologic features: A series of six YAP1-rearranged adnexal skin tumors. J. Cutan. Pathol. 2021, 48, 1139–1149. [Google Scholar] [CrossRef]
- Sekine, S.; Kiyono, T.; Ryo, E.; Ogawa, R.; Wakai, S.; Ichikawa, H.; Suzuki, K.; Arai, S.; Tsuta, K.; Ishida, M.; et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J. Clin. Investig. 2019, 129, 3827–3832. [Google Scholar] [CrossRef]
- Kervarrec, T.; Frouin, E.; Collin, C.; Tallet, A.; Tallegas, M.; Pissaloux, D.; Tirode, F.; Guyétant, S.; Samimi, M.; Gaboriaud, P.; et al. Distinct regulations driving YAP1 expression loss in poroma, porocarcinoma and RB1-deficient skin carcinoma. Histopathology 2023, 82, 885–898. [Google Scholar] [CrossRef]
- Girdwichai, N.; Chanprapaph, K.; Vicharamon, V. Eccrine Poroma Arising Within Nevus Sebaceous. Case Rep. Dermatol. 2016, 8, 80–84. [Google Scholar] [CrossRef]
- Van Hees, C.L.; Van Duinen, C.M.; Bruijin, J.A.; Vermeer, B.J. Malignant eccrine puroma in a patient with Rothniund- Thomson syndrome. Br. J. Dermatol. 1996, 134, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Helmke, B.; Starz, H.; Bachter, D.; Balda, B.R. Metastasising porocarcinoma following exposure to poison gas. Lancet 2002, 359, 1685. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Hovelson, D.H.; Cani, A.K.; Omata, K.; Haller, M.J.; Wang, M.L.; Arps, D.; Patel, R.; Fullen, D.R.; Wang, M.; et al. Porocarcinomas harbor recurrent HRAS-activating mutations and tumor suppressor inactivating mutations. Hum. Pathol. 2016, 51, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Le, L.P.; Dias-Santagata, D.; Pawlak, A.C.; Cosper, A.K.; Nguyen, A.T.; Selim, M.A.; Deng, A.; Horick, N.K.; Iafrate, A.J.; Mihm, M.C.; et al. Apocrine-Eccrine Carcinomas: Molecular and Immunohistochemical Analyses. PLoS ONE 2012, 7, e47290. [Google Scholar] [CrossRef]
- Bosic, M.; Kirchner, M.; Brasanac, D.; Leichsenring, J.; Lier, A.; Volckmar, A.L.; Oliveira, C.; Buchhalter, I.; Stögbauer, F.; Zivkovic-Perisic, S.; et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology 2018, 50, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, A.S.; Sihto, H.; Koljonen, V. Merkel cell polyomavirus is a passenger virus in both poroma and porocarcinoma. J. Cutan. Pathol. 2022, 49, 49–54. [Google Scholar] [CrossRef]
- Puttonen, M.; Isola, J.; Ylinen, O.; Böhling, T.; Koljonen, V.; Sihto, H. UV-induced local immunosuppression in the tumour microenvironment of eccrine porocarcinoma and poroma. Sci. Rep. 2022, 12, 5529. [Google Scholar] [CrossRef] [PubMed]
- Denisova, E.; Westphal, D.; Surowy, H.M.; Meier, F.; Hutter, B.; Reifenberger, J.; Rütten, A.; Schulz, A.; Sergon, M.; Ziemer, M.; et al. Whole-exome sequencing in eccrine porocarcinoma indicates promising therapeutic strategies. Cancer Gene Ther. 2022, 29, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Garzarolli, M.; Sergon, M.; Horak, P.; Hutter, B.; Becker, J.C.; Wiegel, M.; Maczey, E.; Blum, S.; Grosche-Schlee, S.; et al. High tumour mutational burden and EGFR/MAPK pathway activation are therapeutic targets in metastatic porocarcinoma. Br. J. Dermatol. 2021, 185, 1186–1199. [Google Scholar] [CrossRef]
- Godillot, C.; Boulinguez, S.; Riffaud, L.; Sibaud, V.; Chira, C.; Tournier, E.; Paul, C.; Meyer, N. Complete response of a metastatic porocarcinoma treated with paclitaxel, cetuximab and radiotherapy. Eur. J. Cancer 2018, 90, 142–145. [Google Scholar] [CrossRef]
- Lee, K.A.; Cioni, M.; Robson, A.; Bataille, V. Metastatic porocarcinoma achieving complete radiological and clinical response with pembrolizumab. BMJ Case Rep. 2019, 12, e228917. [Google Scholar] [CrossRef]
