New Technologies in the Assessment of Carotid Stenosis: Beyond the Color-Doppler Ultrasound—High Frame Rate Vector-Flow and 3D Arterial Analysis Ultrasound
Abstract
:1. Introduction
2. Methodology Section
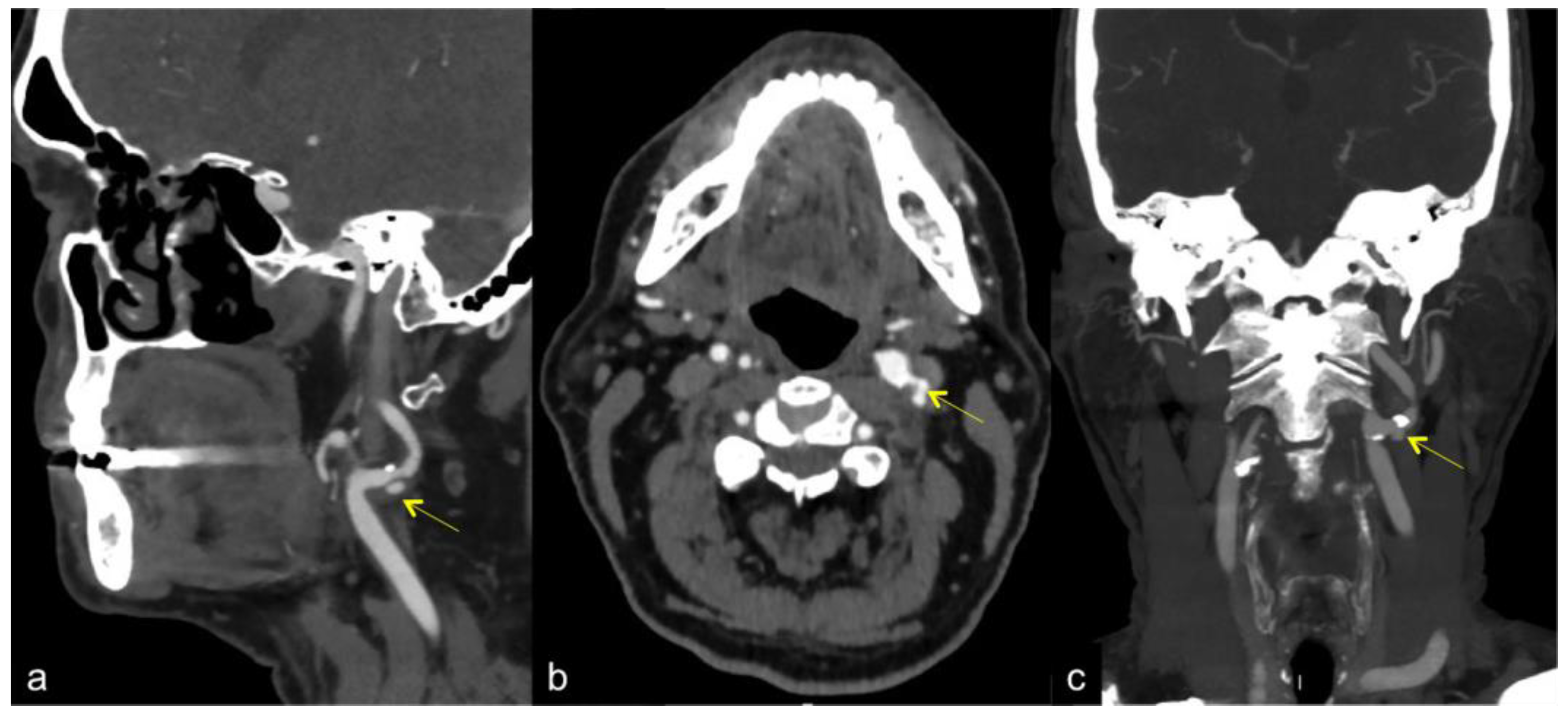
3. Computed Tomography Angiography (CTA) and Magnetic Resonance Angiography (MRA)
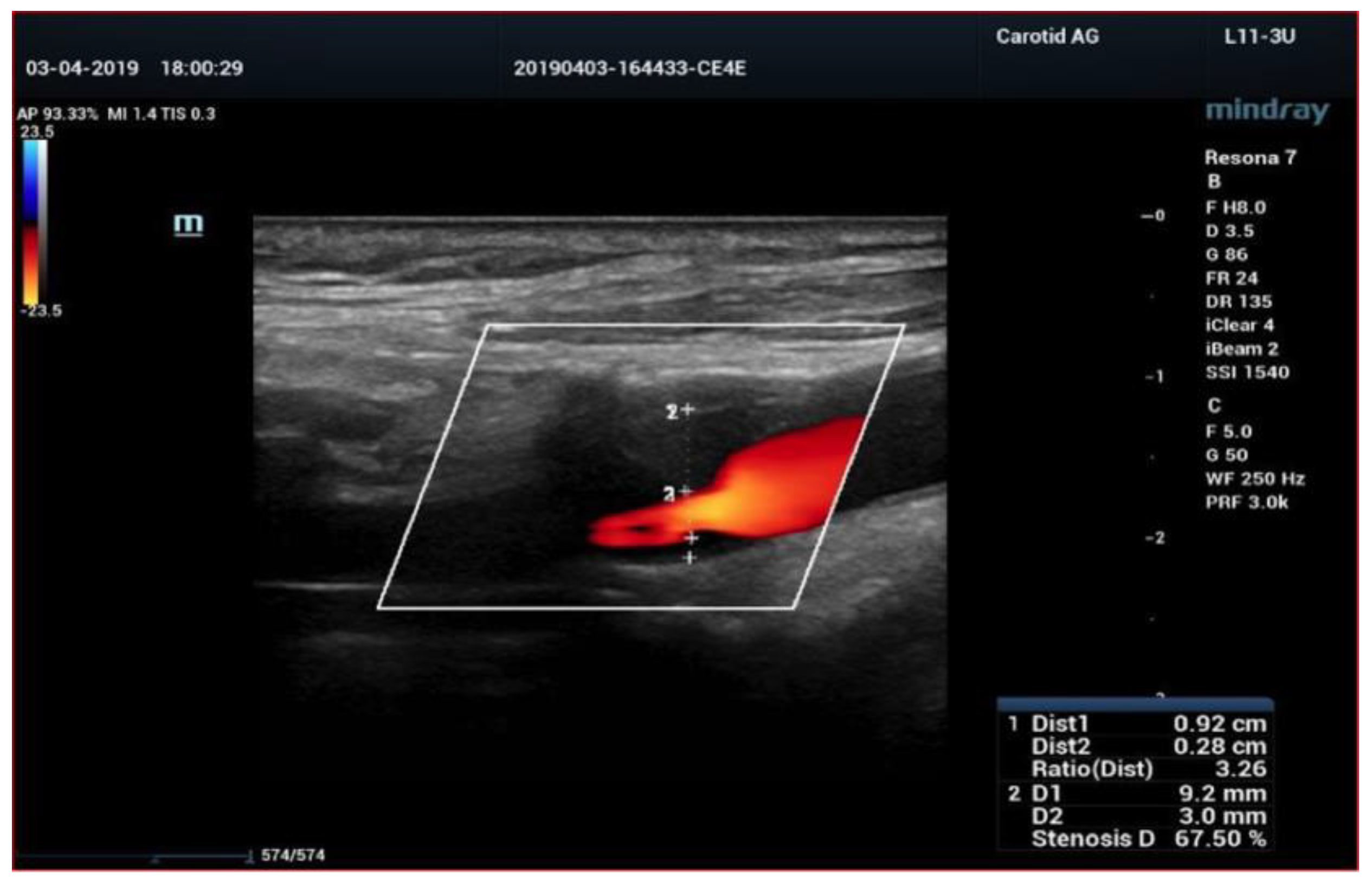
4. Color-Doppler Ultrasound (CDUS)
5. Contrast Enhanced Ultrasound (CEUS)
6. High Frame Rate Vector Flow (V-Flow) and 3D Arterial Analysis Ultrasound (3D-US)
7. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.; Dong, Y.; Mao, F.; Zhang, Q.; Yang, D.; Chen, K.; Shi, S.; Zuo, D.; Tian, X.; Yu, L.; et al. High-Frame Rate Vector Flow Imaging Technique: Initial Application in Evaluating the Hemodynamic Changes of Carotid Stenosis Caused by Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 617391. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, 721–729. [Google Scholar] [CrossRef]
- Nemoto, S. Diagnostic imaging of carotid stenosis: Ultrasound, magnetic resonance imaging, and computed tomography angiography. Nihon Geka Gakkai Zasshi 2011, 112, 371–376. [Google Scholar]
- Chappell, F.M.; Wardlaw, J.M.; Young, G.R.; Gillard, J.H.; Roditi, G.H.; Yip, B.; Pell, J.P.; Rothwell, P.M.; Brown, M.M.; Gough, M.J.; et al. Carotid artery stenosis: Accuracy of noninvasive tests–individual patient data meta-analysis. Radiology 2009, 251, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Barlinn, K.; Alexandrov, A.V. Vascular imaging in stroke: Comparative analysis. Neurotherapeutics 2011, 8, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Horev, A.; Honig, A.; Cohen, J.E.; Goldbart, A.; Dizitzer, Y.; Star, M.; Gomori, J.M.; Zlotnik, Y.; Ifergane, G.; Borodetsky, V.; et al. Overestimation of carotid stenosis on CTA—Real world experience. J. Clin. Neurosci. 2021, 85, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Baradan, H.; Gupta, A. Carotid Vessel Wall Imaging on CTA. AJNR Am. J. Neuroradiol. 2020, 41, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, W.S.; Hatsukami, T.; Yuan, C.; Zhao, X.Q. MRI of carotid atherosclerosis. AJR Am. J. Roentgenol. 2013, 200, W304–W313. [Google Scholar] [CrossRef]
- Demarco, J.K.; Ota, H.; Underhill, H.R.; Zhu, D.C.; Reeves, M.J.; Potchen, M.J.; Majid, A.; Collar, A.; Talsma, J.A.; Potru, S.; et al. MR carotid plaque imaging and contrast-enhanced MR angiography identifies lesions associated with recent ipsilateral thromboembolic symptoms: An in vivo study at 3T. AJNR Am. J. Neuroradiol. 2010, 31, 1395–1402. [Google Scholar] [CrossRef]