- Belin, E.; Ezzedine, K.; Stanislas, S.; Lalanne, N.; Beylot-Barry, M.; Taieb, A.; Vergier, B.; Jouary, T. Factors in the surgical management of primary eccrine porocarcinoma: Prognostic histological factors can guide the surgical procedure. Br. J. Dermatol. 2011, 165, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Riera-Leal, L.; Guevara-Gutiérrez, E.; Barrientos-García, J.G.; Madrigal-Kasem, R.; Briseño-Rodríguez, G.; Tlacuilo-Parra, A. Eccrine porocarcinoma: Epidemiologic and histopathologic characteristics. Int. J. Dermatol. 2015, 54, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Juay, L.; Choi, E.; Huang, J.; Jaffar, H.; Ho, S.A.J.E. Unusual Presentations of Eccrine Porocarcinomas. Skin. Appendage Disord. 2022, 8, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Warner, C.L.; Cockerell, C.J. The new seventh edition American Joint Committee on Cancer staging of cutaneous non-melanoma skin cancer: A critical review. Am. J. Clin. Dermatol. 2011, 12, 147–154. [Google Scholar] [CrossRef]
- Scampa, M.; Merat, R.; Kalbermatten, D.F.; Oranges, C.M. Head and Neck Porocarcinoma: SEER Analysis of Epidemiology and Survival. J. Clin. Med. 2022, 11, 2185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, A.; Moon, K.C.; Seo, S.H.; Kim, I.H.; Kim, A.; Baek, Y.S. Eccrine porocarcinoma: A multicenter retrospective study with review of the literatures reported in Korea. Ann. Dermatol. 2020, 32, 223–229. [Google Scholar] [CrossRef]
- Gerber, P.A.; Schulte, K.W.; Ruzicka, T.; Bruch-Gerharz, D. Eccrine porocarcinoma of the head: An important differential diagnosis in the elderly patient. Dermatology 2008, 216, 229–233. [Google Scholar] [CrossRef]
- Pinheiro, R.; Oliveira, A.; Mendes-Bastos, P. Dermoscopic and reflectance confocal microscopic presentation of relapsing eccrine porocarcinoma. J. Am. Acad. Dermatol. 2017, 76, S73–S75. [Google Scholar] [CrossRef]
- Sgouros, D.; Routsi, E.; Almpanis, Z.; Korogiannos, A.; Katoulis, A. Dermatoscopic Features of a Metastatic Eccrine Porocarcinoma Arising on Lymphedema. Dermatol. Pract. Concept 2022, 12, e2022079. [Google Scholar] [CrossRef]
- Mahdar, I.; Lembarki, G.; Jamil, J.; Safi-Eddine, H.; Najib, B.; Sabiri, M.; Labied, M.; El Manjra, S.; Samira, L.; Diouri, M.; et al. Eccrine porocarcinoma: An extremely rare cutaneous tumor from a radiological point of view—Case report and review of the literature. BJR Case Rep. 2022, 8, 20220044. [Google Scholar] [CrossRef]
- Koh, M.; Telang, G.; Fonseca, A.; Ghanian, S.; Walker, J. Clear Cell Differentiation in Eccrine Porocarcinoma as a High-Risk Feature: Epidemiologic and Pathologic Features of Eccrine Porocarcinoma in a Single-Center Case Series. Am. J. Dermatopathol. 2021, 43, 647–652. [Google Scholar] [CrossRef]
- Shiohara, J.; Koga, H.; Uhara, H.; Takata, M.; Saida, T. Eccrine porocarcinoma: Clinical and pathological studies of 12 cases. J. Dermatol. 2007, 34, 516–522. [Google Scholar] [CrossRef]
- Goto, K.; Takai, T.; Fukumoto, T.; Anan, T.; Kimura, T.; Ansai, S.I.; Oshitani, Y.; Murata, Y.; Sakuma, T.; Hirose, T. CD117 (KIT) is a useful immunohistochemical marker for differentiating porocarcinoma from squamous cell carcinoma. J. Cutan. Pathol. 2016, 43, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Ishikawa, M.; Hamada, K.; Muramatsu, K.; Naka, M.; Honma, K.; Sugino, T.M. Comparison of Immunohistochemical Expression of Cytokeratin 19, c-KIT, BerEP4, GATA3, and NUTM1 between Porocarcinoma and Squamous Cell Carcinoma. Am. J. Dermatopathol. 2021, 43, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Mita, H.; Takigawa, M.; Iwatsuki, K.; Tokura, Y.M.; Inoue, K.; Yamada, M. An immunohistoiogic study on eccrine gland carcinoma. J. Am. Acad. Dermatol. 1989, 20, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.; Chan, M.P.; Wang, G.; Patel, R.M.; Andea, A.A.; Bresler, S.C.; Harms, P.W. Altered Rb, p16, and p53 expression is specific for porocarcinoma relative to poroma. J. Cutan. Pathol. 2019, 46, 659–664. [Google Scholar] [CrossRef]
- Mitsuishi, T.; Ansai, S.; Ueno, T.; Kawana, S. Pigmented poroid neoplasm mimicking nodular melanoma. J. Dermatol. 2010, 37, 542–544. [Google Scholar] [CrossRef]
- Cazzato, G.; Cascardi, E.; Colagrande, A.; Belsito, V.; Lospalluti, L.; Foti, C.; Arezzo, F.; Dellino, M.; Casatta, N.; Lupo, C.; et al. PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience. Diagnostics 2022, 12, 2197. [Google Scholar] [CrossRef]