- Qiao, Y.; Etesami, M.; Malhotra, S.; Astor, B.C.; Virmani, R.; Kolodgie, F.D.; Trout, H.H.; Wasserman, B.A. Identification of intraplaque hemorrhage on MR angiography images: A comparison of contrast-enhanced mask and time-of-flight techniques. AJNR Am. J. Neuroradiol. 2011, 32, 454–459. [Google Scholar] [CrossRef]
- Chu, B.; Ferguson, M.S.; Chen, H.; Hippe, D.S.; Kerwin, W.S.; Canton, G.; Yuan, C.; Hatsukami, T.S. Magnetic resonance imaging features of the disruption-prone and the disrupted carotid plaque. JACC Cardiovasc. Imaging 2009, 2, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Di Leo, N.; David, E.; Clevert, D.A. Role of CEUS in Vascular Pathology. Ultraschall Med. 2021, 42, 348–366. [Google Scholar] [CrossRef] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- ECST Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Williams, M.A.; Nicolaides, A.N. Predicting the normal dimensions of the internal and external carotid arteries from the diameter of the common carotid. Eur. J. Vasc. Surg. 1987, 1, 91–96. [Google Scholar] [CrossRef]
- Murray, C.S.G.; Nahar, T.; Kalashyan, H.; Becher, H.; Nanda, N.C. Ultrasound assessment of carotid arteries: Current concepts, methodologies, diagnostic criteria, and technological advancements. Echocardiography 2018, 35, 2079–2091. [Google Scholar] [CrossRef]
- Grajo, J.R.; Barr, R.G. Duplex Doppler Sonography of the Carotid Artery: Velocity Measurements in an Artery With Contralateral Stenosis. Ultrasound Q. 2007, 23, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A. Estimation of Blood Velocities Using Ultrasound: A Signal Processing Approach; Cambridge University Press: New York, NY, USA, 1996. [Google Scholar]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar]
- Di Leo, N.; Venturini, L.; de Soccio, V.; Forte, V.; Lucchetti, P.; Cerone, G.; Alagna, G.; Caratozzolo, M.; Messineo, D.; Di Gioia, C.; et al. Multiparametric ultrasound evaluation with CEUS and shear wave elastography for carotid plaque risk stratification. J. Ultrasound 2018, 21, 293–300. [Google Scholar] [CrossRef]
- Ten Kate, G.L.; van Dijk, A.C.; van den Oord, S.C.H.; Hussain, B.; Verhagen, H.J.; Sijbrands, E.J.; van der Steen, A.F.; van der Lugt, A.; Schinkel, A.F. Usefulness of Con- trast-Enhanced Ultrasound for Detection of Carotid Plaque Ulceration in Patients With Symptomatic Carotid Atherosclerosis. Am. J. Cardiol. 2013, 112, 292–298. [Google Scholar] [CrossRef]
- Li, C.; He, W.; Guo, D.; Chen, L.; Jin, X.; Wang, W.; Huang, B.; Wang, W. Quantification of Carotid Plaque Neovascularization Using Contrast-Enhanced Ultrasound With Histopathologic Validation. Ultrasound Med. Biol. 2014, 40, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Clevert, D.A.; Helck, A.; Paprottka, P.M.; Reiser, M.F.; Jung, E.M. Contrast-enhanced ultrasound imaging of the carotid artery. Radiologe 2011, 51, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, X.; Du, L. Identification of carotid artery dissection by contrast enhanced ultrasonography. A case report. Med. Ultrason. 2015, 17, 564–565. [Google Scholar] [PubMed]
- Dempsey, R.J.; Varghese, T.; Jackson, D.C.; Wang, X.; Meshram, N.H.; Mitchell, C.C.; Hermann, B.P.; Johnson, S.C.; Berman, S.E.; Wilbrand, S.M. Carotid atherosclerotic plaque insta- bility and cognition determined by ultrasound-measured plaque strain in asymptomatic patients with significant stenosis. J. Neurosurg. 2017, 128, 111–119. [Google Scholar] [CrossRef]
- Rafailidis, V.; Chryssogonidis, I.; Xerras, C.; Nikolaou, I.; Tegos, T.; Kouskouras, K.; Rafailidis, D.; Charitanti-Kouridou, A. A comparative study of color Doppler imaging and contrast- enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur. Radiol. 2019, 29, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Fresilli, D.; Di Leo, N.; Martinelli, O.; Di Marzo, L.; Pacini, P.; Dolcetti, V.; Del Gaudio, G.; Canni, F.; Ricci, L.I.; De Vito, C.; et al. 3D-Arterial analysis software and CEUS in the assessment of severity and vulnerability of carotid atherosclerotic plaque: A comparison with CTA and histopathology. Radiol. Med. 2022, 127, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Clevert, D.A.; Sommer, W.H.; Helck, A.; Saam, T.; Reiser, M. Improved carotid atherosclerotic plaques imaging with contrast-enhanced ultrasound (CEUS). Clin. Hemorheol. Microcirc. 2011, 48, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yiu, B.Y.; Lai, S.S.; Yu, A.C. Vector projectile imaging: Time-resolved dynamic visualization of complex flow patterns. Ultrasound Med. Biol. 2014, 40, 2295–2309. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.Q.; Karuna, T.; He, X.Y.; Wang, X.M.; Li, X.F.; Liu, W.C.; Li, R.; Guo, S.Q.; Chen, Y.C.; et al. The role of wall shear stress in the parent artery as an independent variable in the formation status of anterior communicating artery aneurysms. Eur. Radiol. 2019, 29, 689–698. [Google Scholar] [CrossRef]
- Fenster, A.; Blake, C.; Gyacskov, I.; Landry, A.; Spence, J.D. 3D ultrasound analysis of carotid plaque volume and surface morphology. Ultrasonics 2006, 44 (Suppl. 1), e153–e157. [Google Scholar] [CrossRef]