- Skowron, F.; Poulhalon, N.; Balme, B.; Touzet, S.; Thomas, L. Primary eccrine porocarcinoma: A clinicopathological study of 50 cases. Ann. Dermatol. Venereol. 2014, 141, 258–264. [Google Scholar] [CrossRef]
- Goyal, A.; Marghitu, T.; Goyal, N.; Rubin, N.; Patel, K.; Goyal, K.; O’leary, D.; Bohjanen, K.; Maher, I. Surgical management and lymph-node biopsy of rare malignant cutaneous adnexal carcinomas: A population-based analysis of 7591 patients. Arch. Dermatol. Res. 2021, 313, 623–632. [Google Scholar] [CrossRef]
- Martinez, S.R.; Barr, K.L.; Canter, R.J. Rare Tumors Through the Looking Glass An Examination of Malignant Cutaneous Adnexal Tumors. Arch. Dermatol. 2011, 147, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Winn, R.T.; Gazzani, P.; Venables, Z.C.; Shah, F.; Gkini, M.; Jeetle, S.; Oliphant, T.; Wijesuriya, N.; Martin-Clavijo, A.; Husain, A.; et al. Variation in management of porocarcinoma: A 10-year retrospective review of 75 cases across three UK tertiary centres. Clin. Exp. Dermatol. 2023, 48, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Tolkachjov, S.N.; Hocker, T.L.; Camilleri, M.J.; Baum, C.L. Treatment of Porocarcinoma with Mohs Micrographic Surgery: The Mayo Clinic Experience. Dermatol. Surg. 2016, 42, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Yanagi, T.; Maeda, T.; Ujiie, H. Diagnosis and Management of Porocarcinoma. Cancers 2022, 14, 5232. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.; Onishi, M.; Maeda, F.; Akasaka, T.; Sugai, T.; Amano, H. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm. Venereol. 2019, 99, 691–692. [Google Scholar] [CrossRef]
- Roka, F.; Kittler, H.; Cauzig, P.; Hoeller, C.; Hinterhuber, G.; Wolff, K.; Pehamberger, H.; Diem, E. Sentinel node status in melanoma patients is not predictive for overall survival upon multivariate analysis. Br. J. Cancer 2005, 92, 662–667. [Google Scholar] [CrossRef]
- Fujimura, T.; Hashimoto, A.; Furudate, S.; Kambayashi, Y.; Haga, T.; Aiba, S. Successful treatment of eccrine porocarcinoma metastasized to a cervical lymph node with cyberknife radiosurgery. Case Rep. Dermatol. 2014, 6, 159–163. [Google Scholar] [CrossRef]
- Mandaliya, H.; Nordman, I. Metastatic Eccrine Porocarcinoma: A Rare Case of Successful Treatment. Case Rep. Oncol. 2016, 9, 454–456. [Google Scholar] [CrossRef]
- Fionda, B.; Di Stefani, A.; Lancellotta, V.; Gentileschi, S.; Caretto, A.A.; Casa, C.; Federico, F.; Rembielak, A.; Rossi, E.; Morganti, A.G.; et al. The role of postoperative radiotherapy in eccrine porocarcinoma: A multidisciplinary systematic review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1695–1700. [Google Scholar]
- Avraham, J.B.; Villines, D.; Maker, V.K.; August, C.; Maker, A.V. Survival after resection of cutaneous adnexal carcinomas with eccrine differentiation: Risk factors and trends in outcomes. J. Surg. Oncol. 2013, 108, 57–62. [Google Scholar] [CrossRef]
- Singh, A.; Nguyen, L.; Everest, S.; Vinogradov, M. Metastatic Porocarcinoma Effectively Managed by Pembrolizumab. Cureus 2021, 13, e20004. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiogka, A.; Koumaki, D.; Kyriazopoulou, M.; Liopyris, K.; Stratigos, A.; Gregoriou, S. Eccrine Porocarcinoma: A Review of the Literature. Diagnostics 2023, 13, 1431. https://doi.org/10.3390/diagnostics13081431
Tsiogka A, Koumaki D, Kyriazopoulou M, Liopyris K, Stratigos A, Gregoriou S. Eccrine Porocarcinoma: A Review of the Literature. Diagnostics. 2023; 13(8):1431. https://doi.org/10.3390/diagnostics13081431
Chicago/Turabian StyleTsiogka, Aikaterini, Dimitra Koumaki, Maria Kyriazopoulou, Konstantinos Liopyris, Alexander Stratigos, and Stamatios Gregoriou. 2023. "Eccrine Porocarcinoma: A Review of the Literature" Diagnostics 13, no. 8: 1431. https://doi.org/10.3390/diagnostics13081431
APA StyleTsiogka, A., Koumaki, D., Kyriazopoulou, M., Liopyris, K., Stratigos, A., & Gregoriou, S. (2023). Eccrine Porocarcinoma: A Review of the Literature. Diagnostics, 13(8), 1431. https://doi.org/10.3390/diagnostics13081431