- Giangregorio, F.; Garolfi, M.; Mosconi, E.; Ricevuti, L.; Debellis, M.G.; Mendozza, M.; Esposito, C.; Vigotti, E.; Cadei, D.; Abruzzese, D. High frame-rate contrast enhanced ultrasound (HIFR-CEUS) in the characterization of small hepatic lesions in cirrhotic patients. J. Ultrasound. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Jung, E.M.; Moran, V.O.; Engel, M.; Krüger-Genge, A.; Stroszczynski, C.; Jung, F. Modified contrast-enhanced ultrasonography with the new high-resolution examination technique of high frame rate contrast-enhanced ultrasound (HiFR-CEUS) for characterization of liver lesions: First results. Clin Hemorheol Microcirc. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Van Helvert, M.; Engelhard, S.; Voorneveld, J.; van der Vee, M.; Bosch, J.G.; Versluis, M.; Groot Jebbink, E.; Reijnen, M.M.P.J. High-frame-rate contrast-enhanced ultrasound particle image velocimetry in patients with a stented superficial femoral artery: A feasibility study. Eur. Radiol. Exp. 2022, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Averkiou, M.A.; Bruce, M.F.; Powers, J.E.; Sheeran, P.S.; Burns, P.N. Imaging methods for ultrasound contrast agents. Ultrasound Med. Biol 2020, 46, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Leow, C.H.; Bazigou, E.; Eckersley, R.J.; Yu, A.C.H.; Weinberg, P.D.; Tang, M.X. Flow velocity mapping using contrast enhanced high-frame-rate plane wave ultrasound and image tracking: Methods and initial in vitro and in vivo evaluation. Ultrasound Med. Biol. 2015, 41, 2913–2925. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; Bashmail, F.T.; Alzahrani, N.A.; Alharbi, S.I.; Anbar, R.; Alkharaiji, M. Is 3D ultrasound reliable for the evaluation of carotid disease? A systematic review and meta-analysis. Med. Ultrason. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Johri, A.M.; Nambi, V.; Naqvi, T.Z.; Feinstein, S.B.; Kim, E.S.H.; Park, M.M.; Becher, H.; Sillesen, H. Recommendations for the Assessment of Carotid Arterial Plaque by Ultrasound for the Characterization of Atherosclerosis and Evaluation of Cardiovascular Risk: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 917–933. [Google Scholar] [CrossRef]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Messas, E.; Goudot, G.; Halliday, A.; Sitruk, J.; Mirault, T.; Khider, L.; Saldmann, F.; Mazzolai, L.; Aboyans, V. Management of carotid stenosis for primary and secondary prevention of stroke: State-of-the-art 2020: A critical review. Eur. Heart J. Suppl. 2020, 22, M35–M42. [Google Scholar] [CrossRef]
- Naylor, A.R.; Ricco, J.-B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s choice—Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the european society for vascular surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Noubiap, J.J.; Wilman, A.H.; Saqqur, M.; Shuaib, A.; Jickling, G.C. Prevalence of high-risk plaques and risk of stroke in patients with asymptomatic carotid stenosis: A metaanalysis. JAMA Neurol. 2020, 77, 1524–1535. [Google Scholar] [CrossRef]
- Deng, F.; Mu, C.; Yang, L.; Li, H.; Xiang, X.; Li, K.; Yang, Q. Carotid plaque magnetic resonance imaging and recurrent stroke risk: A systematic review and meta-analysis. Medicine 2020, 99, e19377. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.N.; Gallagher, P.; Lovett, J.K.; Rothwell, P.M. Critical cap thickness and rupture in symptomatic carotid plaques: The oxford plaque study. Stroke 2008, 39, 1722–1729. [Google Scholar] [CrossRef]
- Dunmore, B.J.; McCarthy, M.J.; Naylor, A.R.; Brindle, N.P. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J. Vasc. Surg. 2007, 45, 155–159. [Google Scholar] [CrossRef]
- Yamada, K.; Kawasaki, M.; Yoshimura, S.; Shirakawa, M.; Uchida, K.; Shindo, S.; Nishida, S.; Iwamoto, Y.; Nakahara, S.; Sato, Y. High-intensity signal in carotid plaque on routine 3D-TOF-MRA is a risk factor of ischemic stroke. Cerebrovasc. Dis. 2016, 41, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Veken, B.; De Meyer, G.R.; Martinet, W. Intraplaque neovascularization as a novel therapeutic target in advanced atherosclerosis. Expert Opin. Ther. Targets 2016, 20, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Cantisani, V.; Grazhdani, H.; Clevert, D.A.; Iezzi, R.; Aiani, L.; Martegani, A.; Fanelli, F.; Di Marzo, L.; Wlderk, A.; Cirelli, C.; et al. EVAR: Benefts of CEUS for monitoring stent-graft status. Eur. J. Radiol. 2015, 84, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Fischer, T.; Rückert, R.I.; Oberwahrenbrock, T.; Harms, L.; Kronenberg, G.; Kunte, H. Identifcation of neovascularization by contrast-enhanced ultrasound to detect unstable carotid stenosis. PLoS ONE 2017, 12, e0175331. [Google Scholar] [CrossRef]
- Huang, R.; Abdelmoneim, S.S.; Ball, C.A.; Nhola, L.F.; Farrell, A.M.; Feinstein, S.; Mulvagh, S.L. Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J. Am. Soc. Echocardiogr. 2016, 29, 491–502. [Google Scholar] [CrossRef]
- Owen, D.R.; Shalhoub, J.; Miller, S.; Gauthier, T.; Doryforou, O.; Davies, A.H.; Leen, E.L. Infammation within carotid atherosclerotic plaque: Assessment with late-phase contrast-enhanced US. Radiology 2010, 255, 638–644. [Google Scholar] [CrossRef]
- Feinstein, S.B. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J. Am. Coll. Cardiol. 2006, 48, 236–243. [Google Scholar] [CrossRef]
- Feinstein Steven, B. The powerful microbubble: From bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am. J. Physiol.-Heart Circ. Physiol. 2004, 287, H450–H457. [Google Scholar] [CrossRef]
- Hoogi, A.; Akkus, Z.; van den Oord, S.C.; ten Kate, G.L.; Schinkel, A.F.; Bosch, J.G.; de Jong, N.; Adam, D.; van der Steen, A.F. Quantitative analysis of ultrasound contrast fow behavior in carotid plaque neovasculature. Ultrasound Med. Biol. 2012, 38, 2072–2083. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, C.S.; Li, H.X.; Shi, J.Q.; Liu, J.; Liu, Q.; Su, X.; Zhang, F.; Cheng, X.; Lu, G. Radiomics versus conventional assessment to identify symptomatic participants at carotid comupted tomography angiography. Cerebrovasc. Dis. 2022, 51, 647–654. [Google Scholar] [CrossRef] [PubMed]





| CTA | MRA | CDUS | DSA | |
|---|---|---|---|---|
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
| CEUS | |
|---|---|
| Advantages |
|
| Disadvantages |
|
| V-Flow | 3D-US | |
|---|---|---|
| Advantage |
|
|
| Disadvantage |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E.; Martinelli, O.; Pacini, P.; Di Serafino, M.; Huang, P.; Dolcetti, V.; Del Gaudio, G.; Barr, R.G.; Renda, M.; Lucarelli, G.T.; et al. New Technologies in the Assessment of Carotid Stenosis: Beyond the Color-Doppler Ultrasound—High Frame Rate Vector-Flow and 3D Arterial Analysis Ultrasound. Diagnostics 2023, 13, 1478. https://doi.org/10.3390/diagnostics13081478
David E, Martinelli O, Pacini P, Di Serafino M, Huang P, Dolcetti V, Del Gaudio G, Barr RG, Renda M, Lucarelli GT, et al. New Technologies in the Assessment of Carotid Stenosis: Beyond the Color-Doppler Ultrasound—High Frame Rate Vector-Flow and 3D Arterial Analysis Ultrasound. Diagnostics. 2023; 13(8):1478. https://doi.org/10.3390/diagnostics13081478
Chicago/Turabian StyleDavid, Emanuele, Ombretta Martinelli, Patrizia Pacini, Marco Di Serafino, Pintong Huang, Vincenzo Dolcetti, Giovanni Del Gaudio, Richard G. Barr, Maurizio Renda, Giuseppe T. Lucarelli, and et al. 2023. "New Technologies in the Assessment of Carotid Stenosis: Beyond the Color-Doppler Ultrasound—High Frame Rate Vector-Flow and 3D Arterial Analysis Ultrasound" Diagnostics 13, no. 8: 1478. https://doi.org/10.3390/diagnostics13081478





